Abstract
Objectives
Light therapy has shown promise as a nonpharmacological treatment to help regulate abnormal sleep-wake patterns and associated behavioral issues prevalent among individuals diagnosed with Alzheimer’s disease and related dementia (ADRD). The present study investigated the effectiveness of a lighting intervention designed to increase circadian stimulation during the day using light sources that have high short-wavelength content and high light output.
Methods
Thirty-five persons with ADRD and 34 caregivers completed the 11-week study. During week 1, subjective questionnaires were administered to the study participants. During week 2, baseline data were collected using Daysimeters and actigraphs. Researchers installed the lighting during week 3, followed by 4 weeks of the tailored lighting intervention. During the last week of the lighting intervention, Daysimeter, actigraph and questionnaire data were again collected. Three weeks after the lighting intervention was removed, a third data collection (post-intervention assessment) was performed.
Results
The lighting intervention significantly increased circadian entrainment, as measured by phasor magnitude and sleep efficiency, as measured by actigraphy data, and significantly reduced symptoms of depression in the participants with ADRD. The caregivers also exhibited an increase in circadian entrainment during the lighting intervention; a seasonal effect of greater sleep efficiency and longer sleep duration was also found for caregivers.
Conclusions
An ambient lighting intervention designed to increase daytime circadian stimulation can be used to increase sleep efficiency in persons with ADRD and their caregivers, and may also be effective for other populations such as healthy older adults with sleep problems, adolescents, and veterans with traumatic brain injury.
Keywords: Alzheimer’s disease, ADRD, dementia, sleep, light therapy, short-wavelength light, circadian rhythms, depression
Background
As Alzheimer’s disease and related dementia (ADRD) progresses, families are sometimes forced to move their loved ones from home to assisted living facilities or nursing homes. Often the precipitating factor is disturbed sleep-wake cycles, in which the person with ADRD is awake at night, causing tremendous stress and fatigue to family caregivers. These unpredictable wake episodes at night and associated wandering and disruptive behaviors tend to increase as ADRD progresses and are among the most prevalent reasons for institutional placement of persons with ADRD.1
Compared to normal older adults, persons with ADRD demonstrate lower sleep efficiency and more frequent arousals, with the severity of sleep disturbances paralleling progression of the disease.2, 3 Physiological studies have demonstrated fragmented circadian rhythms, phase delays, and diminished regularity of circadian temperature and hormonal cycles in persons with ADRD.4, 5 Several mechanisms have been postulated for these effects such as degeneration of the retinal ganglion cells6, 7 and loss of functionality of the “biological clock” located in the suprachiasmatic nuclei.8, 9 Moreover, optical changes to the aging eye, particularly smaller pupils and denser lenses, reduce retinal illuminance by over two-thirds relative to young adults. Exacerbating these neurological and optical factors, persons with ADRD are often exposed to low light levels during the day.10
Light therapy has shown great promise as a nonpharmacological treatment to help regulate sleep and improve cognition in individuals with ADRD. Studies have demonstrated that daytime light exposure can consolidate sleep at night and increase nighttime sleep efficiency, while increasing daytime wakefulness and reducing evening agitation.11–14 One landmark study showed that light can improve sleep as well as cognition.15 Longer and better sleep during the night can reduce disruptive behaviors associated with ADRD and, by extension, have a positive impact on caregivers, both in institutions and at home.
Sleep-wake cycles respond differentially to the spectral power distributions of light. Human melatonin suppression has a peak sensitivity to light close to 460 nm;16, 17 thus, light with relatively more energy at short wavelengths will be relatively more effective at affecting the circadian clock. Light sources typically used in eldercare facilities do not necessarily provide efficacious stimulation of the circadian system. Recently, it was shown that high correlated color temperature (CCT) polychromatic light sources (bluish-white light) during daytime hours decreased depression and agitation scores in those with ADRD living in long-term care facilities.18 The same lighting improved subjective and objective measures of sleep.
Not all of the studies to date showed positive results of light therapy for persons with ADRD. Colenda et al19 did not see an effect of a light visor on sleep patterns and Fontana Gasio et al20 did not see an effect of a dawn simulator on circadian rhythm disturbances in persons with ADRD. Sloane et al21 did not show an effect of a tailored lighting system on measures of sleep and behavior of persons with ADRD, but there was a significant improvement in sleep quality in caregivers. The authors hypothesized that personal light exposures collected one day during the intervention period and one day during the control period showed that exposures during the intervention period, while higher than those experienced during the control period, did not seem to be high enough to elicit a biological response in this population.
A recent Cochrane review included 8 studies that met their criteria for inclusion in their review. The authors concluded that there is not enough evidence to justify the use of light therapy to improve sleep and behavior in persons with ADRD.22 However, the authors analyzed studies that used a variety of light therapy approaches and, critically, it is uncertain how the actual light doses received by the study participants were measured or monitored. This is an important point to consider, because in studies where carefully controlled light stimulus was delivered, researchers in fact did find a positive impact of light on the sleep quality of persons with ADRD.18, 23
The goal of the present study was to extend those by Figueiro et al18 and Sloane et al21 by investigating the effectiveness of a lighting intervention designed to increase circadian stimulation during the day using light sources that have high short-wavelength content and high light output. Based upon calculation,24 the use of “bluish-white” light sources allowed us to reduce light levels to about one-third of those used in previous studies, where “warm” light sources were used.
Participants and Methods
Participant Selection
Thirty-five participants with ADRD (9 females; mean age 80.8 ± 7.9 years) and 34 caregivers (27 females; mean age 71.8 ± 12.3 years) completed the study and had usable data. The participants with ADRD lived at home with their caregivers, except one who did not have a caregiver, and were diagnosed with mild to moderate ADRD based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria, categorized with a Clinical Dementia Rating (CDR) of 1 to 2 (mild or moderate), and had a score from the Mini-Mental State Examination (MMSE) between 12 and 24. The control group consisted of the cohabitating, non-ADRD caregiver spouses or relatives of the participants with ADRD, who were also pre-assessed with the same clinical tools. Caregivers with an MMSE < 24 were excluded. Once pre-qualified, dyads were accepted or excluded from the study using the following criteria: 1) Inclusion criteria: To be eligible for the study, physicians of potential participants must have confirmed a diagnosis of mild-moderate dementia based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV) criteria and agreed that their patient was suitable for participation in the study. Participants with ADRD taking anti-depressants were included and the types of medicine and dosage intake were monitored. In each dyad, the caregiver lived in the same household and provided primary care for the participant with ADRD, and the household was located within a 20-mile radius of Case Western Reserve University (CWRU) main campus. There were no exclusions based on age, gender, race, or ethnicity; 2) Exclusion criteria for all participants with ADRD included major organ failure, major illness including psychiatric disorders, history of head injury, or uncontrolled generalized disorders such as hypertension or diabetes. Exclusion criteria also included sleep apnea, use of psychotropic (sleep aid) medicine, obstructing cataracts, macular degeneration, blindness, and caregiver cognitive impairment. If the caregiver did not provide informed consent, was not willing to participate in key aspects of the study protocol, was in unstable health (based on physician’s report), was unable to communicate adequately with study staff, or had a MMSE score of < 24, the dyad was excluded. Both members of the dyad received compensation for study participation. There were two seasonal data collection periods, ‘summer’ and ‘winter.’ Participant dyads were eligible to participate in both periods. Many dyads declined this option citing family schedules, a household move, or illness.
