Abstract
Objective
Addiction is often conceptualized as a behavioral strategy for avoiding negative experiences. In rodents, opioid intake has been associated with abnormal acquisition and extinction of avoidance behavior. Here, we tested the hypothesis that these findings would generalize to human opioid-dependent subjects.
Method
Adults meeting DSM-IV criteria for heroin-dependence and treated with opioid medication (n=27), and healthy controls (n=26), were recruited between March–October 2013 and given a computer-based task to assess avoidance behavior. On this task, subjects controlled a spaceship and could either gain points by shooting an enemy spaceship, or hide in safe areas to avoid on-screen aversive events.
Results
While groups did not differ on escape responding (hiding) during the aversive event, heroin-dependent males (but not females) made more avoidance responses during a warning signal that predicted the aversive event (ANOVA, sex × group interaction, p=0.007). This group was also slower to extinguish the avoidance response when the aversive event no longer followed the warning signal (p=0.011). This behavioral pattern resulted in reduced opportunity to obtain reward without reducing risk of punishment. Results suggest that differences in avoidance behavior cannot be easily explained by impaired task performance or by exaggerated motor activity in male patients.
Conclusion
This study provides evidence for abnormal acquisition and extinction of avoidance behavior in opioid-dependent patients. Interestingly, data suggest abnormal avoidance is demonstrated only by male patients. Findings shed light on cognitive and behavioral manifestations of opioid addiction, and may facilitate development of therapeutic approaches to help affected individuals.
Keywords: Addiction, avoidance, extinction, opioid dependence, heroin dependence, sex differences
Introduction
Addiction is often conceptualized as an avoidance behavior: alcohol addicts often drink to avoid dysphoric emotions or negative mood,1 gamblers often gamble to block out their problems,2 and substance users report using addictive substances in an attempt to cope with stress, escape reality, as well as to avoid the aversive drug-withdrawal symptoms.3–8 Indeed, escape and avoidance of negative affect was argued to be the principal motive for addictive drug use, where addicts attempt to reduce aversive internal states.9, 10 Surprisingly, while both avoidance behavior and substance misuse are strategies for coping with negative and painful effects, evidence for the link between these two constructs in humans is scant and is based on self-report measures.11–13 The animal literature, however, has provided important empirical parallels between avoidance behavior and drug intake, and suggests addictive behavior to be a form of avoidance learning.14 One type of addiction that has been extensively studied in animals, including in the context of avoidance behavior, is opioid addiction.5–7, 15–24 While reports often showed increased avoidance behavior in rodents that were given opioids,15–19 this was not always the case.20–22, 24
Extinction of conditioned avoidance behavior, i.e., refraining from avoidance responding when the aversive event no longer occurs, may also be affected by opioid intake. In rodents, opioid receptors in the midbrain have been shown to regulate extinction of aversive conditioning,25 opioid agonists decreased avoidance during extinction of free-operant avoidance,24 and opiate seeking behavior is extinguished slowly, with a high risk of relapse.26 Indeed, evidence suggests that rodents with history of opioid use tend to respond to drug cues even when drugs are absent.27, 28
Importantly, avoidance paradigms often include an appetitive component, which might compete with the avoidance response. Thus, any observed impairment on avoidance behavior might be the result of reduced motivation to obtain reward, rather than an increased motivation to avoid punishment. One might argue that such motivational imbalance represents anhedonia, impaired capacity to experience pleasure. Anhedonia is a symptom in various psychiatric conditions including substance use disorders.29 Since anhedonic patterns might affect avoidance behavior, it is of importance to dissociate the appetitive versus aversive components of the observed behavior.
In this study, we assess the balance between reward-seeking and avoidance behavior in treated heroin-dependent patients, as compared with healthy controls. By using a simple computer-based task that captures several key features of common animal avoidance paradigms,30–32 we attempt to bridge the gap between human and non-human opioid addiction research. We hypothesize that, as in the animal literature, patients will show abnormal acquisition and/or extinction of avoidance behavior.
Methods
Subjects
The patient group consisted of 27 individuals with history of heroin addiction (mean age=41.3 years, SD=10.6; 44.4% female), recruited from the Opioid Treatment Program Clinic at the Drug Health Services at the Royal Prince Alfred Hospital in Sydney, Australia. Opioid dependence was confirmed using DSM-IV criteria and urine drug screening; dependence for substances other than heroin was an exclusion criteria. All patients were being treated with opioid medication; 22 were on methadone (mean dose=66.7 mg, SD=42) and five were on buprenorphine (mean dose=19.6 mg, SD=6.4). One patient was transferred to another site after testing and his medical record was not available. For the remaining 26 patients: mean admission time to the clinic was 3.8 years (SD=4.2) before the experiment, and testing was conducted 1–6 hours after daily dose. These patients reported mean heroin addiction duration of 15.8 years (SD=10.5), with a daily dose of 353.8 mg (SD=248.6) before treatment. Twelve patients were diagnosed with no other DSM-IV psychiatric disorders (Axis I or Axis II), while others were diagnosed with schizophrenia (7), depression (4), panic disorder (1), bipolar disorder (1) and cluster B personality disorder (1). Clinical diagnosis was based on interview with a psychiatrist and retrieved from patients’ medical records. There were no differences in sex or age between patients who were or were not diagnosed with other disorders.
The control group consisted of 26 healthy adults recruited from the community via referrals and word of mouth (mean age=38.3 years, SD=11.1; 65.4% female). Subjects who reported current substance dependence or other DSM-IV psychiatric disorders were excluded. No differences were observed between patients and controls on age and sex. Ethics approval was obtained from the Royal Prince Alfred Hospital Ethics Committee and from the Ethics Committee at the University of Western Sydney. All subjects provided written informed consent and the experiment was conducted in accordance with guidelines established by the Declaration of Helsinki for the protection of human subjects.
