Abstract
Objective
To compare the efficacy of ketorolac nasal spray (NS) vs placebo and sumatriptan NS for the acute treatment of migraine.
Methods
This was a randomized, double-blind, placebo and active-comparator, crossover study. Adult migraineurs were randomized to ketorolac NS 31.5 mg, sumatriptan NS 20 mg, or placebo to treat three moderate to severe migraine attacks and switched treatments with each attack. Patients seeking headache care at a headache center or in response to community advertisement were recruited. Adult participants with episodic migraine who experienced ≥2 migraine attacks per month were eligible for the Ketorolac vs Sumatriptan vs Placebo Nasal Spray migraine study. Participants were randomized to treatment arms by a research pharmacist, in a 1:1:1 ratio using computer-generated lists. The primary outcome was 2-hour pain relief. Secondary outcomes included 2-hour pain freedom and absence of migraine associated symptoms, and 24-hour sustained pain relief and pain freedom.
Results
Of the 72 randomized participants, 54 (75%) treated at least one attack and 49 (68%) completed all three treatments, for a total of 152 treated migraine attacks. Both ketorolac NS (72.5%, P < .001) and sumatriptan NS (69.4%, P=.001) were more effective than placebo (38.3%) for 2-hour pain relief and 2-hour pain freedom (ketorolac: 43.1%, P=.004; sumatriptan: 36.7%, P=.046; placebo: 18.4%). Ketorolac NS, but not sumatriptan NS, was more effective than placebo in 2-hour absence of nausea. Both ketorolac NS and sumatriptan NS were more effective than placebo for 24-hour sustained pain relief (ketorolac: 49%, P < .001; sumatriptan: 31%, P=.01, placebo: 20%). Only ketorolac NS was superior to placebo for 24-hour (ketorolac: 35.3%, P=.003; sumatriptan: 22.4%, P=.18, placebo: 12.2%) sustained pain freedom. Nasal burning and dysgeusia were the most common adverse effects for active treatments.
Conclusions
This study supports that ketorolac NS is superior to placebo and that it is non-inferior to sumatriptan NS for the acute abortive treatment of migraine.
Keywords: intranasal, migraine, moderate to severe pain, nonsteroidal anti-inflammatory, triptan, ketorolac, sumatriptan, nasal spray, treatment
INTRODUCTION
Migraine is a highly prevalent and often disabling neurological disorder affecting 10–15% of the general population worldwide.1 A typical migraine attack is characterized by unilateral head pain that is throbbing in character, aggravated by routine activity, and is moderate to severe in intensity. During an acute attack, several migraine-associated symptoms often develop, including photophobia, phonophobia, nausea and/or vomiting, and allodynia.2,3
Two of the most common classes of pharmacological interventions to treat acute migraine attacks include nonspecific abortive treatments, such as nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit prostaglandin synthesis, and migraine-specific treatments such as triptans, which are selective serotonin agonists. Both classes of medication have demonstrated efficacy for migraine in adults.4
Ketorolac is a mixed cyclooxygenase 1/2-inhibitor. It is FDA approved for moderate to severe pain in an oral, intravenous, and more recently, a nasal spray (NS) formulation.5 NS formulations in general offer several advantages to migraine patients; these include faster absorption than oral agents as well as the ability to utilize such medication formulations even when patients are nauseated and cannot or do not want to swallow a tablet or use more invasive parenteral therapies.6 While data support that parenteral ketorolac may be as effective or even more effective as some triptans and other acute abortive therapies,4,7 no study has directly compared the less invasive, intranasal ketorolac formulation to any migraine-specific therapy. We hypothesized that the efficacy, tolerability, and safety of 31.5 mg ketorolac NS would be comparable to that of 20 mg sumatriptan NS and greater than that of placebo.
METHODS
Trial Design and Participants
This was a prospective, randomized, double-blind, double-dummy, placebo and active comparator, crossover, non-inferiority trial conducted at a single outpatient headache center in Baltimore, Maryland from March 2013 to December 2014. Consecutive patients who sought care for headache or in response to community advertisement were screened by headache specialists (A.S.R., P.D., and B.L.P.) and enrolled by the primary investigator (B.L.P.) or research coordinator. Participants were eligible for the Ketorolac vs Sumatripan vs Placebo Nasal Spray (KSPN) migraine study if they were ≥18 years of age, had a history of episodic migraine (according to the International Classification of Headache Disorders, 2nd edition3) for at least 1 year, and experienced 2–10 migraine attacks per month. Participants taking migraine preventative medication were allowed to enter the study provided that their prescribed daily dose had not changed during the 3 months prior to enrollment and throughout the study period.
