Abstract
Tumor-infiltrating lymphocytes (TIL) has been associated with improved survival in cancer patients. Within the tumor microenvironment, regulatory cells and expression of co-inhibitory immune checkpoint molecules can lead to the inactivation of TIL. Hence, there is a need to develop strategies that disrupt these negative regulators to achieve robust anti-tumor immune responses. We evaluated the blockade of immune checkpoints and their effect on T cell infiltration and function. We examined the ability of TIL to induce tumor-specific immune responses in vitro and in vivo. TIL isolated from tumor bearing mice were tumor-specific and expressed co-inhibitory immune checkpoint molecules. Administration of monoclonal antibodies against immune checkpoints led to a significant delay in tumor growth. However, anti-PD-L1 antibody treated mice had a significant increase in T cell infiltration and IFN-γ production compared to other groups. Adoptive transfer of in vitro expanded TIL from tumors of anti-PD-L1 antibody treated mice led to a significant delay in tumor growth. Blockade of co-inhibitory immune checkpoints could be an effective strategy to improve TIL infiltration and function.
Introduction
Infiltration of T cells into tumors has been correlated with improved survival in cancer patients. Although T cells are able to adequately infiltrate tumors, they are ineffective at eradicating patients’ tumors. Studies have shown that T cell infiltration in to tumor tissues are associated with reduction in the tumor burden and improved clinical prognosis [1,2]. Over the last decade, adoptive transfer of tumor infiltrating lymphocytes (TIL) has emerged as a promising approach to induce effective anti-tumor immunity and tumor regression in various cancers [3,4]. TIL therapy resulted in objective response rate of 40–50% in treated melanoma patients. [5,6]. Tumor-specific T cells may be inactivated in vivo by immunosuppressive factors in the local tumor microenvironment, such as T-regulatory and myeloid derived suppressor cells, or by signaling through of co-inhibitory molecules that modulate T cell activation. There are an increasing number of co-inhibitory signals in the tumor microenvironment that have been demonstrated to inhibit anti-tumor T cell responses. Activated T cells express multiple co-inhibitory receptors including lymphocyte activations gene 3 (LAG-3), B and T lymphocyte attenuator (BTLA), cytotoxic T lymphocyte antigen 4 (CTLA-4), and programmed death (PD-1) [7–11]. While these immune checkpoint receptors maintain T cell homeostasis, when expressed by tumor-specific T cells, they represent a significant barrier for the induction of effective anti-tumor immune responses. Blockade of these receptors has been shown to improve anti-tumor immune T cell responses. CTLA-4 (CD152) is a cell surface molecule that is expressed on activated T cells. Ipilimumab, approved by the FDA in 2011, targets the CTLA-4 receptor [12]. Lag-3 is a cell-surface molecule that is involved in T cell activation and function [13]. Studies have shown that LAG-3 is expressed on T regulatory cells (Tregs) and blockade of LAG-3 affects Treg function [14]. It has been shown that an in vitro suppression assay using CD4+CD25highLAG-3+ T cells showed that this subset of cells is endowed with potent suppressor activity and their frequency is enhanced in the PBMCs of patients with cancer and is expanded at tumor sites [15]. LAG3 expression is upregulated on TILs and blockade of LAG3 can enhance anti-tumour T cell responses [16,17]. BTLA belongs to CD28 family and is structurally similar to CTLA-4 and PD-1 [10]. BTLA expression on lymphocytes has been shown to attenuate T cell activation and proliferation. T cells from BTLA-deficient mice display a proliferative phenotype in response to T or B cell activation [18]. Expression of the negative regulator, programmed death ligand 1 (PD-L1) on tumor cells inhibits the activation of T cells upon binding to its receptor PD-1, thereby preventing effective anti-tumor immunity [19–22]. Monoclonal antibodies against PD-L1 have been explored in patients with cancer [21]. A previous study in our laboratory has shown that blockade of PD-L1 signaling enhanced anti-tumor effects in a melanoma tumor model [23]. Blockade of negative regulators on T cells in the tumor microenvironment may improve anti-tumor T cell responses and lead to improved immunotherapeutic strategies for cancer.
TIL therapy depends on the expansion of tumor-specific T cells from tumor fragments. Strategies to increase the number of T cells, expand reactive T cells at tumor site may improve and increase the probability of expanding tumor-specific T cells. In this study, we examined whether co-inhibitory blockade improves T cells for adoptive transfer and improves anti-tumor immune responses.
Materials and Methods
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at the University of South Florida (#A4100-01). Mice were humanely euthanized by CO2 inhalation according to the American Veterinary Medical Association Guidelines. Mice were observed daily and humanely euthanized if a solitary subcutaneous tumor exceeded 1.5cm in diameter or mice showed signs referable to metastatic cancer. All efforts were made to minimize suffering. Female C57BL.6 mice (6–8 weeks old) were purchased from Harlan Laboratories (Indianapolis, IN). Mice were housed at the Animal Research Facility of the H. Lee Moffitt Cancer Center and Research Institute.