All study materials and procedures were reviewed and approved by the Institutional Review Board at Rensselaer Polytechnic Institute, Louis Stokes Cleveland Veterans Affairs Medical Center, and University Hospitals Case Medical Center. Informed written consent was obtained from both members of the dyad after full explanation of the procedures, in accordance with the Helsinki Declaration of 1975.25
Methods
Field Monitoring Procedures
a. Daysimeter
The Daysimeter is a small device that continuously records personal light exposures (using red-green-blue [RGB] solid-state photosensor package), and activity levels.26 Each study participant wore a Daysimeter device as a pendant (at chest length) during waking hours and placed the device next to their bed during sleep. Participants were instructed not to cover the device with blankets, coats or sweaters. Upon downloading, the RGB values were converted into illuminance, circadian light (CLA), and circadian stimulus (CS) levels. Briefly, illuminance is irradiance weighted by the photopic luminous efficiency function (V(λ)), an orthodox measure of the spectral sensitivity of the human fovea, peaking at 555 nm. CLA is irradiance weighted by the spectral sensitivity of the retinal phototransduction mechanisms stimulating the response of the circadian clock, based on nocturnal melatonin suppression. CS is a transformation of CLA into relative units from 0, the threshold for circadian system activation, to 0.7, response saturation, and is directly proportional to nocturnal melatonin suppression after 1 hour of exposure (0% to 70%). A value of 0.7 is equivalent to exposure to approximately 2,000 lux at the cornea, which is comparable to morning daylight exposure.
Rest-activity patterns are recorded from a 3-axis, monolithic solid-state accelerometer calibrated in g-force (1 g-force = 9.8 m/s2) with an upper frequency limit of 6.25 Hz. An activity index (AI) is determined using the following formula:
where SSxi, SSyi, and SSzi are the sum of squared differences from the mean over a 30-second interval; n is the number of 30-second intervals in the logging period (for a logging period of 90 seconds, n=3), and k is the factor equal to 0.0039 g/count. Logging intervals for both light and activity were set at 90 seconds.
Rea et al27 have proposed a quantitative technique to measure circadian disruption, known as phasor analysis, which quantifies circadian disruption in terms of the phase and the amplitude relationship between the measured light-dark stimulus pattern and measured activity-rest response pattern. Phasor analysis makes it possible to interpret the light and activity data, sampled together over consecutive multiple days, in terms of circadian entrainment and disruption. To quantify circadian disruption using the Daysimeter data, we used the measured CS light-dark pattern and the AI rest-activity pattern. Conceptually, each data set is joined end-to-end in a continuous loop. Correlation values (r) between the patterns of light-dark and rest-activity are then computed as one set of data is rotated with respect to the other (e.g., every 5 minutes). A fast Fourier transform (FFT) analysis is then applied to the circular correlation function to determine the 24-hour amplitude and phase relationships between the light-dark data and the rest-activity data. The resulting vector, or phasor, quantifies, in terms of the 24-hour frequency, how closely tied the 24-hour light-dark and activity-rest patterns are to one another (phasor magnitude) as well as their relative temporal relationship (phasor angle). Because the Daysimeter was removed at night, the data analyses probing sleep and rest-activity parameters were performed using wrist actigraphy, as described below.
b. Wrist actigraph
Participants with ADRD were asked to wear an actigraph (AMI Basic MotionLogger) on the non-dominant wrist that monitored their rest-activity patterns. Caregivers were asked to keep a diary with observations obtained during the one-week data collection period. The actigraph data were used to calculate interdaily stability (IS) and intradaily variability (IV).14 IS quantifies the extent to which all recorded 24-hour activity profiles resemble each other, which represents the day-by-day regularity of the sleep-wake pattern. IV quantifies the fragmentation of the rhythm, that is, the frequency and extent of transitions between periods of rest and activity. The actigraph data were also used to obtain estimates of sleep parameters, including total sleep time, sleep efficiency (percentage of actual sleep between sleep onset and final awakening), and sleep onset latency (the time between lights out and sleep onset).
c. Sleep diary
Caregivers kept a sleep diary (bed/wake-up times, naps) for both members of the dyad.
Subjective Scales
The following subjective scales were used to probe depression and sleep quality in the dyads.
a. Cornell Scale for Depression in Dementia (CSDD)
The CSDD is a 19-item tool designed to rate symptoms of depression in persons with dementia.28 This tool evaluates the presence and extent of mood-related signs (anxiety, sadness, irritability), behavioral disturbances (agitation, loss of interest), physical signs (loss of appetite, weight loss), cyclic functions (mood variation, sleep quality), and ideational disturbances (suicidal thoughts, poor self-esteem). Each item is scored 0 (not present), 1 (mild or intermittent symptom), or 2 (severe symptom). A score over 12 indicates depression. Caregivers filled out the CSDD for the participants with ADRD.
b. Geriatric Depression Scale-Short Form (GDS-SF)
The GDS was developed to measure depression in healthy adults;29 it is now also routinely used as a clinical screening tool for persons with dementia.30 A short version of the GDS, which consists of 15 questions, was used. Of the 15, ten questions indicate the presence of depression when answered positively, while the other five questions indicate depression when answered negatively. A score over 6 indicates depression. Participants with ADRD completed their own questionnaires with the assistance of the researchers.
c. Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a tool that can be used to measure sleep quality in clinical populations.31 It is composed of 19 items that generate seven component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction). The sum of the seven component scores yields one global score from 0 to 21. A person with a global score above 5 is considered to have sleep disturbances. Caregivers only were asked to fill out the PSQI questionnaire.