Participants were excluded if they had contraindications to NSAIDs or triptans, including basilar and hemiplegic migraine, cerebrovascular disease, cardiovascular disease, uncontrolled hypertension, use of any ergotamine-containing medication or monoamine oxidase inhibitor, classification as treatment resistant by the investigator, chronic pain disorders other than migraine, bleeding dyscrasias, chronic renal or hepatic impairment, substance abuse, opioid use in the past two months, chronic pulmonary disorders including nasal polyps and asthma, and a history of upper respiratory tract infection or other respiratory tract condition that could interfere with absorption or assessment of adverse effects, history of nasal surgery. Based on the information provided, participants excluded had similar characteristics as those included for randomization.
Participants were required to be headache free for at least 48 hours prior to utilization of study drug and were instructed to administer the study medication when they experienced a migraine attack with moderate or severe pain. Rescue medications were permitted 2 hours after study treatment and included: sumatriptan 20 mg NS, sumatriptan 100 mg oral, sumatriptan 4 mg subcutaneously, ketorolac 10 mg orally, antihistamines (eg, diphenhydramine 25 mg orally) and/or dopamine antagonists (eg, metoclopramide 10 mg orally).
Randomization and Masking
Participants were randomized using computer-generated lists by a research pharmacist, in a 1:1:1 ratio, to one of six sequences of ketorolac NS 31.5 mg, sumatriptan NS 20 mg, or placebo NS in blocks of 6 to treat three acute migraine attacks. Treatment sequences were as follows: (1) ketorolac NS, sumatriptan NS, placebo NS, (2) ketorolac NS, placebo NS, sumatriptan NS, (3) sumatriptan NS, ketorolac NS, placebo NS, (4) sumtriptan NS, placebo NS, ketorolac NS, (5) placebo NS, ketorolac NS, sumatriptan NS, (6) placebo NS, sumatriptan NS, ketorolac NS. Thus, participants switched study drugs with each attack, such that each participant received active drug for two of the three treated attacks (sumatriptan NS for one, ketorolac NS for the other active treatment) and placebo for one of the three treated attacks. Study drug was prepared at each visit by the research pharmacy staff following the randomization sequence where the drug was prepared and delivered in a double-blinded fashion as described below. All participants, study investigators, and study site personnel, other than the research pharmacist, were blinded to treatment allocation throughout the study. Unblinding took place after data collection was complete.
Procedures
A double-dummy design was utilized given that sumatriptan NS is administered by administering one spray in one nostril and ketorolac NS is administered by providing one spray in each nostril. For each treated attack participants utilized two study treatments (A and B), with study treatment A being administered as one spray in each nostril and study treatment B being administered as one spray in one nostril. Participants randomized to ketorolac NS administered ketorolac NS as treatment A and placebo NS study as treatment B. Participants randomized to sumatriptan NS administered placebo NS as treatment A and sumatriptan NS as treatment B. Participants randomized to placebo received placebo NS for both study treatments.
Assessments
All participants completed a standardized baseline questionnaire to assess baseline demographics and headache characteristics including disability (Headache Impact Test [HIT]-6) and allodynia (allodynia symptom checklist [ASC]-12).8–10 Additionally, all participants recorded assessments of headache and migraine associated characteristics (including photophobia, phonophobia, nausea), allodynia, disability, use of rescue medications, and any adverse effects during the 48 hours following the use of each study treatment.
During acute attacks, headache severity, migraine associated symptoms (nausea, photophobia, phonophobia), and participant self-assessment of disability were assessed using 4-point scales (none, mild, moderate, and severe) at onset of a moderate to severe migraine before treatment and repeated at 10, 15, 30, 60, and 120 minutes, as well as 24 and 48 hours after study treatment. Pain relief was defined as reduction of pain to none or mild from moderate to severe using the 4-point scale (none, mild, moderate, severe). Pain freedom was defined as an absence of pain from moderate to severe using the 4-point scale (none, mild, moderate, severe). Additionally during acute attacks, the presence of allodynia was assessed based on a series of 8 questions inquiring as to the presence of allodynia (eg, Is your scalp tender to touch?). Participants answering two or more questions positively were considered to have allodynia as previously described.9–11 For participants who reported pain-relief (reduction of pain to none or mild) or pain freedom (no pain) 2 hours after study treatment, without the use of rescue medication, the presence or absence of headache worsening within 2–24 hours and 2–48 hours was evaluated. Tolerability and safety were assessed by adverse event reports, which were assessed at each time point up to 48 hours.