Escape-avoidance task
To test avoidance behavior, subjects were administered a simple computer-based task recently developed by our group,31, 32 and based on earlier work by Molet et al.30 On this task (Figure 1), subjects controlled a spaceship and were instructed to gain points by shooting and destroying an enemy spaceship that randomly appeared on the screen. Every 20 s, rectangles appeared for 5 s at the top of the screen (warning period). On each of the 12 acquisition trials, a warning period was always followed by appearance of a bomb for another 5 s (bomb period). During the bomb period there was an explosion of the subjects’ spaceship and a reduction of points. The bomb period was followed by a 10-s intertrial period during which subjects could gain points without any risk of aversive events. Twelve extinction trials followed, during which no bombs appeared. At the bottom corners of the screen, there were two “safe areas” where subjects could protect themselves from the aversive events, but were unable to gain points.
Figure 1.
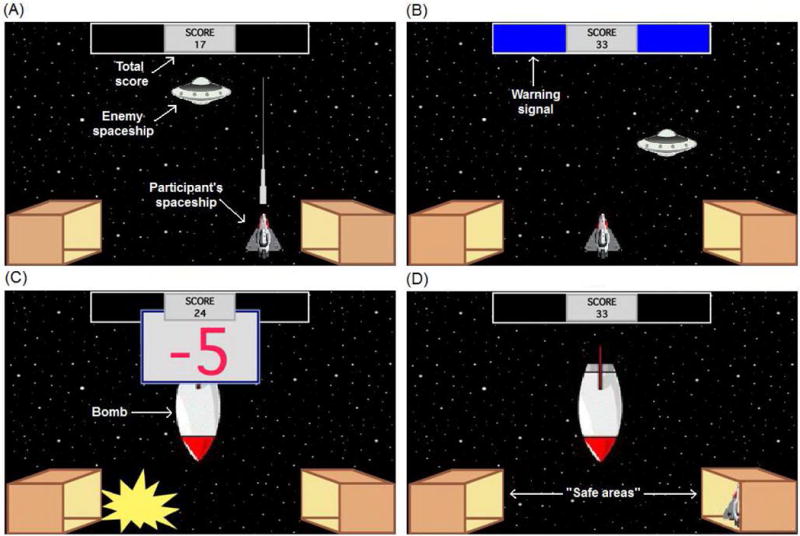
Computer-based escape-avoidance task. (A) An enemy spaceship appears in one of six locations on the screen, approximately every 1 s. The participant’s goal is to gain points by shooting and destroying this spaceship (1 point for each hit). (B) The warning signal is two colored rectangles at the top of the screen, which appear every 20 s and remain visible for 5 s (warning period). (C) The warning signal is always followed by appearance of a bomb, which remains onscreen for 5 s (bomb period). The bomb period is divided into five segments of equal duration; during each segment there is an explosion and loss of 5 points to a maximum of 25 points. (D) At the bottom corners of the screen, there are two box-shaped areas representing “safe areas.” Moving the subject’s spaceship to one of those boxes is defined as “hiding.” While hiding, the subject’s spaceship cannot be destroyed and no points can be lost, but neither can the subject shoot the enemy spaceship and gain points. Subjects were not given any explicit instructions about the safe areas or the hiding response. Labels shown in white text are for illustration only and do not appear on the screen during the task.
Variations of this task have been previously used to test different aspects of human avoidance behavior.30, 33–35 Importantly, recent work using a similar task revealed that subjects with increased anxiety vulnerability demonstrated greater avoidance.31, 32
Data analysis
For each trial, the program computed the percentage of time the subject spent hiding during the 5-s warning period, the 5 s that followed the warning period, and the remaining 10-s intertrial period. On acquisition trials, the bomb period follows the warning period, whereas on extinction trials there is no bomb period, and the intertrial period is extended to 15 s for consistency with the acquisition trials. Hiding during the bomb period represents an escape response, and terminates point loss, while hiding during the warning period represents an avoidance response that might completely prevent any point loss. To assess overall performance on the task, total points gained during the entire session, number of shooting attempts (presses on the FIRE key) and subjects’ motor activity (presses on the LEFT/RIGHT keys), were recorded.
To test behavioral differences between groups, we used mixed analysis of variance (ANOVA) with within-subject factor of trial (12 trials per phase) and between-subject factors of group (patients versus controls) and sex. Dependent variables were percentage of time spent hiding during acquisition and extinction phase on each period (warning, bomb and intertrial). Sphericity was checked by Mauchly’s test and Greenhouse-Geisser correction was used when sphericity was violated. Univariate ANOVA was used to analyze total points, shooting and motor activity, with group and sex as the independent variables.
Results
We first analyzed hiding during the 5-s warning period (Figure 2). On the acquisition phase, mixed ANOVA revealed main effects of Trial [F(6.2,301.4)=2.348, p=0.030], Sex [F(1,49)=5.022, p=0.030] and Group [F(1,49)=12.567, p=0.001], and a Sex × Group interaction [F(1,49)=7.974, p=0.007]. On the extinction phase, analyses revealed main effects of Trial [F(7.9,385.8)=2.441, p=0.014] and Group [F(1,49)=10.824, p=0.002], and a Sex × Group interaction [F(1,49)=6.945, p=0.011]. Tukey’s HSD tests revealed that male patients hid more than all the other groups on both the acquisition and extinction phases (all p<0.010).
Figure 2.
