Abstract
Accelerator Mass Spectrometry (AMS) is the most sensitive method for quantitation of 14C in biological samples. This technology has been used in a variety of low dose, human health related studies over the last 20 years when very high sensitivity was needed. AMS helped pioneer these scientific methods, but its expensive facilities and requirements for highly trained technical staff have limited their proliferation. Quantification of 14C by cavity ring-down spectroscopy (CRDS) offers an approach that eliminates many of the shortcomings of an accelerator-based system and would supplement the use of AMS in biomedical research. Our initial prototype, using a non-ideal wavelength laser and under suboptimal experimental conditions, has a 3.5-modern, 1-σ precision for detection of milligram-sized, carbon-14-elevated samples. These results demonstrate proof of principle and provided a starting point for the development of a spectrometer capable of biologically relevant sensitivities.
Keywords: CRDS, spectroscopy, carbon-14
1. Introduction
Upon the adoption of Accelerator Mass Spectrometry (AMS) to the measurement of carbon-14, scientists realized the possible implications to biomedical aplications [1]. Since this time, AMS has facilitated biomedical studies where sensitive detection of carbon-14 is required. Many experiments, utilizing carbon-14 microdoses and microtracers, would not have been possible without AMS. However, AMS's complexity, cost, and limited throughput have become a bottle neck for biomedical studies. Together with other scientific applications of carbon-14, biomedical research has driven interest in the development of a laser based method to quantify concentrations of carbon-14. Starting with a paper by Labrie and Reid in 1981, several groups have explored the possibility of measuring carbon-14 with laser spectroscopy [2, 3, 4, 5]. Of particular interest is the work by Galli et. al., which demonstrated a detection limit of .043 fraction modern [6]. This experimental setup leveraged a state-of-the-art laser system utilizing an optical frequency comb and a saturated-absorption cavity ring-down spectroscopy (SCAR) technique. While the milestone study by Galli et al demonstrates the efficacy of laser based carbon-14 measurements, the complexity of the laser system used prohibits its ease of distribution to biomedical labs.
Our goal is to develop an instrument that balances complexity and sensitivity to enable biomedical studies. Using simple, robust hardware the laser-based instrument would not replace but would supplement AMS in biomedical studies. Here we present the preliminary results of our initial cavity ring-down spectroscopy (CRDS) system. These initial experiments were conducted at non-ideal wavelengths and temperatures, and carbon-14 sensitivities necessary for biomedical work were not expected. However, this initial prototype elucidated many of the design hurdles needed for developing a biomedical CRDS carbon-14 spectrometer.
2. Method
This initial prototype used a quantum-cascade laser (QCL) to measure at the non-ideal P(40) transition wavelength of the 14C16O2 antisymetric stretch rovibrational band. The instrument consists of a heavily modified Picarro CRDS. These instruments utilize a tunable QCL laser (Hamamatsu), an etalon based wavelength monitor, a traveling-wave optical cavity, and an HgCdTe photodetector (Vigo). Modification were made to enable low-temperature experimental conditions. However, all measurements reported here were made near room temperature. Further details can be found in “Development of a low-temperature cavity ring-down spectrometer for the detection of carbon-14” McCartt et al. [7].
Glucose mixtures with 1-, 50-, and 100-modern levels of carbon-14 were prepared by diluting carbon-14 elevated glucose in contemporary glucose. These samples were later measured with AMS to determine the exact carbon-14 concentration, however for simplicity, they will be referred to as 1-, 50-, and 100-modern samples for the remainder of the paper. CRDS measurements of the combusted sugar samples were made for several breakpoint vials for each carbon-14 concentration. Breakpoint vials typically contained 5 mg of carbon, however only approximately .5 mg is necessary to make a measurement.
All tests gases measured with the CRDS system were cryogenically purified before measurement. The test gases first flow through an annular piping component which is partially submerged in a dry ice and isopropyl alcohol cooling bath. This cryogenic element freezes out any remaining H2O from the test samples[8]. The second cryogenic element is a liquid nitrogen cold finger. The carbon dioxide is frozen onto the cold finger, and the remaining gaseous components, such as carbon monoxide and nitrogen, are evacuated to vacuum. Unfortunately some species have phase transitions similar to carbon dioxide. These species cannot be filtered out cryogenically and must be accounted for spectroscopically.