Lighting Intervention
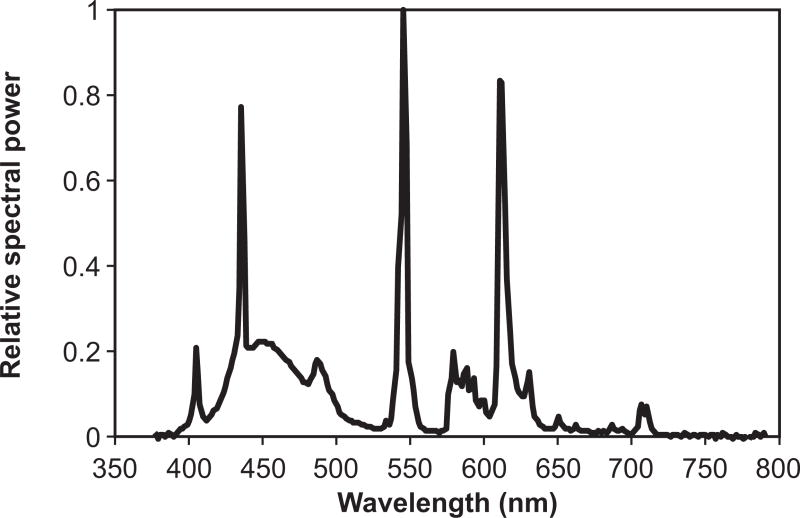
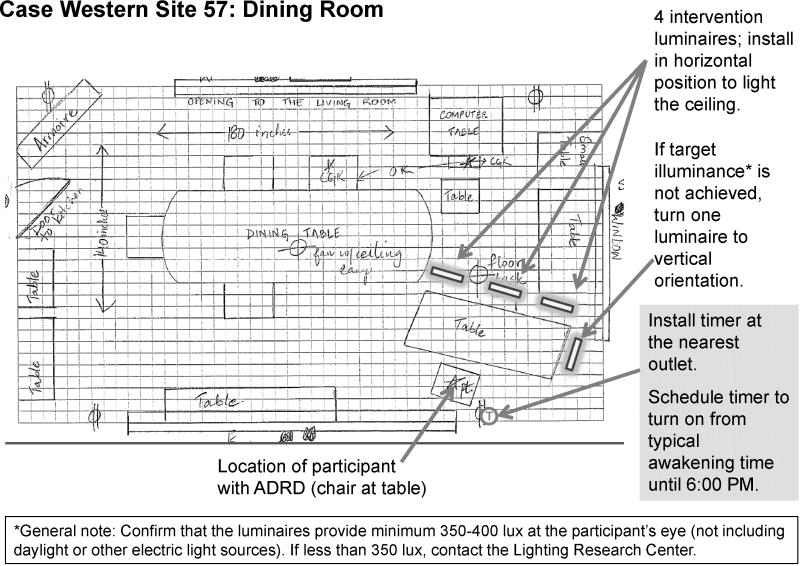
Custom luminaires were built for the study using parts currently available on the market. Two GE 45851 F55BX/AR/FS fluorescent lamps (GE Lighting, Cleveland, OH, USA) were inserted in a luminaire head (ETC 454 Line Voltage T5 Fluorescent Wall Washer; ELCO Lighting, Los Angeles, CA, USA). Figure 1 shows the relative SPD of the light source used in the study. The measured CCT of the light source was 9325 K. To save energy, all luminaires were plugged into a GE 15079v2 SunSmart Digital Timer (GE Lighting, Cleveland, OH, USA). This timer automatically turned all luminaires on when study participants reported typically waking up and turned off at 6:00 PM. During the day when the luminaires were turned on, an additional layer of control was added by installing a passive infrared (PIR) motion sensor (OSFHU-ITW; Leviton Mfg. Company Inc., Melville, NY, USA) directly onto each luminaire, automatically turning the lamps off after 20 minutes without detection of occupant movement. The luminaires were energized from a standard 120VAC wall power supply through a carefully concealed, strain-release extension cord. The luminaire was affixed to a hinged gimbal on an 86-centimeter (cm) tall microphone stand; a quick release on the stand could extend the pole to 157 cm. During installation, two 2.25-kilogram sandbag weights were wrapped around the base to prevent tipping and all electrical cords were positioned out of walkways. In order to minimize glare, the luminaire was tilted to direct light upwards to the ceiling. Using architectural and lighting measurements, luminaires were strategically installed in the main daytime living area for the person with ADRD to provide a minimum of 350–400 lux at the participant’s eye (not including daylight or other electric light sources). There was considerable variation in the participants’ home settings, all of which factored into the number and placement of luminaires. Features evaluated included room size, wall and furnishing colors, amount and placement of furniture, and the orientation, size, and coverings of the windows. Figure 2 shows an example of an installation.
Figure 1.
Relative spectral power distribution of the light source used during the intervention period
Figure 2.
Schematic of a dining room with tailored lighting intervention
Due to changes in the aging eye, older people are slightly less sensitive to short wavelengths than young observers for any source of light, but the differential effect can be estimated. The optical density of a normal, 60-year-old person’s crystalline lens is about 0.2 greater at short wavelengths than a 20-year-old observer’s; thus, the relative crystalline lens transmission for a normal 60-year-old at short wavelengths would be 63% of that of a normal 20-year-old. This age-dependent differential density of the lens at short wavelengths is comparable to having the 60-year-old observer view a blue light source 25% closer than the 20-year-old observer. Taking lens transmission into consideration, the intervention was predicted to deliver a CS of 0.375, which is based upon a measured melatonin suppression of 37.5% after 1-hour exposure during the night. Since the intervention was delivered for a period longer than 1 hour per day, the overall circadian light dose was inevitably increased.
Experimental Protocol
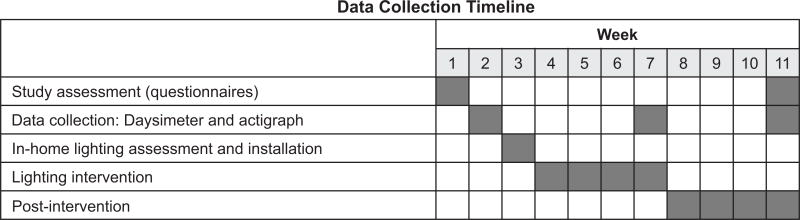
Figure 3 illustrates the experimental protocol. During week 1, once participants were selected and had signed consent documents, a pre-test assessment was performed using the questionnaires listed above. Researchers performed the in-home lighting assessment. During week 2, both members of the dyad wore the Daysimeter and participants with ADRD wore the wrist actigraph to obtain baseline data. A researcher brought the devices into the participants’ homes and instructed caregivers on how to use them. Daily phone calls were made to check on the participants. During week 3, researchers performed a home visit to retrieve the Daysimeter and actigraph data and supplement the lighting in the participants’ homes (lighting intervention installation). During weeks 4–7, the dyads adapted to the lighting intervention; research personnel were available for consult but no data were collected. During week 7, with the experimental luminaires still in place, the post-intervention assessment was performed. The data collection protocol was identical to week 2. At the end of week 7, the battery of tests that had been completed during week 1 was repeated and the lighting installation was removed and the original lighting scheme was returned to the participants’ homes. Anecdotally, the experimental lighting was well received and almost all caregivers remarked on how ‘dull’ their room looked after removal of the experimental lighting. During weeks 8–11, the experimental lighting was removed. During week 11, a third data collection (post-intervention assessment) was performed. Both members of the dyad were again asked to wear the Daysimeter and participants with ADRD wore the wrist actigraph for 7 consecutive days to check if participants returned to baseline levels collected during week 1. The same subjective questionnaires were administered again.
Figure 3.
Experimental data collection timeline
Statistical Analyses
All measurements described in this section were submitted to mixed-model linear regressions using IBM® SPSS® 22.0 statistical software. As independent variables in each regression, participant was entered as a random factor, while treatment phase and season were entered as fixed factors. The season factor, and interactions between season and treatment phase, were not statistically significant for most measures; we do not refer to these factors in the following results unless they were significant. No other interactions were significant. Measures were considered significant if the associated p-value was less than 0.05. Effect sizes for significant measures are expressed as Cohen’s d.
Results and Discussion
Daysimeter Data
Complete Daysimeter data were available for analysis for 28 participants with ADRD and 24 caregivers.