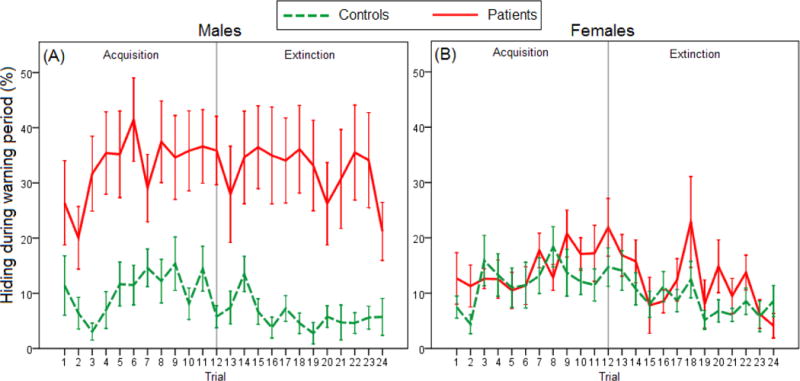
Acquisition and extinction of hiding behavior during the warning period in (A) male patients versus male controls (n=15 and 9, respectively), and in (B) female patients versus female controls (n=12 and 17, respectively). On the acquisition phase, there were main effects of Trial, Sex and Group, as well as a Sex x Group interaction (mixed ANOVA, all p<0.050). On the extinction phase, analyses revealed main effects of Trial and Group and a Sex x Group interaction (all p<0.050). Tukey’s HSD tests revealed that male patients hid more than all the other groups on both the acquisition and extinction phases. Error bars indicate SEM.
We next analyzed hiding during the 5 s that followed the warning signal (Figure 3). On the acquisition phase, when this period is the bomb period, mixed ANOVA revealed a main effect of trial [F(4.9,242)=22.869, p<0.001]. While a Trial × Group interaction was also found [F(4.9,242)=2.663, p=0.024], post-hoc investigation found no differences between groups on any of the trials (independent t-tests, all p>0.100). On the extinction phase, when this period is the first 5-s of the intertrial period, analyses revealed a main effect of Group [F(1,49)=8.274, p=0.006] and a Sex × Group interaction [F(1,49)=4.749, p=0.034]. Tukey’s HSD test revealed that, during extinction, male patients hid more than male and female controls (both p<0.010) and tended to hide more that female patients (p=0.052).
Figure 3.
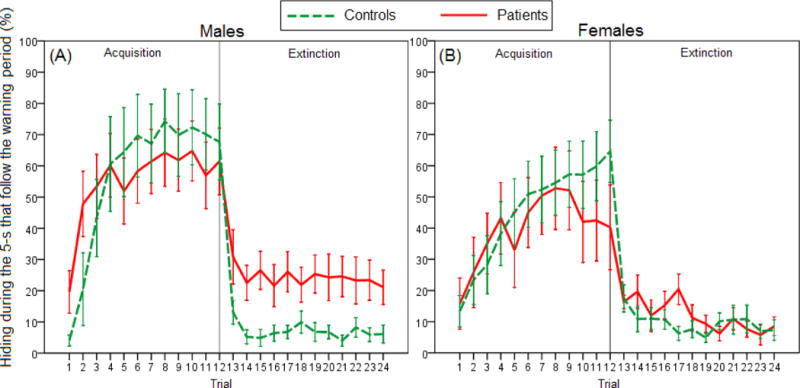
Acquisition and extinction of hiding behavior during the 5 s that follow the warning period in (A) male patients versus male controls (n=15 and 9, respectively), and in (B) female patients versus female controls (n=12 and 17, respectively). On the acquisition phase, when this period was a bomb period, there was a main effect of Trial and Trial x Group interaction (mixed ANOVA, both p<0.050), although post-hoc investigation of the interaction did not show significant effects. On the extinction phase, when this period was the first 5 s of the intertrial period, there were main effects of Group and Sex × Group interaction (both p<0.050), with male patients hiding more than male and female controls and a tending to hide more than female patients. Error bars indicate SEM.
We then analyzed hiding during the intertrial period (Figure 4). On the acquisition phase, mixed ANOVA revealed a main effect of Group, with patients hiding more than controls [F(1,49)=6.250, p=0.016], while the effect of Sex approached significance [F(1,49)=3.911, p=0.080]. On the extinction phase, only the main effect of Group appeared again [F(1,49)=6.012, p=0.018].
Figure 4.
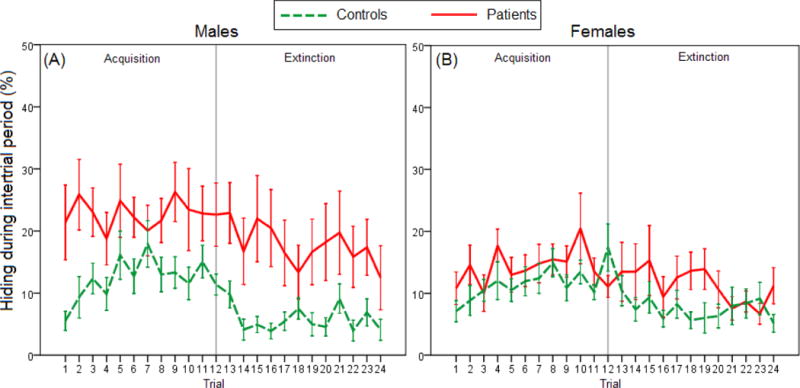
Hiding behavior during the 10-s intertrial period in (A) male patients versus male controls (n=15 and 9, respectively), and in (B) female patients versus female controls (n=12 and 17, respectively). On both acquisition and extinction phases, there was a main effect of Group, with patients hiding more than controls overall (mixed ANOVA, both p<0.050). Error bars indicate SEM.
We also tested overall task performance. Univariate ANOVA on total points gained during the entire session revealed a main effect of Group [F(1,49)=16.911, p<0.001] and Sex × Group interaction [F(1,49)=6.180, p=0.016]. Tukey’s HSD tests showed that male controls gained more points than all the other groups (all p<0.050; Figure 5A). Similarly, when shooting was analyzed, while a main effect of Sex approached significance [F(1,49)=3.458, p=0.069], a significant Sex × Group interaction was shown [F(1,49)=6.175, p=0.016]. Tukey’s HSD tests revealed that male controls shot more than female controls (p=0.022) and tended to shoot more than male patients (p=0.057; Figure 5B). However, when we analyzed motor activity, no significant main effects or interactions were found (all p>0.100; Figure 5C).