The cavity temperature was maintained at 300K, and a measurement pressure of 10 torr was selected based on HITRAN simulations of the spectroscopic region[9].
The details of the data acquisition routine are covered in “Model-based, closed-loop control of PZT creep for cavity ring-down spectroscopy” McCartt et al.[10].
3. Results
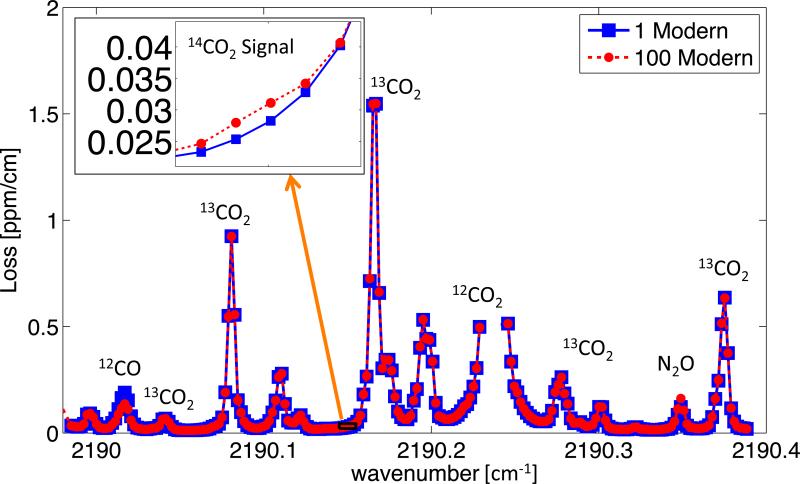
Figure 1 below gives an overview of the carbon-14 measurement spectra.
Figures 1.
Overview of carbon-14 measurement spectra. Inset shows loss from P(40) 14CO2 line for both contemporary and 100-modern carbon dioxide samples.
The surrounding spectra is composed primarily of carbon dioxide isotopologues and some other interfering species. The inset shows the P(40) 14CO2 line location and the total loss for both the 1- and 100-modern samples. The 1- and 100-modern data represents the average of 30-minute of measurements from a single break-point-vial. The error from both measurements amounts to a 5% error on the 1- to 100-modern sample peak signal (error bars were smaller than the data points in the inset of Figure 1). Subtracting the 1-modern spectra loss from elevated samples provides a simple analysis method but cannot account for variations in the sample carbon matrix, interfering species concentrations, or measurement wavelength. Using a spectroscopic model to quantify the 14C16O2 concentration can account for these variations.
4. 14CO2 P(40) Spectroscopic Fit and Comparison to AMS
Data was quantified using a spectroscopic model developed from HITRAN and measurements of surrounding interfering spectra. While beyond the scope of this short form paper, extensive characterization of the surrounding interfering species spectra was conducted. A more detailed description can be found in the first authors thesis[7].
Figure 2 shows example fits of the 14CO2 P(40) line for 1-, 50- and 100-modern samples.
Figure 2.
Spectroscopic model fits of the 14CO2 P(40) line. Individual 1-, 50- and 100-modern fits are shown from top to bottom in Panels A, B, and C respectively. Error bars represent the 1-σ confidence from the ring-down events at each measurement wavelength. Shaded regions represent the species contribution to total loss at a given wavenumber.