Circadian Stimulus
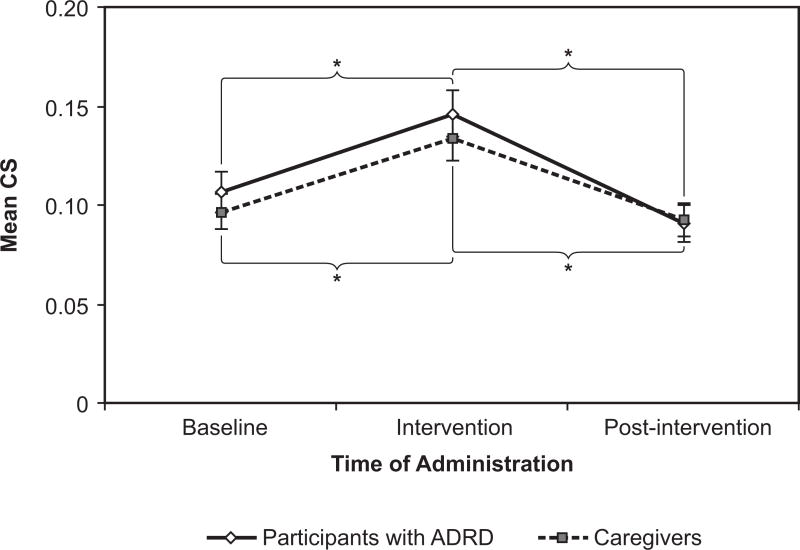
The intervention increased the measured CS that both participants with ADRD and caregivers received (Figure 4). The mean +|− SEM CS of the participants with ADRD was 0.11 +|− 0.01 at baseline. The CS increased to 0.15 +|− 0.01 during the intervention phase, then fell back to 0.09 +|− 0.01 in the post-intervention phase. The rise and fall of the CS was significant (F(2, 51) = 5.35, p < 0.00001). Pairwise comparisons showed that the difference between CS at baseline and at intervention was significant (p < 0.0001, d = −0.72), as well as the difference between intervention and post-intervention (p < 0.0001, d = 1.09). The d values suggest that the light intervention had a relatively large effect on CS for participants with ADRD.
Figure 4.
Circadian stimulus (CS) rose significantly for both the participants with ADRD and the caregivers during the intervention phase. As expected, it fell to near baseline levels after the intervention ceased. Error bars represent standard error of the mean. *= statistical significance
For caregivers, the mean +|− SEM CS was 0.10 +|− 0.01 at baseline. During intervention, CS increased to 0.13 +|− 0.01; in the post-intervention phase, CS was reduced to just 0.09 +|− 0.01. The main effect of the intervention showed the same pattern as for the participants with ADRD; it was also statistically significant (F(2, 41) = 10.37, p < 0.0001). A planned pairwise comparison of the difference between baseline and intervention was significant (p < 0.0001, d = −0.66), as was the difference between intervention and post-intervention (p < 0.0001, d = 0.88). The difference between baseline and post-intervention, however, was not significant. The d values suggest that the effect of the light intervention on CS was also relatively large for this group.
It is important to note that, although CS values were significantly higher during the intervention period than during baseline and post-intervention periods, these values were much lower than a one-time photometric measurement that was performed soon after the lighting installation. According to the photometric measurements, the CS values of the installation were between 0.25 and 0.4. Although caregivers were asked to make sure Daysimeter devices were not covered, the low levels recorded by the device suggest otherwise.
Phasor Magnitude
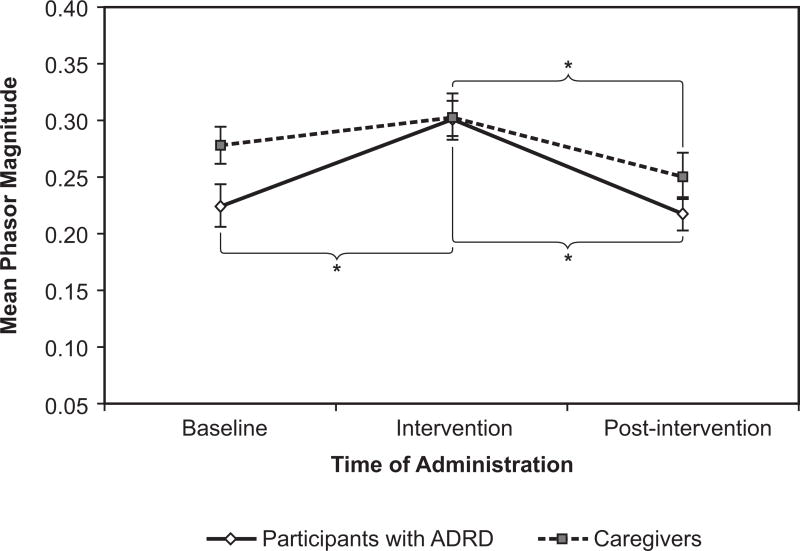
Increases in phasor magnitude can be interpreted as increases in circadian entrainment. Phasor magnitudes for the participants with ADRD and the caregivers increased from baseline through intervention, then returned to lower levels after the intervention ended (Figure 5).
Figure 5.
Phasor magnitude was significantly affected for both the participants with ADRD and the caregivers. Error bars represent standard error of the mean. * = statistical significance
This finding indicates that the participants’ activity was more synchronized with the presence of the circadian effective light during the intervention, but became less so, perhaps becoming unrelated to daytime light exposure (e.g., nighttime wandering and/or daytime napping), when the intervention was withdrawn.
For the participants with ADRD, the mean +|− SEM phasor magnitude was 0.22 +|− 0.02 at baseline, rising to 0.30 +|− 0.02 in the intervention phase, and falling back to 0.22 +|− 0.01 in the post-intervention phase. This effect was highly statistically significant (F(2, 51) = 16.15, p < 0.0001). A comparison of the difference between baseline and intervention was significant (p < 0.0001, d = −0.88), as was the difference between intervention and post-intervention (p < 0.0001, d = 1.00).
The caregivers’ phasor magnitude pattern was also statistically significant (F(2, 42) = 3.36, p = 0.04). The mean +|− SEM phasor magnitude was 0.28 +|− 0.02 at baseline, 0.30 +|− 0.02 in the intervention phase, and 0.25 +|− 0.02 in the post-intervention phase. The effect did not occur between baseline and intervention, as the pairwise comparison of the difference between the phases was not statistically significant, but was due solely to the difference between the intervention and post-intervention phases (p = 0.012, d = 0.53).
As hypothesized, the lighting intervention increased the resonance between the light-dark and activity-rest patterns, as shown by an increase in phasor magnitude. Phasor magnitudes in participants with ADRD were similar to those observed by Figueiro et al32 who showed that persons with ADRD have a phasor magnitude of 0.22 in winter months and 0.35 in summer months. These results are also consistent with those by Higgins et al33 who showed that a caregiver of a person with ADRD also exhibited low phasor magnitude, suggesting that caregivers of persons with ADRD who are still living at home are just as disrupted as the persons with ADRD. The intervention significantly increased phasor magnitudes of the participants with ADRD, but as expected, it did not bring them to levels comparable to those found in regular, daytime workers. For comparison, day-shift nurses had a mean phasor magnitude of 0.46, whereas rotating-shift nurses, who are known to be disrupted, had a mean phasor magnitude of 0.30.