Tumor Cell Lines
B16 and MC38 murine colon cancer cell lines were maintained by serial in vitro passages in Complete medium (CM). CM consisted of RPMI 1640 supplemented with 10% heat-inactivated FCS, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM fresh L-glutamine, 100 mg/ml streptomycin, 100 U/mL penicillin, 50 mg/mL gentamycin, 0.5 mg/mL fungizone (all from Life Technologies, Rockville, MD), and 0.05 mM 2-ME (Sigma-Aldrich, St. Louis, MO).
Monoclonal Antibodies
The monoclonal antibodies anti-LAG3 (clone C9B7W), anti-BTLA (clone 6A6), anti-CTLA-4 (clone 9H10), anti-PD-1 (clone RMP1-14), and anti PDL-1 (clone 10F.9G2) were all purchased from BioXcell (West Lebanon, NH). Normal ratIgG or hamster IgG was used as isotype controls.
In Vivo Treatment Model
A total of 1x105 MC-38 colon cancer tumor cells were injected subcutaneously (s.c.) in C57BL/6 mice. Three days later, mice were injected with 300 ug of monoclonal antibodies (isotype, anti-PD-1, anti-PD-L1, anti-Lag-3, anti-CTLA-4 or anti-BTLA) intraperitoneally (i.p.). Mice continued to receive this treatment every 3–4 days until the tumor reached a size of 400mm2. Tumor size was measured and recorded every two days. In another set of experiments, mice were euthanatized at day 21 after tumor injection. Tumors and splenocytes were harvested for in vitro assays.
Mouse TIL Isolation
Tumor cell suspensions were prepared from solid tumors by enzymatic digestion in HBSS (Life Technologies) containing 1 mg/ml collagenase, 0.1 mg/ml DNAse I, and 2.5 U/ml of hyaluronidase (all from Sigma-Aldrich) with constant stirring for 2 hours at room temperature. The resulting suspension was passed through a 70-um cell strainer, washed once with HBSS and resuspended in PBS + 3% BSA to a concentration of 1 x 106 cells/ml for flow cytometric analysis. Cells were labeled with anti-CD90 microbeads according to the manufacturers’ instructions (Miltenyi Biotec) and purified using an autoMACS. After autoMACS purification, TIL were cultured for 5 days in the presence of IL-2 (3000 IU/ml). On day 5, TIL were collected for in vitro assays.
Chromium Release Assay
A 51Cr release assay was done as described previously [3]. MC-38 cells were used as targets and B16 tumor cells were used as a control. TIL purified from tumors were used as effector cells. T cell purity was measured by flow cytometry and cells were 95% positive for CD90 (data not shown). Briefly, target MC-38 or B16 tumor cells were labeled with 100 μCi of 51Cr (Amersham Corp.) in 0.2 mL of medium at 37°C in a 5% CO2 atmosphere for one and a half hours. The labeled tumor cells were washed three times and added to the effector cells in triplicate wells of 96-well round-bottomed microplates at 50:1 and 25:1 effector to target ratios. After 5 hours, the percentage of specific 51Cr release was determined by the following equation: (experimental cpm − spontaneous cpm)/ (total cpm incorporated − spontaneous cpm) × 100. All determinations were done in triplicate, and the SE of all assays was calculated and was typically 5% of the mean or less.
Elispot Assay
Briefly, TIL were plated at 1x104 and co-cultured with 1x 103 irradiated MC-38 or B16 tumor cells and incubated for 48 hours at 37°C. The number of IFN-γproducing cells in response to stimulation was evaluated in an ELISPOT assay. The number of spots was counted in triplicates and calculated using an automatic ELISPOT counter.
Flow Cytometry
Spleens or TIL were harvested under sterile conditions. Single-cell suspensions were prepared, and red blood cells were removed using ACK lysis buffer. For analysis of immune cell populations, one million cells (splenocytes or tumor digest suspension) were incubated for 20 minutes on ice in staining medium with relevant antibodies for surface expression analysis according to the manufacturer’s instructions (all from BD Biosciences). Samples were analyzed using an LSRII (BD Biosciences) and the data was analyzed using FlowJo software (Tree Star).
Adoptive Transfer of TIL
TIL were purified from either NrIgG or anti- PD-L1 antibody treated mice as described above. After autoMACS purification, TIL were cultured for 5 days in the presence of IL-2 (3000IU/ml) and TIL were used for in vivo studies. On day 4 following tumor injections, 5x106 TIL per mouse were i.v. transferred. Beginning on day 4 and continuing every 12 hours for three days, mice also received 2.5e5 IU of IL-2 i.p. Following this treatment, tumor size was measured and recorded every 2 days.
Statistical Analysis
A Mann-Whitney test (unpaired) or a Student’s t-test was used to compare between two treatment groups. All statistical evaluations of data were performed using Graph Pad Prism software. Statistical significance was achieved at p<0.05.