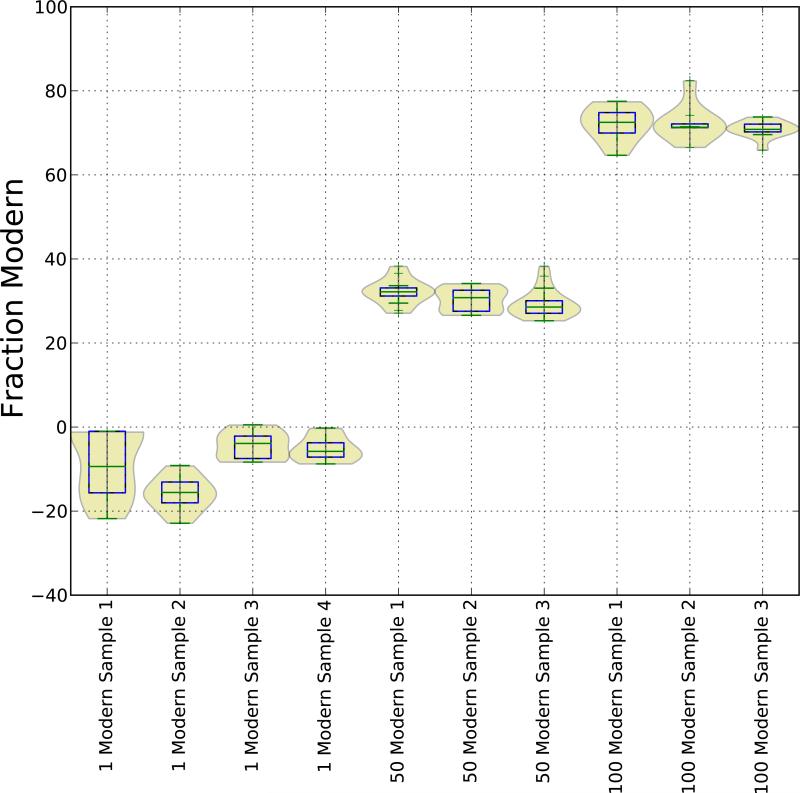
The shaded regions represent the portion of the total loss that the fitter attributed to the given species. Non-14C16O2 species concentrations were determined from fits of surrounding spectra. Error bars represent the 1-σ confidence from all ring-down events at each measurement wavelength, which should not be confused with the total sample error. Figure 3 shows the distribution of measured fraction modern for all sugar-sample measurements. Calculation of the fraction modern values employed a linestrength of S300K = 6:27(30) × 10−18 [cm−1/cm−2molec] from the literature and the isotopic ratio Modern≡1.18 × 10−12 [14 C/C] [6].
Figure 3.
Fraction Modern of glucose samples as determined by spectroscopic model fits. Sample numbers refer to individual breakpoint vials and are numbered chronologically. Distributions represent the spectroscopic fit output for all measurements of a breakpoint vial.
Figure 4 plots the carbon-14 CRDS measurements versus the AMS measurements. Error bars represent the 1-σ confidence interval for total sample error of the indicated carbon-14 concentration. AMS error is less than the datapoint size.
Figure 4.
Comparison of CRDS and AMS carbon-14 measurements. Error bars represent the 1-σ confidence interval for all measurements of the indicated carbon-14 concentration. AMS error is less than the datapoint size.
5. Discussion and Conclusion
This preliminary data represents the starting point for the development of a carbon-14 CRDS analyzer. While promising, there are a multitude of issues that must be dealt with before such an analyzer can be realized. The spectroscopic fitter clearly underpredicts the carbon-14 levels in the sugar samples (see Figure 4). The complexity of the model and measured spectra make it difficult to identify the cause of the error. However, the CRDS results have a linear response to concentration, and the inaccuracies of scale could be explained by something as simple as an error in the estimated linestrength. However, the 35% error is well above the stated confidence of HITRAN and estimated 14C16O2 linestrengths, and the inaccuracies are most likely the result of a combination of contributing factors[6, 9].
The negative fraction modern values for the 1-modern samples are an artifact of the spectroscopic fitter. The error bars in Figure 2 Panel A clearly indicate that the spectral fit cannot accurately quantify 1-modern carbon-14 samples. For most fits, the curvature of the measured spectra is slightly more concave than the spectroscopic model at the 14CO2 P(40) line location. This error in curvature of the background spectrum drives the fitter towards a non-physical, negative strength for the P(40) line. This spurious result is most likely partially responsible for the underpredicted CRDS carbon-14 concentrations.