Standard Protocol Approvals, Registrations, and Patient Consents
The KSPN Migraine Study was registered at http://www.clinicaltrials.gov/ (NCT 01807234) and approved by the Johns Hopkins School of Medicine Institutional Review Board. All participants gave informed written consent.
Outcomes
The primary outcome was 2-hour headache relief. Secondary outcomes included 2-hour: (1) pain freedom; (2) absence of migraine associated symptoms including photophobia, phonophobia, and nausea; (3) absence of allodynia; (4) participant self-assessment of disability; as well as (5) 24 and 48 sustained pain relief (SPR) and sustained pain freedom (SPF); and (6) the time to pain relief, as defined as the time when pain relief was first observed and maintained through 2 hours with no rescue medication use at or prior to this point. Additionally, time-to-rescue medication use and efficacy in presence vs absence of allodynia were included as exploratory analyses.
Statistical Analysis
Baseline demographic and clinical characteristics were summarized using means (± standard deviation) for continuous variables and counts and percentages for categorical variables. For skewed variables median [interquartile range] were provided. To evaluate the effect of the three treatment arms on the primary outcome, a mixed-effects model was used in which the treatment group (sumatriptan, ketorolac, placebo) was a fixed factor and subjects were random factors. Separate models were built using maximum likelihood estimation for the primary and each secondary outcome described below.
Further, we utilized survival analysis (time-to-pain- relief and time-to-rescue medication use) for all time points (between 10 minutes to 2 hours for both survival analyses as well as 10 minutes to 48 hours for time-to-rescue medication use) to calculate a hazard ratio comparing sumatriptan NS and ketorolac NS treatment arms to placebo using Cox proportional hazards models. All reported P-values are 2-sided and deemed statistically significant at α=0.05. All analyses were performed using STATA 13.0 statistical software for Windows (Statacorp, College Station, TX, USA).
Power and Sample Size
The power and sample size calculation was based on the primary endpoint of the study, pain relief at 2 hours after treatment. Based on prior studies, we estimated that 50 patients would be required for a 2-tailed significance level of 0.05 and a power of 80% to detect a 28% difference in pain relief for treatment groups vs placebo.12–14
RESULTS
Participant Enrollment and Demographics
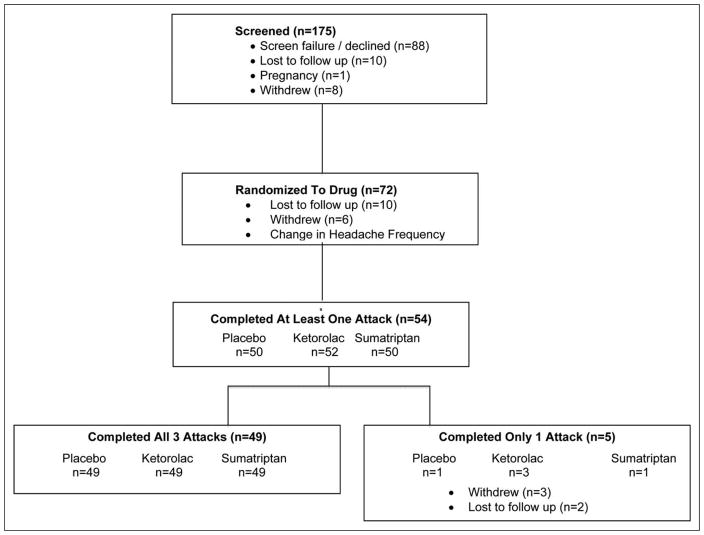
Of the 72 participants who were randomized, 54 utilized at least one dose of study medication, for a total of 152 treated migraine attacks included in analyses (Fig. 1). Demographic and headache characteristics of participants are displayed in Table 1. Participants’ medical diagnoses and medication usage are displayed in Supporting Information Table e-1.
Fig. 1.
Consort flow diagram for the Ketorolac vs Sumatripan vs Placebo Nasal Spray migraine study.
Table 1.