Phasor Angle
Phasor angles describe the temporal relationship between light-dark and activity-rest patterns. Individuals who have higher phasor angles tend to have activity extended into the evening, after sunset, while those with lower phasor angles tend to have early activity in the morning, before sunrise.
For the participants with ADRD, mean phasor angle did vary, falling from baseline to intervention and rising again in the post-intervention phase. Mean angle was 1.34 +|− 0.34 at baseline, 1.05 +|− 0.36 during intervention, and 1.29 +|− 0.47 at post-intervention. However, this variation did not reach statistical significance.
The mean phasor angle for the caregivers did not vary significantly in any of the conditions. At baseline the mean +|− angle was 0.94 +|− 0.21; during the intervention phase, the mean angle was 0.93 +|− 0.35; during the post-intervention phase, the mean fell to 0.82 +|− 0.47.
Actigraph Data
Complete actigraph data were collected for 34 participants with ADRD and 33 caregivers. Twenty-one persons with ADRD and 20 caregivers participated in the summer phase of data collection; 13 persons with ADRD and 13 caregivers participated in the winter. The actigraph was worn day and night, removed only while bathing. From the 24-hour data, IV and IS were calculated. From the nighttime data, measures of sleep duration, sleep minutes, and sleep efficiency were calculated.
Interdaily Stability and Intradaily Variability
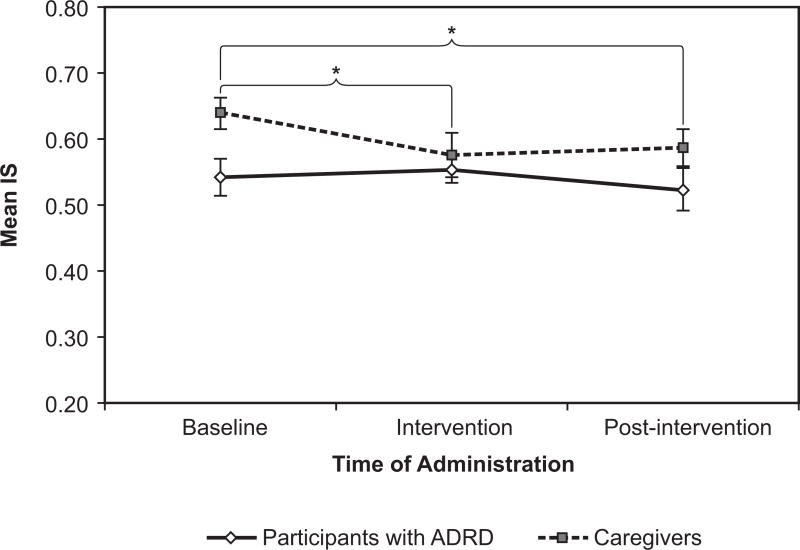
For the participants with ADRD, IS remained almost the same; it was 0.54 +|− 0.03 at baseline, 0.55 +|− 0.02 at intervention, and 0.52 +|− 0.03 at post-intervention. The caregivers’ IS, however, did show statistically significant changes, albeit in the direction opposite of our hypothesis (Figure 6). For this group, IS was 0.64 +|− 0.02 at baseline, 0.58 +|− 0.03 at intervention, and 0.59 +|− 0.03 at post-intervention. The differences were significant (F(2, 42) = 3.24, p = 0.049). The decrease between baseline and intervention was significant in a pairwise comparison (p = 0.011, d = 0.42), as well as the decrease from baseline to post-intervention (p = 0.018, d = 0.40), but not the difference between intervention and post-intervention. The d values indicate a moderate effect of the intervention on caregivers.
Figure 6.
Interdaily stability (IS) fell and rose significantly over the course of the study for the caregivers, but not for the participants with ADRD. Error bars represent standard error of the mean. * = statistical significance
IV was not significantly affected by the intervention. IV for the participants with ADRD was generally higher than for caregivers: it was 0.91 +|− 0.06 at baseline, 0.91 +|− 0.05 at intervention, and 0.89 +|− 0.06 at post-intervention, while IV for caregivers was 0.67 +|− 0.04 at baseline, 0.70 +|− 0.03 at intervention, and 0.72 +|− 0.04 at post-intervention.
These results were not consistent with those by van Someren et al14 who demonstrated that persons with ADRD showed an increase in IS and a decrease in IV after 4 weeks of bright light intervention. However, Sloane et al21, 34 did not find any significant change in IS and IV scores after persons with ADRD received bright light and a tailored lighting intervention similar to the one used in the present study. Consistently, Figueiro et al18 using the same protocol as in the present study in persons with ADRD living in nursing homes, did not show any effect of the lighting intervention on IS and IV scores.
Sleep Duration
Sleep duration is measured as the number of minutes from sleep onset to waking. It is calculated as the time elapsed between the sleep start time and the sleep end time, as reported by the sleep logs. For the participants with ADRD, sleep duration did vary over the course of the study: it was 588.08 +|− 18.07 minutes at baseline, 584.02 +|− 22.51 minutes at intervention, and 599.41 +|− 22.27 minutes at post-intervention. Caregivers’ sleep duration decreased; mean duration +|− SEM was 511.26 minutes +|− 13.04 at baseline, 492.67 minutes +|− 15.42 at intervention, and 494.92 minutes +|− 17.07 at post-intervention. None of these changes reached statistical significance, however.
These results are consistent with Ancoli-Israel et al35 and Sloane et al21 who showed that daytime light exposure did not increase nighttime sleep duration in persons with ADRD. These results are not consistent with those from Figueiro et al18, Lyketsos et al12, and Sloane et al34 who showed a significant increase in sleep duration in persons with ADRD living in nursing homes. It is interesting to observe that studies that showed an increase in sleep parameters were performed in more controlled environments, suggesting perhaps that those still living at home may be more mobile and spend more time outdoors than those living in nursing homes.
Sleep Minutes
Sleep minutes are the actual time spent asleep during sleep duration. Sleep minutes were determined by summing the number of epochs that do not exceed a sensitivity threshold and multiplying that value by the total epoch length. For the participants with ADRD, sleep minutes increased slightly, from 387.06 +|− 17.20 at baseline, to 394.64 +|− 20.58 at intervention, and 404.17 +|− 20.18 at post-intervention. Caregivers’ sleep minutes fell, going from 353.35 minutes +|− 12.61 at baseline, to 332.12 minutes +|− 12.55 at intervention, but rising again to 342.58 minutes +|− 9.77 at post-intervention. As with duration, these changes were not significant, but for the participants with ADRD, were in the direction predicted by our hypothesis.