Figure 5.
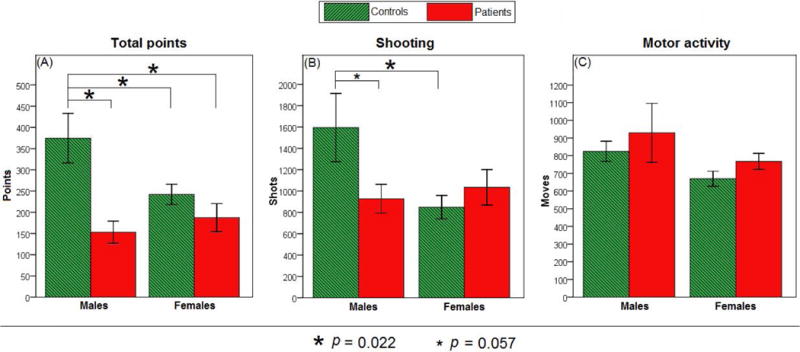
Overall performance on the computer-based task in male and female patients (n=15 and 12, respectively) versus male and female controls (n=9 and 17, respectively). (A) Total points gained during the entire session. Main effect of Group and Sex × Group interaction were shown (both p<0.050); male controls gained more points than all the other groups (all p<0.050). (B) Number of shooting attempts (FIRE keypresses). A Sex × Group interaction was shown (p<0.050); male controls shot more than female controls (p=0.022) and tended to shoot more than male patients (p=0.057). (C) Motor activity (LEFT and RIGHT keypresses). No differences were found (all p>0.100).
When analyses were repeated on only the methadone treatment group, behavioral differences remained the same (data not shown). It is also important to note that patients’ medication maintenance dose did not correlate with any of the described behavioral variables (all p>0.100).
Lastly, we tested whether comorbidity with other DSM-IV psychiatric disorders affected behavior. During the warning signal on acquisition phase, patients with comorbidities made approximately twice more hiding responses than patients without comorbidities (mixed ANOVA, p=0.013). Thus, we repeated analysis of hiding during this period (Figure 2; acquisition phase), with the inclusion of comorbid status as a covariate. As in the original analysis, mixed ANCOVA revealed a Sex × Group interaction [F(1,47)=6.303, p=0.016], with male patients hiding more than other groups. Hiding during other task periods, as well as overall task performance, did not differ between the two comorbidity groups (all p>0.090).
Discussion
The purpose of the current study was to examine acquisition and extinction of avoidance behavior in opioid-dependent patients. Consistent with prior results in the animal literature, these findings show abnormal learning of avoidance behavior in humans treated for heroin dependence. Specifically, male patients demonstrated more overall hiding during the warning period for the acquisition and extinction trials and more hiding during the 5-s that follow the warning period for the extinction trials. Exaggerated hiding during these periods represents non-optimal behavior in the current paradigm, as it prevents the ability to obtain reward (points), without minimizing punishment (explosions and point loss). This behavioral pattern is reminiscent of the compulsive nature of substance addiction, where drug use continues despite negative consequences.10,36
Male patients’ high levels of hiding during the warning periods on the acquisition trials represent exaggerated learning of avoidance behavior. Such responding, before the initiation of the aversive event, might relate to impaired impulse control that could contribute to addicts’ difficulty in inhibiting drug-taking action.37 Increased learning of the association between the warning signal and the following aversive event is also consistent with the idea of exaggerated associative learning in addicts, where increased tendency to associate discrete stimuli with specific drugs might underlie addictive behavior.38, 39 Moreover, male patients extinguished more slowly, and exhibited more hiding during the extinction phase. Continued responding during extinction is believed to represent increased impulsivity, impaired disinhibition,28, 37 might result in responding to drug-related cues when the drugs themselves are no longer available, and closely resembles the diagnostic criterion for substance dependence that addresses the subject’s difficulty in restricting drug use.40
Many prior studies examining opioid-dependence in humans have been based solely on male addicts.41–43 However, females compose a significant portion of the general addict population,44 and often show distinct personal characteristics and patterns of abuse.45, 46 In the current study, while all patients demonstrated increased overall hiding responding compared to controls, exaggerated avoidance was demonstrated only by male patients. It is possible that female patients are more sensitive to the reward in the current task, leading them to hide less.47, 48 However, neither female patients nor female controls gained more points or shot more times than their male counterparts, arguing against the idea of higher reward sensitivity in females. While interpretation of the observed sex effect remains speculative and awaits further investigation, the current results suggest that including both sexes should be of a high priority in any addiction research.
Differences in reinforcement sensitivities might also be involved in the unique avoidance pattern in male patients. The current task is characterized by a motivational conflict between the need to hide to avoid possible punishment and the option to stay at the center, to shoot the enemy spaceship and obtain point reward. Thus, the exaggerated avoidance in male patients might actually be the result of decreased reward-seeking, rather than increased tendency to avoid punishment. This idea of decreased motivation for reward is partially supported by fewer total points and fewer shooting attempts in male patients compared to male controls, and is consistent with a large literature that shows reduced reward sensitivity (i.e., anhedonia)29, 49, 50 and undervaluation of nondrug-related reward51, 52 in substance users. However, decreased reward-seeking behavior is insufficient to explain avoidance differences on the current task, since female controls obtained less reward (fewer points) than male controls but showed no differences on avoidance responding. It is possible that male patients had abnormal learning of both the appetitive and aversive components of the current task53 or had lower learning rates, which impaired the ability of reinforcement to alter their behavior.54
One can also argue that the exaggerated hiding by male patients in the current study might be the result of elevated baseline responding, rather than a specific learning pattern.22 To address such a possibility, we analyzed motor activity, as indicated by subjects’ tendency to move their spaceship, and found no group differences. Furthermore, male patients showed generally more hiding during the warning period than during the intertrial period, and demonstrated a clear understanding of the protective nature of the hiding response, as demonstrated by their rapid learning during the bomb period. All these suggest that exaggerated avoidance behavior in male patients is a learned response that can not be simply explained by increased motor activity.