Demographic and Headache Characteristics of Ketorolac vs Sumatripan vs Placebo Nasal Spray Migraine Study Participants
| Characteristics | n | % | ||
|---|---|---|---|---|
|
|
|
|||
| Age | Mean (SD) | 36.3 (9.8) | ||
| Race | Caucasian | 43 | 79.6 | |
| African American | 7 | 12.9 | ||
| Other | 4 | 7.4 | ||
| Sex | Female | 53 | 98.1 | |
| Male | 1 | 1.9 | ||
| Marital status | Single | 20 | 37.0 | |
| Married | 27 | 50.0 | ||
| Other | 7 | 13.0 | ||
| Education | HS | 17 | 1.5 | |
| College | 24 | 44.4 | ||
| Post Grad | 12 | 44.2 | ||
| Unknown | 1 | 1.9 | ||
| Income | <50 K | 18 | 33.3 | |
| >50 K | 35 | 64.8 | ||
| Unknown | 1 | 1.9 | ||
| Baseline HA Characteristics | ||||
| Age at 1st HA | Median (IQR) | 12 (6) | ||
| Age at Mig Dx | Median (IQR) | 22 (14) | ||
| Monthly HA frequency | Median (IQR) | 6 (4) | ||
| ASC total | Median (IQR) | 2 (6) | ||
| HIT-6 total | Mean (SD) | 60.4 (7.7) | ||
| Acute Attack HA Characteristics (Pretreatment) | ||||
| Nausea | 25 | 46.3 | ||
| Vomiting | 1 | 1.3 | ||
| Photophobia | 42 | 78.3 | ||
| Phonophobia | 38 | 70.4 | ||
| Aura | 14 | 28.6 | ||
| Number of alloydnia sx | Median (IQR) | 1 (2) | ||
| Disability | Mild | 19 | 37.5 | |
| Moderate/Severe | 23 | 45.4 | ||
Dx = diagnosis; HA = headache; IQR = interquartile ratio; SD = standard deviation; sx = symptoms.
Efficacy
For the primary endpoint of 2-hour pain relief, both ketorolac NS (72.5%; CI: 59.9–85.2, P<.001) and sumatriptan NS (69.4%; CI: 56.0–82.8, P=.001) were superior to placebo (38.8%; CI: 24.6–52.9) (Table 2, Supporting Information Fig. 1). In other words, ketorolac NS was 33.7% (95%CI: 15.4–52.1) and sumatriptan NS 30.6% (95%CI: 11.8–49.3) more effective than placebo for 2-hour pain relief. For secondary endpoints, both ketorolac NS (43.1%; CI: 29.1–57.2, P=.004) and sumatriptan NS (36.7%; CI: 22.7–50.7, P=.046) were superior to placebo (18.4%; CI: 7.1–29.6) for 2-hour pain freedom (Table 2, Supporting Information Fig. 2). Additionally, both ketorolac NS and sumatriptan NS were superior to placebo for time-to-pain-relief (Fig. 2, Supporting Information Table e-2), 2-hour freedom from photophobia and 2–24 hour SPR.
Table 2.
Percentage of Patients (95% CI) With Pain Relief and Pain Freedom in the Ketorolac vs Sumatripan vs Placebo Nasal Migraine Study
| Placebo % (95% CI) | Ketorolac NS % (95% CI) | P-Value* vs Placebo | Sumatriptan NS % (95% CI) | P-Value vs Placebo | P-Value vs Ketorolac | |
|---|---|---|---|---|---|---|
| 2-hour pain relief | 38.8 (24.6–52.9) | 72.5 (59.9–85.2) | <.001 | 69.4 (56.0–82.8) | .001 | .724 |
| 2–24-hour sustained pain relief | 20.4 (8.7–32.1) | 49.0 (34.8–63.2) | <.001 | 40.8 (26.5–55.1) | .011 | .070 |
| 2–48-hour sustained pain relief | 20.4 (8.7–32.1) | 49.0 (34.8–63.2) | <.001 | 30.6 (17.2–43.9) | .200 | .313 |
| 2-hour pain freedom | 18.4 (7.1–29.6) | 43.1 (29.1–57.2) | .004 | 36.7 (22.7–50.7) | .046 | .513 |
| 2–24-hour sustained pain freedom | 12.2 (2.7–21.7) | 35.3 (21.7–48.9) | .003 | 22.4 (10.3–34.5) | .184 | .381 |
| 2–48-hour sustained pain freedom | 12.2 (2.7–21.7) | 33.3 (19.9–46.7) | .006 | 18.4 (7.1–29.6) | .060 | .915 |
P-values computed using a mixed longitudinal model.