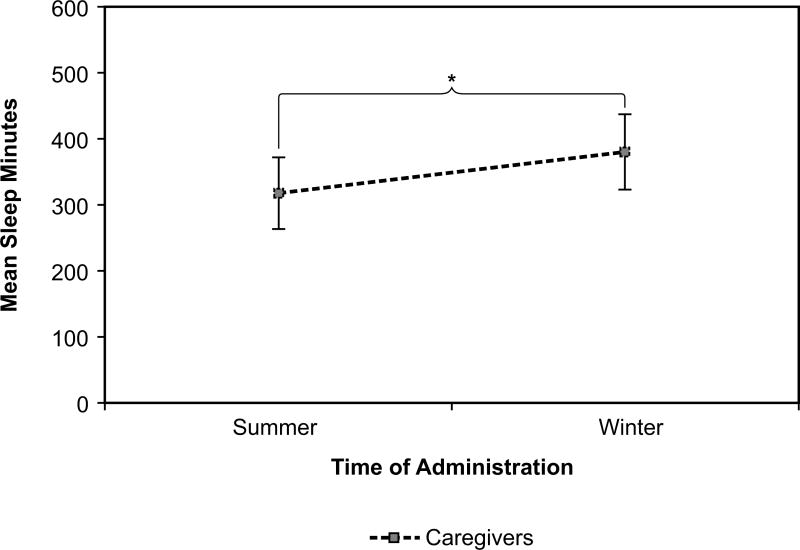
However, season did affect the number of minutes caregivers slept (F(1, 31) = 13.72, p = 0.001) as shown in Figure 7. Caregivers slept an average of 318 +|− 54.56 minutes per night over all phases of the study in the summer. They slept an average of 382 +|− 57.33 minutes per night in the winter. For participants with ADRD, season did not affect the number of minutes slept.
Figure 7.
Caregivers slept significantly longer in the winter than in the summer, averaged over all phases of the study. Error bars represent standard error of the mean. * = statistical significance
Sleep Efficiency
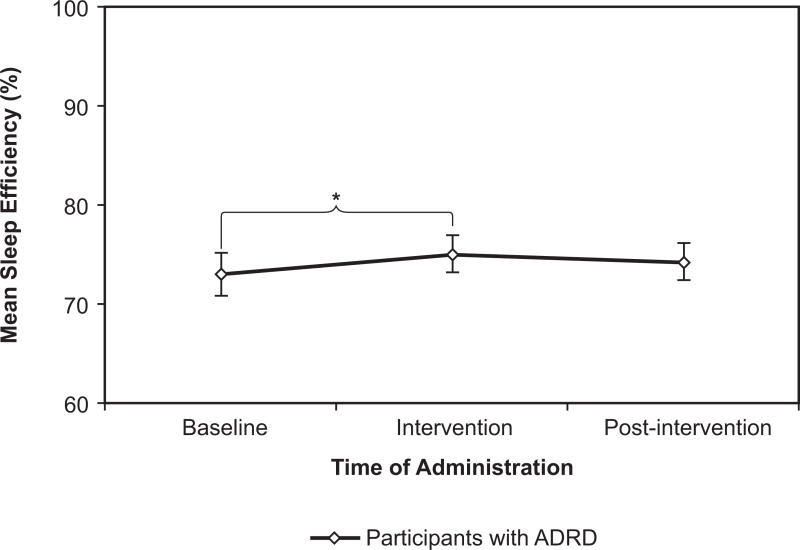
Sleep efficiency was measured as percentage of total sleep time divided by the total amount of time spent in bed, as reported by the sleep logs. As expected, mean sleep efficiency rose during the intervention phase for the participants with ADRD (though not for caregivers), from 73% +|− 2.2% at baseline to 75% +|− 1.8% (Figure 8). Efficiency fell a little, to 74% +|− 1.9%, after the intervention ended. The slight rise from baseline to intervention was significant (p = 0.033 in a planned comparison), although the overall effect of the intervention was not quite significant.
Figure 8.
For the participants with ADRD, sleep efficiency showed a slight rise, as expected, during the intervention phase. Error bars represent standard error of the mean. * = statistical significance
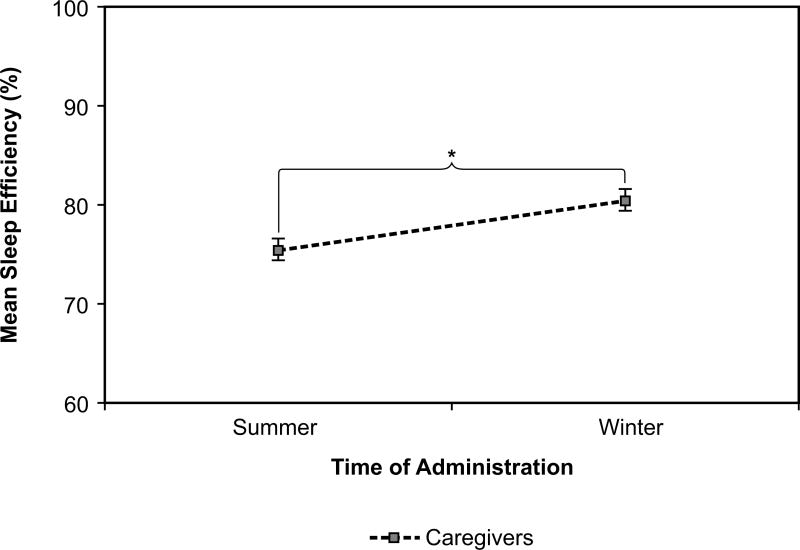
Caregivers’ sleep efficiency +|−SEM was 77% +|− 1.5% at baseline, 77% +|− 1.5% at intervention, and 78% +|− 1.4% at post-intervention. This measure showed a significant effect of season (F(1, 31) = 4.16, p = 0.05, d = −0.69). Sleep efficiency was greater in winter than in summer; it was 80% +|− 1.1% in winter and 75% +|− 1.1% in summer (Figure 9). This effect was relatively large. Although for the participants with ADRD, sleep efficiency was slightly more variable, it did not demonstrate the same seasonal effect (F(1, 32) = 1.70, p = 0.20).
Figure 9.
Sleep efficiency increased significantly for caregivers from summer to winter. Error bars represent standard error of the mean. * = statistical significance
Questionnaire Data
Geriatric Depression Scale
Thirty-five participants with ADRD and 29 caregivers each rated their own affect and symptoms on the GDS.
Caregivers’ scores did not vary significantly over the course of the study. Their mean score +|− SEM was 1.83 +|− 0.38 at baseline, 2.00 +|− 0.50 at intervention, and 2.07 +|− 0.38 at post-intervention.