This study has important implications for therapy. First, while previous reports of avoidance behavior in addicts have relied on self-report,11–13 the current study presents a more objective tool to assess specific behavior patterns that might be abnormal in this population. Second, better understanding of sex-related differences in heroin patients might help explain why males and females often differ on treatment outcomes,45, 55 and why sex-specific treatments should be considered.56 Specifically, since treatment strategies often focus on facilitating extinction of drug-related memories,26, 57–59 the current results suggest that male patients might have more trouble extinguishing and thus, might better benefit from such therapies. Future work could also test whether the addition of specific “safety signals” during therapy could attenuate the exaggerated avoidance behavior, as suggested by a recent study examining the effect of adding such “safety signals” to this task.32
This study comes with the following limitations. First, 53.8% of patients in the current study reported comorbidity with other DSM-IV psychiatric disorders. While some previous studies reported comparable rates (e.g. 47–55%60, 61), other studies reported higher rates (e.g. 70–75%62, 63). Indeed, a review of 14 studies found that among treatment-seeking opioid-users, comorbidity rates are typically between 40–80%.64 Such heterogeneity in reported comorbidities might be associated with variations in methods and populations,60, 65 as well as with increasing availability of opioid substitution clinics, which could result in the admission of individuals with milder symptoms.60 Low prevalence of psychiatric comorbidity in the current study might also be due to the treatment itself, as previously suggested by a study that reported a comorbidity rate of 57.6% in patients receiving treatment for drug use.66 Importantly, since avoidance behavior is a predominant symptom in anxiety disorders, such comorbidity could potentially affect avoidance behavior in the current study. To address this possibility, we repeated analyses only for those subjects without comorbidity and showed that the group differences were maintained, suggesting a basic association between opioid addiction and avoidance behavior. The low comorbidity rate in the current study further supports this association, irrespective of other confounding variables. To promote generalizability of the results to clinical populations, future studies could examine larger and more heterogenic patient groups, or alternatively, specifically target and compare groups of opioid addicts with different diagnosed comorbidities. A special attention to comorbidity with schizophrenia would also be important, as participants in the current study had a comorbidity rate two-fold higher than previously reported.67
Second, as subjects were tested a relatively short time after daily medication dose (1–6 hours), the acute and the chronic effects of the medication cannot be dissociated.68 To this end, future work could better control dosing and testing times and test patients immediately after and just before the daily medication dose,69 as well as analyzing withdrawal symptoms that might be differentially experienced during the inter-dosing interval.70 It would also be important to dissociate the overall effects of opioid medication in treated addicts from the behavior that characterizes treatment-naïve addicts. Furthermore, although both methadone and buprenorphine are opioid medications that have been recommended and shown to provide positive effects in opioid-dependent patients,71–75 differences do exist.72–74 While the current study included both medications in the patient group, when analyses were repeated only for the methadone group, the behavioral differences remained the same. Future studies could specifically compare different medication groups, or alternatively, focus on one specific medication.
Another issue is whether sex differences in avoidance are related to treatment outcomes. Assuming that drug-taking involves a desire to avoid an aversive state, successful maintenance therapy should normalize or at least decrease avoidance behavior. It is interesting to note that female addicts might have better treatment outcomes than male addicts,76 so what emerged as a sex effect in the current study could actually reflect a treatment effect. Future studies could specifically examine whether reduced avoidance on the computer task is correlated with treatment success, perhaps via a longitudinal study that compared baseline versus post-treatment performance as a function of treatment success. However, it is also entirely possible that the current results reflect a true sex difference, particularly given known gender differences in drug pharmacokinetics and pharmacodynamics.45 Other physiological, psychological and cultural factors could also contribute to different treatment effects in males and females.45, 55 However, these ideas remain speculative and require further work that would specifically study the effects of medication in opioid addicts.
It is also important to address the validity of the described computer-based task. The use of such “spaceship” tasks to study human avoidance behavior has been gaining popularity in recent years.30–35 These prior reports suggest that subjects are generally motivated to gain points and successfully learn to avoid on-screen aversive events on these tasks. They further suggest that the tasks can be used to study specific aspects of avoidance behavior (e.g., passive avoidance,33 active avoidance,30–32 differential effects of reinforcement contingencies and contextual variables,34 and discriminative learning and context-dependent latent inhibition35). Our recent work has demonstrated that this task is also adequate for studying individual differences, specifically showing that anxiety vulnerable individuals demonstrate more hiding on both the acquisition and extinction phases of the task.31, 32 However, all these prior studies tested undergraduate students in European or American institutions, while the current study examined opioid-dependent patients and healthy controls in Australia. Additional large-scale multi-site studies with healthy and psychiatric populations, including more racial and ethnic diversity, would be useful to establish normative values for the various dependent variables on this task. Thus, rather than proposing a diagnostic tool where numerical values are the focus (i.e., defining cutoffs for diagnosis criteria), we here targeted relative group differences that could teach us about basic mechanisms responsible for pathological outcomes in addicts.
It should be noted that the current study has primarily targeted the differences between patients and healthy controls. The reported interaction with sex is interesting, but should be treated with caution and awaits further confirmation from studies with larger group sizes of males and females in each experimental condition. Further, while overall task performance in male versus female controls (Figure 5) is generally consistent with recent findings,32 prior studies in healthy young adults reported longer avoidance duration in females than males,31, 32 a pattern which was not observed in the current study. Such discrepancy could be the result of different demographic characteristics, as well as the overall lower hiding rates by control subjects in the current study; specific investigations of sex-related differences in various healthy populations should be performed. Lastly, future studies would also benefit from inclusion of self-report questionnaires regarding subjects’ experience with computer games and incentive for good performance,77 as well as the change in their experience of negative affect (between baseline and directly after task completion)78, 79 - factors that could bias performance on the computer task.