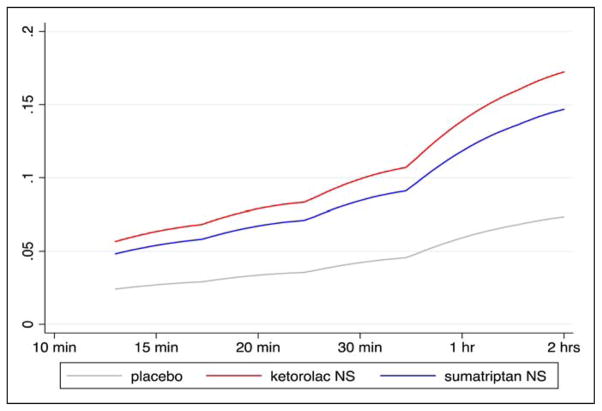
Fig. 2.
Time-to-pain-relief. The time-to-pain-relief in the Ketorolac vs Sumatripan vs Placebo Nasal Spray migraine study was conducted using survival analysis, an approach taking into account all time points between 10 and 120 minutes. Hazard ratios (HR) are calculated indicating whether each of the two treatment groups are superior to placebo. Both ketorolac NS (HR 2.34; 95% CI 1.88–3.04) and sumatriptan NS (HR 2.00; 95%CI: 1.53–2.61) had a faster time to pain relief at any time in the 2-hour period after treatment vs placebo. There was no difference between ketorolac NS and sumatriptan NS, P =.129. Smoothed hazard curves are presented above by treatment group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Only ketorolac NS was superior to placebo for 2-hour freedom from nausea and phonophobia (Table 3), 2–24 hour SPF (ketorolac NS: 35.3%; CI: 21.7–48.9, P=.003; sumatriptan NS: 22.4%; CI: 10.3–34.5, P =.18; placebo: 12.2%; CI: 2.7–21.7), as well as 2–48 hour SPR (ketorolac NS: 49%; CI: 34.8–63.2, P<.001; sumatriptan NS: 30.6%; CI:17.2–43.9, P=.20; placebo: 20.4%; CI: 8.7–32.1) and 2–48 SPF, (Supporting Information Figs. 1 and 2).
Table 3.
Percentage of Patients (95% CI) With 2-Hour Freedom From Migraine Associated Symptoms in the Ketorolac vs Sumatripan vs Placebo Nasal Spray Migraine Study
| Placebo % (95% CI) | Ketorolac NS % (95% CI) | P-Value* vs Placebo | Sumatriptan NS % (95% CI) | P-Value vs Placebo | P-Value vs Ketorolac | |
|---|---|---|---|---|---|---|
| No phonophobia, 2 hrs | 56.0 (46.1–65.9) | 75.0 (66.5–83.4) | .007 | 66.0 (56.5–75.4) | .091 | .278 |
| No photophobia, 2 hrs | 46.0 (36.1–55.9) | 65.4 (56.1–74.7) | .016 | 64.0 (54.4–73.6) | .011 | .848 |
| No nausea, 2 hrs | 66.0 (56.5–75.4) | 82.7 (75.3–90.0) | .036 | 74.0 (65.2–82.7) | .317 | .267 |
| No allodynia, 2 hrs | 69.0 (57.6–83.5) | 70.5 (57.6–83.5) | .477 | 75.5 (63.0–87.9) | .373 | .538 |
P-values computed using a mixed longitudinal model; hrs =hours.
Allodynia
There was no statistically significant difference in 2-hour freedom from allodynia in those treated with either ketorolac NS or sumatriptan NS as compared to placebo (Table 3). Additionally, we explored the extent to which the primary endpoints differ by treatment arms after stratifying according to presence or absence of baseline allodynia. In those with no allodynia the 2-hour pain relief and painfreedom rates were greater for both the ketorolac NS and sumatriptan NS treatment arms as compared to placebo (Table 4). In the presence of allodynia, although there was no statistical difference in 2-hour pain freedom, there were differences in 2-hour pain relief across treatment arms. Specifically, in the presence of moderate to severe allodynia, those treated with ketorolac NS (76.9%; CI: 50.4–99.4, P=.012), but not sumatriptan NS (54.5%; CI: 19.5–89.6, P=.19), had a statistically significant reduction in 2 hour painrelief as compared to placebo (Table 4).
Table 4.