The spectroscopic fit employs a baseline offset to account for wavelength-independent discrepancies in loss between the model and measured spectra. Strong absorption bands present in this region of the mid-IR but not included in the spectroscopic model are the primary source of this loss discrepancy. The far wings from numerous transitions of these strong bands cause the loss offset despite some being many wave numbers away. Furthermore, the Voigt model's approximation of the the physical lineshapes may be contributing to the loss offset. Beyond the observed errors in curvature discussed above, the Voigt model is inaccurate outside of the core of the lineshape, and the modeled contribution from relatively distant lines included in the simulation may be inaccurate[11]. Finally, deviations in the cavity baseline or contributions from molecular scattering not included in the cavity baseline model may be causing some of the offset. Fitting a region four times the FWHM around the P(40) linecenter allows the model to account for these discrepancies while still fitting the 14CO2 line, but an over-predicted baseline offset may contribute to the underpredicted carbon-14 concentrations.
Additionally, these spectral lines may be saturated. For these low pressure measurement conditions, the optical power in the cavity may depopulate the lower states of the spectroscopic transitions. Measurements across a range of pressures are necessary to check for this phenomenon.
Analysis of the room-temperature 14CO2 P(40) measurements with the spectroscopic fitter demonstrates the utility of the method. The fitter was able to improve measurement precision to 3.5-modern (1-σ) from the predicted Allan deviation value of 5-modern. The issues covered above indicate that the model does not perfectly represent the dense and complex measured spectra. Use of a more complex peak model, such as a Galatry or speed-dependent Voigt profile, might more accurately represent the spectra[12, 13]. However, to correctly determine the parameters of these profiles, an independent measurement of wavelength and a multi-spectrum fit would most likely be necessary[13]. Because this prototype did not utilize the ideal wavelength or experimental conditions for a carbon-14 measurement, further improvements to the spectroscopic model and fit were not pursued.
The experiments described herein have provided valuable information that will be utilized in the next design phase. A beta prototype is being built with a design that includes a new cavity and laser. The new QCL laser is capable of accessing the 14CO2 P(20) line location. The P(20) line is 5 times stronger than P(40), located in a less dense spectroscopic region, and better suited for a low temperature measurement. This beta prototype will determine the ultimate sensitivity of this basic hardware setup. With the new cavity, laser, and a faster ring-down rate, the system should be capable of biomedical studies.
Eventually, relatively simple-hardware CRDS systems will be able to measure carbon-14 without low-temperature, test-gas conditions. As the mid-IR mirrors improve, the necessary sensitivity may be achieved. Improvements to QCLs or other laser sources might enable the implementation of more complex spectroscopic techniques, such as SCAR spectroscopy. Efforts have already begun to further decrease the linewidth of QCL lasers, which will allow for more complex spectroscopic techniques[14]. Until these yet unknown technological improvements come to fruition, these prototypes provide an an essential test bed for spectroscopic characterization and instrument development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keilson J, Waterhouse C. Proceedings of the First Conference on Radiocarbon Dating with Accelerators. Vol. 391. University of Rochester; 1978. Possible impact of the new spectrometric techniques on 14C tracer kinetic studies in medicine. [Google Scholar]
- 2.Labrie D, Reid J. Radiocarbon dating by Infrared laser spectroscopy. Applied Physics A. 1981;24(4):381–386. doi:10.1007/bf00899738, URL http://dx.doi.org/10.1007/BF00899738. [Google Scholar]