In sum, while limitations do exist and should be addressed in future work, this is a novel study that assessed escape-avoidance behavior in opioid-dependent patients. As hypothesized, patients showed abnormal learning of this behavior, compared to healthy controls. Overall, the current findings may help bridge the gap between human and non-human research on opioid addiction, promote our understanding of the cognitive and behavioral manifestations of this condition and advance therapeutic approaches to help affected individuals.
Clinical Points.
While addiction in general, and opioid-addiction in particular, are often conceptualized as avoidance strategies, the literature on avoidance behavior in opioid-dependent patients is little and is primarily based on self-report measures.
Consistent with reports from animal literature, opioid-dependent patients in the current study exhibited greater acquisition and impaired extinction of the avoidance behavior. Interestingly, these differences were found only within male subjects. Results support the idea that avoidance might be a mechanism that underlies addiction and contributes to its growth and persistence.
This study demonstrates an objective tool to assess avoidance behavior in opioid addicts. Furthermore, the results suggest abnormal behavior patterns and sex-related differences that might facilitate personalized therapeutic approaches (e.g., exposure-based therapies) in this patient group.
Acknowledgments
The authors want to thank Leonid Sheynin, MD, PhD (Public Health Association and Drug Addiction Department, Ministry of Health, Israel) for valuable discussions and Daniel Barnett, BS and Joseph Phillips, MS (School of Social Sciences and Psychology, University of Western Sydney, Sydney, NSW, Australia) for assistance with data collection. All individuals report no financial or other relationship relevant to the subject of this article.
Funding/support: This work was partially supported by the NSF/NIH Collaborative Research in Computational Neuroscience (CRCNS) Program, by NIAAA (R01 AA018737), and by additional support from the Stress & Motivated Behavior Institute (SMBI).
Role of sponsor: The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The views in this paper are those of the authors and do not represent the official views of the funders, the Department of Veterans Affairs or the U.S. government.
Footnotes
Potential conflict of interest: None reported.
References
- 1.Cahalan D, Cisin IH, Crossley HM. Monographs of the Rutgers Center of Alcohol Studies. 260. New Brunswick, NJ: 1969. American drinking practices: A national study of drinking behavior and attitudes. (Monograph 6). [Google Scholar]
- 2.Wood RT, Griffiths MD, Parke J. Acquisition, development, and maintenance of online poker playing in a student sample. CyberPsychol & Behav. 2007 Jun;10(3):354–361. doi: 10.1089/cpb.2006.9944. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001 Dec;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 4.Bizzarri JV, Sbrana A, Rucci P, et al. The spectrum of substance abuse in bipolar disorder: reasons for use, sensation seeking and substance sensitivity. Bipol Dis. 2007 May;9(3):213–220. doi: 10.1111/j.1399-5618.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- 5.Nichols JR, Headlee CP, Coppock HW. Drug addiction. I. Addiction by escape training. J Am Pharmacists Assoc. 1956 Dec;45(12):788–791. [PubMed] [Google Scholar]
- 6.Wikler A. Conditioning factors in opiate addiction and relapse. In: Wilner DM, Kasselbaum GG, editors. Narcotics. New York: McGraw-Hill; 1965. [Google Scholar]
- 7.Downs DA, Woods JH. Fixed-ratio escape and avoidance-escape from naloxone in morphine-dependent monkeys: effects of naloxone dose and morphine pretreatment. J Exp Anal Behav. 1975 May;23(3):415–427. doi: 10.1901/jeab.1975.23-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blume AW. Negative reinforcement and substance abuse: Using a behavioral conceptualization to enhance treatment. The Behavior Analyst Today. 2001;2(2):86–90. [Google Scholar]
- 9.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004 Jan;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsyth JP, Parker JD, Finlay CG. Anxiety sensitivity, controllability, and experiential avoidance and their relation to drug of choice and addiction severity in a residential sample of substance-abusing veterans. Addict Behav. 2003 Jul;28(5):851–870. doi: 10.1016/s0306-4603(02)00216-2. [DOI] [PubMed] [Google Scholar]
- 12.Simpson T, Jakupcak M, Luterek JA. Fear and avoidance of internal experiences among patients with substance use disorders and PTSD: the centrality of anxiety sensitivity. J Trauma Stress. 2006 Aug;19(4):481–491. doi: 10.1002/jts.20128. [DOI] [PubMed] [Google Scholar]
- 13.Mandelli L, Mazza M, Di Nicola M, et al. Role of substance abuse comorbidity and personality on the outcome of depression in bipolar disorder: harm avoidance influences medium-term treatment outcome. Psychopathology. 2012;45(3):174–178. doi: 10.1159/000330364. [DOI] [PubMed] [Google Scholar]
- 14.Trafton CL, Marques PR. Effects of septal area and cingulate cortex lesions on opiate addiction behavior in rats. J Comp Physiol Psychol. 1971 May;75(2):277–285. doi: 10.1037/h0030810. [DOI] [PubMed] [Google Scholar]
- 15.Mondadori C, Waser PG. Facilitation of memory processing by posttrial morphine: possible involvement of reinforcement mechanisms? Psychopharmacol (Berl) 1979 Jun 21;63(3):297–300. doi: 10.1007/BF00433566. [DOI] [PubMed] [Google Scholar]
- 16.Tramullas M, Martinez-Cue C, Hurle MA. Facilitation of avoidance behaviour in mice chronically treated with heroin or methadone. Behav Brain Res. 2008 Jun 3;189(2):332–340. doi: 10.1016/j.bbr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Holtzman SG. Tolerance to the stimulant effects of morphine and pentazocine on avoidance responding in the rat. Psychopharmacol. 1974;39(1):23–37. doi: 10.1007/BF00421455. [DOI] [PubMed] [Google Scholar]