Two-Hour Pain-Relief and Pain-Freedom From Migraine in Presence or Absence of Baseline Allodynia in the Ketorolac vs Sumatripan vs Placebo Nasal Spray Migraine Study
| Placebo % (95% CI) | Ketorolac NS % (95% CI) | P-Value vs Placebo | Sumatriptan NS % (95% CI) | P-Value vs Placebo | P-Value vs Ketorolac | |
|---|---|---|---|---|---|---|
| No Allodynia | ||||||
| 2-HR Pain Relief | 38.2 (21.0–55.4) | 70.9 (50.0–87.9) | .004 | 70.6 (54.4–86.7) | .004 | .968 |
| 2-HR Pain Freedom | 11.7 (3.5–23.2) | 45.2 (26.6–63.7) | .001 | 32.3 (15.8–48.9) | .042 | .201 |
| Any Allodynia | ||||||
| 2-HR Pain Relief | 40.1(11.9–68.1) | 75.0 (54.2–95.8) | .027 | 66.7 (39.6–93.7) | .114 | .598 |
| 2-HR Pain Freedom | 33.3 (6.3–60.3) | 40.0 (16.5–63.5) | .693 | 46.7 (18.1–75.2) | .449 | .679 |
| Mod-Severe Allodynia | ||||||
| 2-HR Pain Relief | 30.0 (−4.5–64.5) | 76.9 (50.4–99.4) | .012 | 54.5 (19.5–89.6) | .190 | .233 |
| 2-HR Pain Freedom | 30.0 (−4.5–64.5) | 38.4 (7.8–69.1) | .663 | 36.3 (2.5–70.2) | .757 | .905 |
Any allodynia ≥2 allodynic symptoms; moderate to severe: ≥3 allodynic symptoms. HR =hour.
Rescue Medication and Disability
In the first 2 hours after study treatment, participants in the ketorolac NS treatment arm (HR=0.39; 95%CI: 0.18–0.86; P-value=.019) were 61% less likely, and those in the sumatriptan NS treatment arm (HR=0.47; 95%CI: 0.23–0.95; P-value=.036) 53% less likely, to use rescue medication as compared to placebo. However, over the full 48 hours, while participants in the ketorolac NS treatment arm were 43% less likely to use rescue medication (HR 0.57; 95%CI: 0.37–0.86; P-value=.008), there was not a statistically significant reduction in rescue medication use in the sumatriptan NS arm (HR=0.67; 95%CI: 0.45–1.01; P-value=.058). Additionally, participants in the ketorolac NS (OR=0.46, 95%CI: 0.28–0.77; P-value=.003), but not the sumatriptan NS (OR=0.76, 95%CI: 0.49–1.21; P-value=.25) treatment arm, reported a greater reduction in 2-hour disability as compared to placebo.
Additional analyses excluding men did not change significance of primary and secondary aim findings.
Tolerability and Safety
Of the 54 participants who utilized active treatment at least once, no participant withdrew from the study due to adverse events. The most common adverse events reported by participants treated with ketorolac NS were burning of the nose, (mild in 25.5%, moderate in 19.6%, and severe in 3.9%), unusual taste (mild in 2%, moderate in 5.9%, severe 2%), nasal discomfort (8%), burning of the throat (6%), fatigue (4%), dizziness (4%), nausea (2%), and rash (2%). For those treated with sumatriptan NS the most common adverse events were unusual taste (mild in 24.5%, moderate in 12.2%, severe 4.1%), burning of the nose (mild in 6.1%, moderate in 2%), nausea (8%), burning of the throat (6%), nasal discomfort (6%), dizziness (4%), fatigue (4%), and rash (2%). The most common adverse event for placebo were unusual taste (mild in 4%, moderate in 2%), nausea (4%), rash (4%), fatigue (4%), burning of the nose (2%), and dizziness (2%).
DISCUSSION
The KSPN migraine study was a phase 4, randomized, double-blind, double-dummy, crossover, comparative efficacy study evaluating ketorolac NS vs sumatriptan NS vs placebo NS for acute migraine therapy. Both ketorolac and sumatriptan NS treatments were effective for acute migraine treatment. As compared to placebo, both ketorolac NS and sumatriptan NS were superior for the primary endpoint of 2-hour pain relief, as well as several secondary endpoints including 2-hour pain freedom, 2-hour freedom from photophobia, time-to-pain relief, and 24-hour SPR. In addition, acute abortive therapy with ketorolac NS was superior to placebo for 2-hour freedom from nausea and photophobia as well as for both the 24-hour and 48-hour SPR and pain freedom secondary end-points. As with intranasal triptan formulations, the current findings support that ketorolac NS may be appropriate to consider in migraine patients with nausea or rapid onset of moderate to severe attacks.6 Additionally, this study supports that ketorolac NS may be an appropriate consideration for those patients who prefer or need a non-triptan NS abortive agent.