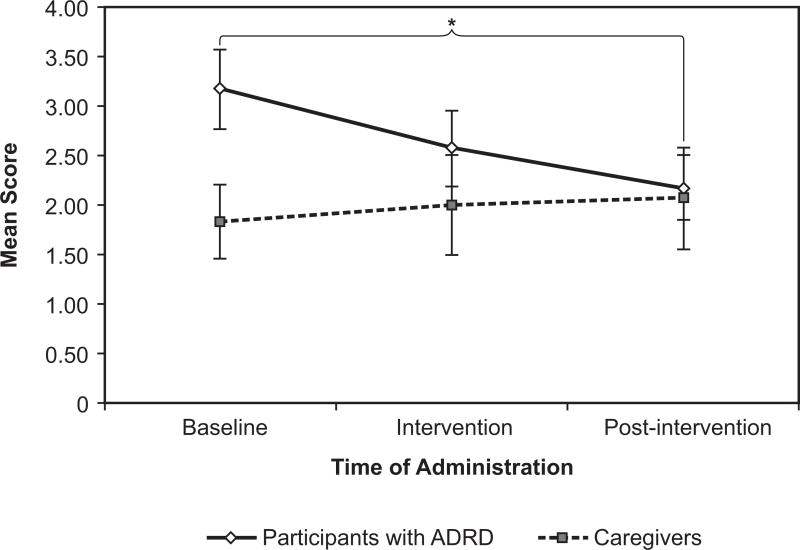
The scores for the participants with ADRD, however, fell as predicted from baseline through post-intervention, indicating less self-reported depression (Figure 10). At baseline, their mean score was 3.17 +|− 0.40, during intervention 2.57 +|− 0.38, and at post-intervention 2.18 +|− 0.32. The overall effect was almost statistically significant (F(2, 65) = 3.08, p = 0.053). The pairwise comparisons showed that the intervention had a long-lasting effect: scores were significantly lower from baseline to post-intervention, although the effect was relatively small, (p = 0.005, d = 0.26), and approached significance from intervention to post-intervention (p = 0.083, d = 0.19). These results are consistent with those from Riemersma-van der Lek et al15 who showed that a lighting intervention reduced symptoms of depression by 19%. The carryover effect of light on subjective ratings of depression after the lighting intervention was removed is consistent with Figueiro et al18 who also showed a carryover effect of the lighting intervention on mood and behavior.
Figure 10.
The Geriatric Depression Scale scores of the participants with ADRD fell over the course of the study, indicating less depression. The difference between baseline and post-intervention was significant. Error bars represent standard error of the mean. * = statistical significance
Cornell Scale for Depression in Dementia
Unlike the method for the GDS, the caregivers evaluated the symptoms for the participants with ADRD on the CSDD scale. Complete data were collected for 33 participants with ADRD. Perhaps because caregivers were less able to evaluate subtle changes in mood for the participants with ADRD, the CSDD scores of the participants with ADRD stayed approximately level throughout the study. At baseline, the mean score +|− SEM was 7.00 +|− 0.79, 7.00 +|− 0.96 at intervention, and 7.16 +|− 0.87 at post-intervention.
For the entire sample, the GDS and CSDD scores indicate a relatively low level of depressed affect. The incongruence of the ratings for the participant with ADRD, between the caregiver’s rating (CSDD) and that of the participant with ADRD (GDS), should be explored in future studies. These differences could be attributed to differences in the psychometric properties of the questionnaires and/or documented discrepancies between proxy and participant data when assessing the psychological well-being of persons with cognitive impairment.37
Pittsburgh Sleep Quality Index
Caregivers’ PSQI global scores fell throughout the phases of the study, but the changes did not reach statistical significance. The mean score +|− SEM at baseline was 6.70 +|− 0.37, during intervention 5.85 +|− 0.50, rising again at post-intervention to 6.22 +|− 0.51. Sloane et al (2014), using a similar lighting intervention and experimental protocol, showed a significant decrease in PSQI scores in the caregivers, but not the participants with ADRD.
Conclusions
The present study extends those from Figueiro et al18 by investigating the effectiveness of a tailored lighting intervention on circadian entrainment, sleep parameters, and mood in persons with ADRD and their caregivers living at home. While these results were less compelling than those from Figueiro et al18 they were consistent, showing that four weeks of lighting intervention resulted in significantly greater circadian entrainment, as measured by phasor magnitude, significantly greater sleep efficiency, as measured by actigraphy data and significantly reduced symptoms of depression in the GDS scale in the participants with ADRD. As expected, sleep duration increased, but this change was not statistically significant. Contrary to our expectations, there was no significant change in IS and IV scores, suggesting no significant changes in the rest-activity rhythms of the participants with ADRD with the treatment. As for the caregivers, we observed the same increase in circadian entrainment during the lighting intervention. Although not hypothesized, it was interesting to observe a seasonal effect on sleep efficiency and sleep duration in caregivers. They slept longer and more efficiently in winter months than in summer months. It is also not known why, contrary to our hypothesis, caregivers had significantly reduced IS scores during the lighting intervention. It may be that, even though the members of each dyad had been expected to cohabitate, some of the caregivers had jobs and other obligations outside the home that precluded them from getting the light treatment. This might also explain why season had a significant effect on caregivers, but not in the participants with ADRD.
Although Figueiro et al26 showed that a pendant device is a good location to measure light as a surrogate for corneal light exposures, the CS values measured using the Daysimeter were lower than static light measurements performed on site. Despite the fact that caregivers were instructed not to cover the devices, they may have been covered while participants were sitting in chairs. Notwithstanding the low absolute values, the Daysimeter data clearly showed that participants received significantly higher circadian effective light during the intervention period.
The increase in sleep efficiency observed in this study was consistent with those observed by Figueiro et al.18 While the results showed only a 2% increase in sleep efficiency, these results may still have clinical relevance, given that recent research suggests that sleep quantity and quality may be a direct consequence of ADRD.38, 39 One study showed that those who had sleep efficiency of 80% had significantly greater beta amyloid deposition in the brain, a marker of ADRD, than those who had sleep efficiency of 84%.40 Therefore, an intervention producing even small increases in sleep efficiency may be of great importance for reducing symptoms associated with ADRD.
The subjective ratings of depression showed contradictory results, but they underscore the methodological issues associated with self and proxy reports. Depression scores that were filled out by the participants with ADRD (GDS) showed a positive effect of the intervention, while the CSDD, which was filled out by caregivers did not. This methodological issue is common when evaluating a population with dementia.
The present results are consistent with those from Sloane et al21 who also showed that a similar lighting intervention delivered to persons with ADRD living at home in North Carolina did not change their sleep patterns. One explanation for these results might have to do with the fact that those living at home are still mobile and can have activities outside the house (or even within the house), while those living in nursing homes have fewer opportunities to spend time outdoors or to move around spaces within the facility. Therefore, a lighting intervention in a more controlled environment may be more effective than the same intervention in the home, because a nursing home resident with ADRD has more opportunities to receive the lighting intervention consistently in an otherwise homogenous lighted environment.
The field study of course has limitations. The sleep logs revealed that some of the participants with ADRD were in bed for 10–12 hours per night, which may explain why there was no significant increase in sleep duration. Given that the PSQI questionnaire was not administered to the participants with ADRD (only to caregivers), it is not possible to determine whether the participants with ADRD in fact had sleep problems. Our expectation was that the effectiveness of the lighting intervention on sleep parameters would be greater in those who have circadian sleep disturbances. The CS value measured by the Daysimeter was low, likely because the devices were covered some of the time. Nevertheless, the present results show that a lighting intervention delivering much lower levels than typically used in light therapy regimes can have a positive impact on mood and circadian entrainment in persons with ADRD living at home.