- 3.Murnick D, Dogru O, Ilkmen E. Intracavity optogalvanic spectroscopy. An analytical technique for 14C analysis with subattomole sensitivity. Analytical chemistry. 2008;80(13):4820–4824. doi: 10.1021/ac800751y. URL http://pubs.acs.org/doi/abs/10.1021/ac800751y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Persson A, Eilers G, Ryderfors L, Mukhtar E, Possnert G, Salehpour M. Evaluation of Intracavity Optogalvanic Spectroscopy for Radiocarbon Measurements. Analytical Chemistry. 2013;85(14):6790–6798. doi: 10.1021/ac400905n. doi:10.1021/ac400905n, URL http://dx.doi.org/10.1021/ac400905n http://pubs.acs.org/doi/abs/10.1021/ac400905n. [DOI] [PubMed] [Google Scholar]
- 5.Galli I, Pastor PC, Di Lonardo G, Fusina L, Giusfredi G, Mazzotti D, Tamassia F, De Natale P. The v3 band of 14C16O2 molecule measured by optical-frequency-comb-assisted cavity ring-down spectroscopy. Molecular Physics. 2011;109(17-18):2267–2272. doi:10.1080/00268976.2011.614284, URL http://dx.doi.org/10.1080/00268976.2011.614284. [Google Scholar]
- 6.Galli I, Bartalini S, Borri S, Cancio P, Mazzotti D, De Natale P, Giusfredi G. Molecular Gas Sensing Below Parts Per Trillion: Radiocarbon-Dioxide Optical Detection. Physical Review Letters. 2011;107(27):270802. doi: 10.1103/PhysRevLett.107.270802. URL http://link.aps.org/doi/10.1103/PhysRevLett.107.270802. [DOI] [PubMed] [Google Scholar]
- 7.McCartt AD. Thesis. Stanford University; 2014. Development of a low-temperature cavity ring-down spectrometer for the detection of carbon-14. [Google Scholar]
- 8.Gordon AJ, Ford RA. The chemist's companion: a handbook of practical data, techniques, and references. Wiley-interscience publication, Wiley; 1972. URL http://books.google.com/books?id=uyhRAAAAMAAJ. [Google Scholar]
- 9.Rothman L, Gordon I, Barbe A, Benner D, Bernath P, Birk M, Boudon V, Brown L, Campargue A, Champion J-P, Chance K, Coudert L, Dana V, Devi V, Fally S, Flaud J-M, Gamache R, Goldman A, Jacquemart D, Kleiner I, Lacome N, Lafferty W, Mandin J-Y, Massie S, Mikhailenko S, Miller C, Moazzen-Ahmadi N, Naumenko O, Nikitin A, Orphal J, Perevalov V, Perrin A, Predoi-Cross A, Rinsland C, Rotger M, Šime£ková M, Smith M, Sung K, Tashkun S, Tennyson J, Toth R, Vandaele A, Vander Auwera J. The HITRAN 2008 molecular spectroscopic database. Journal of Quantitative Spectroscopy and Radiative Transfer. 2009;110(9-10):533–572. ISSN 00224073, doi:10.1016/j.jqsrt.2009.02.013, URL http://linkinghub.elsevier.com/retrieve/pii/S0022407309000727. [Google Scholar]
- 10.McCartt AD, Ognibene TJ, Bench G, Turteltaub KW. Model-based, closed-loop control of PZT creep for cavity ring-down spectroscopy. Measurement Science and Technology. 2014;25(9):095201. doi: 10.1088/0957-0233/25/9/095201. ISSN 0957-0233, doi:10.1088/0957-0233/25/9/095201, URL http://stacks.iop.org/0957-0233/25/i=9/a=095201?key=crossref.d736b1d2fdffe3db44be8f7ebff9641d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann J-M. Collisional Effects on Molecular Spectra. Elsevier; 2008. ISBN 978-0-44-452017-3. [Google Scholar]
- 12.Hartmann J-M, Tran H, Ngo NH, Landsheere X, Chelin P, Lu Y, Liu a. W., Hu S-M, Gianfrani L, Casa G, Castrillo a., Lepère M, Delière Q, Dhyne M, Fissiaux L. Abinitio calculations of the spectral shapes of CO_{2} isolated lines including non-Voigt effects and comparisons with experiments. Physical Review A. 2013;87(1):013403. ISSN 1050-2947, doi:10.1103/PhysRevA.87.013403, URL http://link.aps.org/doi/10.1103/PhysRevA.87.013403. [Google Scholar]
- 13.Casa G, Wehr R, Castrillo A, Fasci E, Gianfrani L. The line shape problem in the near-infrared spectrum of self-colliding CO2 molecules: experimental investigation and test of semiclassical models. The Journal of chemical physics. 2009;130(18):184306. doi: 10.1063/1.3125965. ISSN 1089-7690, doi:10.1063/1.3125965, URL http://www.ncbi.nlm.nih.gov/pubmed/19449920. [DOI] [PubMed] [Google Scholar]
- 14.Bartalini S, Borri S, Pastor PC, Galli I, Giusfredi G, Mazzotti D, De Natale P. Narrow linewidth quantum cascade lasers as ultra-sensitive probes of molecules. Vol. 7945. SPIE; San Francisco, California, USA: 2011. pp. 794505–794506. [Google Scholar]