Although intravenous and intramuscular ketorolac have level B level of efficacy for acute migraine, nasal formulations of ketorolac for migraine are currently only level C.4 Only one previous study has evaluated the efficacy of an intranasal formulation of ketorolac for the acute treatment of migraine.15 In this prior multicenter, double-blind, placebo-controlled study (n=158), Pfaffenrath et al evaluated intranasal ketorolac tromethamine, containing 6% lidocaine (ROX-828) as compared to placebo for the treatment of acute migraine. Both studies included episodic migraine participants with a headache frequency ranging between 2–8 headache days per month in the Pfaffenrath study and 2–10 headache days per month in the current study. In contrast to the current study, 2-hour pain freedom was the primary endpoint in the Pfaffenrath et al study, and was not different in those treated with intranasal ketorolac with lidocaine as compared to placebo. 15 In the KSPN study, 18.4% of those treated with placebo achieved 2-hour pain freedom, as compared to 43% of those treated with ketorolac NS (P=.004) and 37% of those treated with sumatriptan NS (P=.046). However, in both studies 2-hour pain-relief was greater than placebo for the ketorolac NS formulation. In the Pfaffenrath et al study, 52% of those treated with ROX-828 achieved 2-hour pain relief as compared to 32% for placebo.15 In the KSPN migraine study, 72% of those treated with ketorolac NS and 69% of those treated with sumatriptan NS achieved 2-hour pain relief as compared to 39% of those treated with placebo. Both studies also demonstrated a significant reduction in nausea. While the two studies had largely similar inclusion and exclusion criteria, it is possible that subtle differences in the study design of each of these studies contributed to the differences for 2-hour pain relief. In the KSPN migraine study, the ketorolac formulation did not include lidocaine. Additionally, the KSPN migraine study criteria included exclusion of opioid use in the prior 2 months before enrollment, and thus may have limited inclusion of some treatment resistant participants.
There are several limitations and strengths of the KSPN migraine study. While the total number of participants enrolled in this single site study was relatively small (n=54), the cross-over designed allowed for evaluation of over 150 acute migraine attacks and helped to reduce participant variation, such as can occur in multicenter studies and those with parallel treatment arm designs. Additionally although only one primary outcome was set, a wide range of important secondary outcome parameters were a priori specified and evaluated. Finally, the most common adverse effects reported for both ketorolac NS and sumatriptan NS were nasal burning (ketorolac>sumatriptan) and an unusual taste (sumatriptan>ketorolac). Both were mild to moderate for the majority of patients treated with active treatments. Further, although 3.9% of participants reported severe nasal burning with ketorolac NS, no participants withdrew from the study due to this side effect. In the previous trial evaluating ROX-828 vs placebo, nasal discomfort was also the most common adverse event, despite the inclusion of lidocaine.15 Thus, while it is possible that research personnel and participants in the KSPN study may have been able to “guess” which treatment was utilized based on the presence of nasal burning and dysgeusia, given these symptoms were reported by both active treatment arms as well as those given placebo we do not believe it substantially affected the blinding of this study.
The KSPN migraine study demonstrates that the nonsteroidal anti-inflammatory NS formulation of ketorolac is superior to placebo and is non-inferior to the triptan NS formulation of sumatriptan for the acute abortive treatment of moderate to severe migraine. As with triptan intranasal formulations, intranasal ketorolac may be particularly appropriate to consider for acute abortive migraine treatment when nausea or oral medications are not able to be used, and additionally offers an effective alternative for those who cannot or do not want to use a triptan NS.
Supplementary Material
Fig. e-1. Percentage of participants with pain relief over time in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray migraine study.
Fig. e-2. Percentage of participants with pain freedom over time in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray migraine study.
Table e-1: Baseline Medical Diagnoses and Medication Usage by Participants in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray Migraine Study.
Table e-2: Percentage of Patients (95% CI) with Pain Relief and Pain Freedom Between 10 Minutes and 1 Hour in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray Migraine Study.
Acknowledgments
Funding: This study was funded by an investigator initiated grant from Luitpold Pharmaceuticals and the NIH/NINDS (K23-NS078345) to Dr. Peterlin and Egalet Ltd to Dr. Gelaye. Dr. Peterlin serves on the editorial boards for the journals Headache and Neurology.
Footnotes
Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the U.S. government.
Statistical Analysis: Statistical analysis of data was primarily performed by Bizu Gelaye, PhD.