From a sleep health perspective, other studies have demonstrated that low sleep efficiency is associated with worse school achievement in adolescents,41 higher body mass index and body fat,42 negative daily mood in children,43 and worse next-day anxiety and fatigue in persons with irritable bowel syndrome.44 Therefore, an ambient lighting intervention designed to increase circadian entrainment and improve sleep can also impact populations beyond those studied in the present paper.
Acknowledgments
The study was funded by the National Institute on Aging (grant # R01AG034157). GE Lighting donated the lamps and ballasts used in the study. The authors would like to acknowledge Ashritha Epur of Case Western Reserve University and the Louis Stokes Cleveland Veterans Affairs Medical Center. Sharon Lesage, Erin Ryan, Brittany Wood, Rebekah Mullaney, and Dennis Guyon of the Lighting Research Center are also acknowledged for their technical and editorial assistance.
References
- 1.Koyama E, Matsubara H, Nakano T. Bright light treatment for sleep-wake disturbances in aged individuals with dementia. Psychiatry Clin Neurosci. 1999;53:227–9. doi: 10.1046/j.1440-1819.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 2.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 3.Bliwise DL. Observed sleep/wakefulness and severity of dementia in an Alzheimer’s disease special care unit. Journals of Gerontology A: Biological Science and Medical Science. 1995;50A:M303–M6. doi: 10.1093/gerona/50a.6.m303. [DOI] [PubMed] [Google Scholar]
- 4.Prinz PN, Peskind ER, Vitaliano PP, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 5.Satlin A, Teicher MH, Lieberman HR, et al. Circadian locomotor activity rhythms in Alzheimer’s disease. Neuropsychopharmacology. 1991;5:115–26. [PubMed] [Google Scholar]
- 6.Hinton DR, Sadun AA, Blanks JC, et al. Optic-nerve degeneration in Alzheimer’s disease. The New England Journal of Medicine. 1986;315:485–7. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 7.Katz B, Rimmer S, Iragui V, et al. Abnormal pattern electroretinogram in Alzheimer’s disease: Evidence for retinal ganglion cell degeneration? Ann Neurol. 1989;26:221–5. doi: 10.1002/ana.410260207. [DOI] [PubMed] [Google Scholar]
- 8.Moore RY, Swaab DF, Hofman MA, et al. Prog Brain Res. Elsevier; 1992. The organization of the human circadian timing system; pp. 101–17. [PubMed] [Google Scholar]
- 9.Swaab D, Fliers E, Partiman T. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Kripke DF. Now I lay me down to sleep: The problem of sleep fragmentation in elderly and demented residents of nursing homes. Bull Clin Neurosci. 1989;54:127–32. [Google Scholar]
- 11.Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer’s type. Chronobiol Int. 1998;15:647–54. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- 12.Lyketsos C, Lindell Veiel L, Baker A, et al. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–5. [PubMed] [Google Scholar]
- 13.Yamadera H, Ito T, Suzuki H, et al. Effects of bright light on cognitive and sleep–wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54:352–3. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Someren EJW, Kessler A, Mirmirann M, et al. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–63. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 15.Riemersma-van der Lek RF, Swaab DF, Twisk J, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. Journal of the American Medical Association. 2008;299:2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 16.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. The Journal of Physiology. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueiro MG, Plitnick B, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clinical Interventions in Aging. 2014;9:1527–37. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colenda CC, Cohen W, McCall WV, et al. Phototherapy for patients with Alzheimer disease with disturbed sleep patterns: Results of a community-based pilot study. Alzheimer Dis Assoc Disord. 1997;11:175–8. doi: 10.1097/00002093-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Fontana Gasio P, Krauchi K, Cajochen C, et al. Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Exp Gerontol. 2003;38:207–16. doi: 10.1016/s0531-5565(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 21.Sloane P, Figueiro M, Garg S, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Light Res Technol. 2014:1477153513517255. doi: 10.1177/1477153513517255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes D, Blake CM, Thiessen EJ, et al. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. The Cochrane database of systematic reviews. 2014;2:CD003946. doi: 10.1002/14651858.CD003946.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanford N, Figueiro MG. Light therapy and Alzheimer’s disease and related dementia: Past, present, and future. Journal of Alzheimer’s Disease. 2013;33:913–22. doi: 10.3233/JAD-2012-121645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rea MS, Figueiro MG, Bierman A, et al. Circadian light. J Circadian Rhythms. 2010;8:2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Medical Association. Declaration of Helsinki. JAMA. 2000;284:3043–5. [PubMed] [Google Scholar]
- 26.Figueiro MG, Hamner R, Bierman A, et al. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45:421–34. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rea MS, Bierman A, Figueiro MG, et al. A new approach to understanding the impact of circadian disruption on human health. J Circadian Rhythms. 2008;6:7. doi: 10.1186/1740-3391-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ownby RL, Harwood DG, Acevedo A, et al. Factor structure of the Cornell Scale for Depression in Dementia for Anglo and Hispanic patients with dementia. The American Journal of Geriatric Psychiatry. 2001;9:217–24. [PubMed] [Google Scholar]
- 29.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. 1986 [Google Scholar]
- 30.Alzheimer’s Association. Essentials of a Diagnostic Workup. 2015. [Google Scholar]
- 31.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Figueiro MG, Hamner R, Higgins P, et al. Field Measurements of Light Exposures and Circadian Disruption in Two Populations of Older Adults. J Alzheimers Dis. 2012;31:711–5. doi: 10.3233/JAD-2012-120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins PA, Hornick TR, Figueiro MG. Rest-activity and light exposure patterns in the home setting: a methodological case study. American journal of Alzheimer’s disease and other dementias. 2010;25:353–61. doi: 10.1177/1533317510363467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sloane PD, Williams CS, Mitchell CM, et al. High-Intensity Environmental Light in Dementia: Effect on Sleep and Activity. J Am Geriatr Soc. 2007;55:1524–33. doi: 10.1111/j.1532-5415.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 35.Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 37.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: lessons, challenges, and opportunities. J Am Geriatr Soc. 2000;48:1646–54. doi: 10.1111/j.1532-5415.2000.tb03877.x. [DOI] [PubMed] [Google Scholar]
- 38.Benedict C, Byberg L, Cedernaes J, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014 doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- 39.Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging. 2014;35(Suppl 2):S29–34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Ju YS, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical alzheimer disease. JAMA Neurology. 2013;70:587–93. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tonetti L, Fabbri M, Filardi M, et al. Effects of sleep timing, sleep quality and sleep duration on school achievement in adolescents. Sleep Medicine. 2015 doi: 10.1016/j.sleep.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Wirth MD, Hébert JR, Hand GA, et al. Association between actigraphic sleep metrics and body composition. Ann Epidemiol. 2015 doi: 10.1016/j.annepidem.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouros CD, El-Sheikh M. Daily mood and sleep: reciprocal relations and links with adjustment problems. J Sleep Res. 2015;24:24–31. doi: 10.1111/jsr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchanan DT, Cain K, Heitkemper M, et al. Sleep measures predict next-day symptoms in women with irritable bowel syndrome. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;10:1003. doi: 10.5664/jcsm.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]