- 18.Staubli U, Huston JP. Avoidance learning enhanced by post-trial morphine injection. Behav Neural Biology. 1980 Apr;28(4):487–490. doi: 10.1016/s0163-1047(80)91860-9. [DOI] [PubMed] [Google Scholar]
- 19.Shannon HE. Stimulation of avoidance behavior by buprenorphine in rats. Psychopharmacol (Berl) 1983;80(1):19–23. doi: 10.1007/BF00427487. [DOI] [PubMed] [Google Scholar]
- 20.Ageel AM, Chin L, Trafton CL, Jones BC, Picchioni AL. Acute effects of morphine and chlorpromazine on the acquisition of shuttle box conditioned avoidance response. Psychopharmacol. 1976 Apr 15;46(3):311–315. doi: 10.1007/BF00421120. [DOI] [PubMed] [Google Scholar]
- 21.Satinder KP. Differential effects of morphine on two-way avoidance in selectively bred rat strains. Psychopharmacol (Berl) 1976 Jul 28;48(2):235–237. doi: 10.1007/BF00423267. [DOI] [PubMed] [Google Scholar]
- 22.Aguilar MA, Minarro J, Simon VM. Dose-dependent impairing effects of morphine on avoidance acquisition and performance in male mice. Neurobiol Learning and Memory. 1998 Mar;69(2):92–105. doi: 10.1006/nlme.1997.3804. [DOI] [PubMed] [Google Scholar]
- 23.Mucha RF. What is learned during opiate withdrawal conditioning? Evidence for a cue avoidance model. Psychopharmacol (Berl) 1991;104(3):391–396. doi: 10.1007/BF02246041. [DOI] [PubMed] [Google Scholar]
- 24.Fernando A, Urcelay G, Mar A, Dickinson A, Robbins T. Free-Operant Avoidance Behavior by Rats after Reinforcer Revaluation Using Opioid Agonists and D-Amphetamine. J Neurosci. 2014 Apr 30;34(18):6286–6293. doi: 10.1523/JNEUROSCI.4146-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004 Aug 4;24(31):6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrichs SC, Leite-Morris KA, Carey RJ, Kaplan GB. Baclofen enhances extinction of opiate conditioned place preference. Behav Brain Res. 2010 Mar 5;207(2):353–359. doi: 10.1016/j.bbr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacol. 2000 Apr;22(4):413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 28.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000 Aug;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 29.Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molet M, Leconte C, Rosas JM. Acquisition, extinction and temporal discrimination in human conditioned avoidance. Behav Process. 2006 Sep;73(2):199–208. doi: 10.1016/j.beproc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Sheynin J, Beck KD, Pang KC, et al. Behaviourally inhibited temperament and female sex, two vulnerability factors for anxiety disorders, facilitate conditioned avoidance (also) in humans. Behav Process. 2014 Jan 9;103:228–235. doi: 10.1016/j.beproc.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheynin J, Beck KD, Servatius RJ, Myers CE. Acquisition and extinction of human avoidance behavior: Attenuating effect of safety signals and associations with anxiety vulnerabilities. Front Behav Neurosci. 2014;8:323. doi: 10.3389/fnbeh.2014.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arcediano F, Ortega N, Matute H. A behavioural preparation for the study of human Pavlovian conditioning. Q J Exp Psychol B. 1996 Aug;49(3):270–283. doi: 10.1080/713932633. [DOI] [PubMed] [Google Scholar]
- 34.Raia CP, Shillingford SW, Miller HL, Jr, Baier PS. Interaction of procedural factors in human performance on yoked schedules. J Exp Anal Behav. 2000 Nov;74(3):265–281. doi: 10.1901/jeab.2000.74-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byron Nelson J, del Carmen Sanjuan M. A context-specific latent inhibition effect in a human conditioned suppression task. Q J Exp Psychol (Hove) 2006 Jun;59(6):1003–1020. doi: 10.1080/17470210500417738. [DOI] [PubMed] [Google Scholar]
- 36.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nature Rev Neurosci. 2001 Oct;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 37.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005 Nov;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 38.Di Chiara G, Tanda G, Bassareo V, et al. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann New York Acad Sci. 1999 Jun 29;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 39.Frenois F, Le Moine C, Cador M. The motivational component of withdrawal in opiate addiction: role of associative learning and aversive memory in opiate addiction from a behavioral, anatomical and functional perspective. Nature Rev Neurosci. 2005;16(3):255–276. doi: 10.1515/revneuro.2005.16.3.255. [DOI] [PubMed] [Google Scholar]
- 40.Karamustafalioglu OK, Zohar J, Guveli M, et al. Natural course of posttraumatic stress disorder: a 20-month prospective study of Turkish earthquake survivors. J Clin Psychiatry. 2006 Jun;67(6):882–889. doi: 10.4088/jcp.v67n0604. [DOI] [PubMed] [Google Scholar]
- 41.Teh LK, Izuddin AF, M HF, Zakaria ZA, Salleh MZ. Tridimensional personalities and polymorphism of dopamine D2 receptor among heroin addicts. Biol Res Nurs. 2012 Apr;14(2):188–196. doi: 10.1177/1099800411405030. [DOI] [PubMed] [Google Scholar]
- 42.Eisenberg E, Cohen D, Lawental E, Pud D. Personality traits and sensitivity to pain in male chronic opioid addicts. J Opioid Management. 2007 Jul-Aug;3(4):225–230. doi: 10.5055/jom.2007.0008. [DOI] [PubMed] [Google Scholar]
- 43.Gerra G, Bertacca S, Zaimovic A, Pirani M, Branchi B, Ferri M. Relationship of personality traits and drug of choice by cocaine addicts and heroin addicts. Substance Use & Misuse. 2008;43(3–4):317–330. doi: 10.1080/10826080701202726. [DOI] [PubMed] [Google Scholar]
- 44.Eldred CA, Washington MN. Female heroin addicts in a city treatment program: the forgotten minority. Psychiatry. 1975 Feb;38(1):75–85. doi: 10.1080/00332747.1975.11023836. [DOI] [PubMed] [Google Scholar]