Registration: The KSPN Migraine Study was registered at http://www.clinicaltrials.gov/ (NCT 01807234).
STATEMENT OF AUTHORSHIP
Category 1
-
Conception and DesignB. Lee Peterlin, Tobias Kurth
-
Acquisition of DataAruna S. Rao
-
Analysis and Interpretation of DataAruna S. Rao, Bizu Gelaye, Tobias Kurth, Paul D. Dash, Haley Nitchie, B. Lee Peterlin
Category 2
-
Drafting the ManuscriptAruna S. Rao
-
Revising It for Intellectual ContentBizu Gelaye, Tobias Kurth, Paul D. Dash, Haley Nitchie, B. Lee Peterlin
Category 3
-
Final Approval of the Completed ManuscriptAruna S. Rao, Bizu Gelaye, Tobias Kurth, Paul D. Dash, Haley Nitchie, B. Lee Peterlin
Conflict of Interest: Dr. Peterlin: Grant support from the Landenberger Foundation and GSK within the past 5 years for research studies unrelated to current manuscript.
References
- 1.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 2.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. Cephalalgia. (2) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 4.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3–20. doi: 10.1111/head.12499. [DOI] [PubMed] [Google Scholar]
- 5.He A, Hersh EV. A review of intranasal ketorolac tromethamine for the short-term management of moderate to moderately severe pain that requires analgesia at the opioid level. Curr Med Res Opin. 2012;28:1873–1880. doi: 10.1185/03007995.2012.744302. [DOI] [PubMed] [Google Scholar]
- 6.Tepper SJ, Chen S, Reidenbach F, Rapoport AM. Intranasal zolmitriptan for the treatment of acute migraine. Headache. 2013;53(Suppl 2):62–71. doi: 10.1111/head.12181. [DOI] [PubMed] [Google Scholar]
- 7.Taggart E, Doran S, Kokotillo A, Campbell S, Villa-Roel C, Rowe BH. Ketorolac in the treatment of acute migraine: A systematic review. Headache. 2013;53:277–287. doi: 10.1111/head.12009. [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the headache impact test (HIT-6) across episodic and chronic migraine. Cephalalgia. 2011;31:357–367. doi: 10.1177/0333102410379890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakubowski M, Silberstein S, Ashkenazi A, Burstein R. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. 2005;65:1419–1422. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Silberstein S, Jakubowski M, Burstein R. Improved identification of allodynic migraine patients using a questionnaire. Cephalalgia. 2007;27:325–329. doi: 10.1111/j.1468-2982.2007.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tepper SJ, Kori SH, Borland SW, et al. Efficacy and safety of MAP0004, orally inhaled DHE in treating migraines with and without allodynia. Headache. 2012;52:37–47. doi: 10.1111/j.1526-4610.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 12.Ryan R, Elkind A, Baker CC, Mullican W, DeBussey S, Asgharnejad M. Sumatriptan nasal spray for the acute treatment of migraine. Results of two clinical studies. Neurology. 1997;49:1225–1230. doi: 10.1212/wnl.49.5.1225. [DOI] [PubMed] [Google Scholar]
- 13.Peikert A, Becker WJ, Ashford EA, Dahlof C, Hassani H, Salonen RJ. Sumatriptan nasal spray: A dose-ranging study in the acute treatment of migraine. Eur J Neurol. 1999;6:43–49. doi: 10.1046/j.1468-1331.1999.610043.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown C, Moodie J, Bisley E, Bynum L. Intranasal ketorolac for postoperative pain: A phase 3, double-blind, randomized study. Pain Med. 2009;10:1106–1114. doi: 10.1111/j.1526-4637.2009.00647.x. [DOI] [PubMed] [Google Scholar]
- 15.Pfaffenrath V, Fenzl E, Bregman D, Farkkila M. Intranasal ketorolac tromethamine (SPRIX (R)) containing 6% of lidocaine (ROX-828) for acute treatment of migraine: Safety and efficacy data from a phase II clinical trial. Cephalalgia. 2012;32:766–777. doi: 10.1177/0333102412451359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. e-1. Percentage of participants with pain relief over time in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray migraine study.
Fig. e-2. Percentage of participants with pain freedom over time in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray migraine study.
Table e-1: Baseline Medical Diagnoses and Medication Usage by Participants in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray Migraine Study.
Table e-2: Percentage of Patients (95% CI) with Pain Relief and Pain Freedom Between 10 Minutes and 1 Hour in the Ketorolac vs Sumatriptan vs Placebo Nasal Spray Migraine Study.