- 45.Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Women’s Health (London, England) 2008 Jan;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- 46.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008 Jan;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bobzean SA, Denobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014 Feb 6; doi: 10.1016/j.expneurol.2014.01.022. In Press. [DOI] [PubMed] [Google Scholar]
- 48.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trend Pharmacol Sci. 2004 May;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Garfield JB, Lubman DI, Yucel M. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Australian & New Zealand J Psychiatry. 2014 Jan;48(1):36–51. doi: 10.1177/0004867413508455. [DOI] [PubMed] [Google Scholar]
- 50.Martin-Soelch C, Chevalley AF, Kunig G, et al. Changes in reward-induced brain activation in opiate addicts. European J Neurosci. 2001 Oct;14(8):1360–1368. doi: 10.1046/j.0953-816x.2001.01753.x. [DOI] [PubMed] [Google Scholar]
- 51.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 52.Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008 Nov;59(1):164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Gradin VB, Baldacchino A, Balfour D, Matthews K, Steele JD. Abnormal brain activity during a reward and loss task in opiate-dependent patients receiving methadone maintenance therapy. Neuropsychopharmacol. 2014 Mar;39(4):885–894. doi: 10.1038/npp.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychol Med. 2010 Mar;40(3):433–440. doi: 10.1017/S0033291709990468. [DOI] [PubMed] [Google Scholar]
- 55.Nelson-Zlupko L, Kauffman E, Dore MM. Gender differences in drug addiction and treatment: implications for social work intervention with substance-abusing women. Soc Work. 1995 Jan;40(1):45–54. [PubMed] [Google Scholar]
- 56.Hodgins DC, el-Guebaly N, Addington J. Treatment of substance abusers: single or mixed gender programs? Addiction. 1997 Jul;92(7):805–812. [PubMed] [Google Scholar]
- 57.Myers KM, Carlezon WA., Jr Extinction of drug- and withdrawal-paired cues in animal models: relevance to the treatment of addiction. Neurosci Biobehav Rev. 2010 Nov;35(2):285–302. doi: 10.1016/j.neubiorev.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacol (Berl) 2013 Apr;226(4):659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNally GP. Extinction of drug seeking: Neural circuits and approaches to augmentation. Neuropharmacol. 2014 Jan;76(Pt B):528–532. doi: 10.1016/j.neuropharm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997 Jan;54(1):71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 61.Milby JB, Sims MK, Khuder S, et al. Psychiatric comorbidity: prevalence in methadone maintenance treatment. Am J Drug and Alcohol Abuse. 1996 Feb;22(1):95–107. doi: 10.3109/00952999609001647. [DOI] [PubMed] [Google Scholar]
- 62.Rounsaville BJ, Weissman MM, Kleber H, Wilber C. Heterogeneity of psychiatric diagnosis in treated opiate addicts. Arch Gen Psychiatry. 1982 Feb;39(2):161–168. doi: 10.1001/archpsyc.1982.04290020027006. [DOI] [PubMed] [Google Scholar]
- 63.Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, Mulvaney FD. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001 Feb 1;61(3):271–280. doi: 10.1016/s0376-8716(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 64.Strain EC. Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients. Clin J Pain. 2002 Jul-Aug;18(4 Suppl):S14–27. doi: 10.1097/00002508-200207001-00003. [DOI] [PubMed] [Google Scholar]
- 65.Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010 Mar;30(2):155–166. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez-Llera MC, Domingo-Salvany A, Brugal MT, Silva TC, Sanchez-Niubo A, Torrens M. Psychiatric comorbidity in young heroin users. Drug Alcohol Depend. 2006 Sep 1;84(1):48–55. doi: 10.1016/j.drugalcdep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 67.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990 Nov 21;264(19):2511–2518. [PubMed] [Google Scholar]
- 68.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin Pharmacol Ther. 1999 Jun;65(6):685–694. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 69.Langleben DD, Ruparel K, Elman I, et al. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008 Mar;165(3):390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- 70.Dyer KR, White JM. Patterns of symptom complaints in methadone maintenance patients. Addiction. 1997 Nov;92(11):1445–1455. [PubMed] [Google Scholar]
- 71.Whelan PJ, Remski K. Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012 Jan;3(1):45–50. doi: 10.4103/0976-3147.91934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007 Mar;11(9):1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 73.Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- 74.Giacomuzzi SM, Ertl M, Kemmler G, Riemer Y, Vigl A. Sublingual buprenorphine and methadone maintenance treatment: a three-year follow-up of quality of life assessment. Scientific World Journal. 2005 May 24;5:452–468. doi: 10.1100/tsw.2005.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maremmani I, Pani PP, Pacini M, Perugi G. Substance use and quality of life over 12 months among buprenorphine maintenance-treated and methadone maintenance-treated heroin-addicted patients. J Subst Abuse Treat. 2007 Jul;33(1):91–98. doi: 10.1016/j.jsat.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Green CA, Polen MR, Lynch FL, Dickinson DM, Bennett MD. Gender differences in outcomes in an HMO-based substance abuse treatment program. J Addict Dis. 2004;23(2):47–70. doi: 10.1300/J069v23n02_04. [DOI] [PubMed] [Google Scholar]
- 77.Ko CH, Liu GC, Hsiao S, et al. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. 2009 Apr;43(7):739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988 Jun;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 79.Egloff B, Schmukle SC, Burns LR, Schwerdtfeger A. Spontaneous emotion regulation during evaluated speaking tasks: associations with negative affect, anxiety expression, memory, and physiological responding. Emotion. 2006 Aug;6(3):356–366. doi: 10.1037/1528-3542.6.3.356. [DOI] [PubMed] [Google Scholar]