Results
TIL Isolated from Tumor Bearing Mice Are Tumor-Specific with High Cytotoxic Function
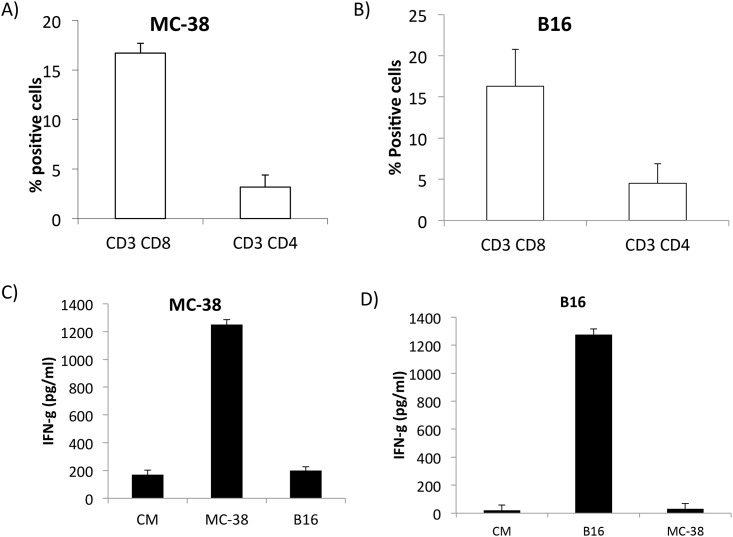
To examine the phenotype of infiltrating lymphocytes in tumors of B16 and MC-38-bearing mice, CD4+ and CD8+ T cells were analyzed by flow cytometry by gating on viable cells. As shown in Fig 1A and 1B, TIL isolated from MC-38 and B16 tumors had increased CD8+ T cell infiltration compared to CD4+ T cells. We next tested the tumor specificity and function of TIL isolated from MC-38 and B16 tumor bearing mice. TIL isolated from MC-38 (Fig 1C) and B16 (Fig 1D) when co-cultured in the presence of specific tumor cells had significant levels of IFN-γ production compared to irrelevant tumor cells. This data shows that TIL isolated from MC-38 and B16 tumors contain tumor-specific CD8+ T cells.
Fig 1. MC-38 and B16 TIL are tumor specific.
A& B) Bar graph represents mean+ SD of CD8+ and CD4+ T cells infiltrating tumors (n = 8); C& D) MC-38 or B16 TIL were co-cultured with specific or irrelevant tumor cells for 48 hours. IFN-γ production was measured in culture supernatants by ELISA.
Immune Checkpoint Receptors Expression on TIL
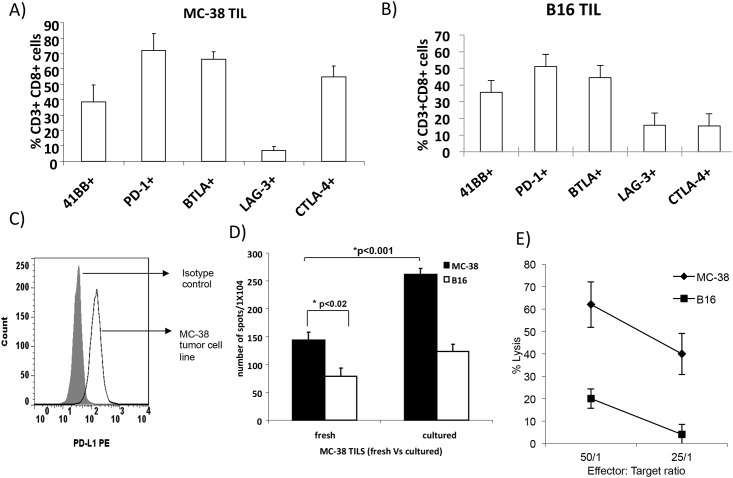
Inhibitory receptors such as PD-1, CTLA-4, Lag-3 and BTLA and expressed on T cells and ligands such as PD-L1 have been shown to contribute to immune mediated suppression. We examined the expression of inhibitory immune checkpoint receptors on CD8+ T cells isolated from B16 and MC-38 tumors and measured expression of PD-1, CTLA-4, BTLA, and LAG-3. We also measured 41BB expression on TIL to determine activation status (Fig 2A and 2B). Reports have shown that PD-L1 expression on tumor cells mediates negative signaling through PD-1 interaction on T cells. Previous study from our lab has shown that B16 tumor cells express PD-L1 on their surface [23]. We examined PD-L1 expression on MC-38 tumor cells. As shown in Fig 2C, MC-38 expresses PD-L1.
Fig 2. Expression of immune checkpoint receptors on MC-38 and B16 TIL.
A&B) Flow cytometry analysis of PD-1, CTLA-4, BTLA, and LAG-3 expression on MC-38 (A) and B16 (B) TIL; C) PD-L1 expression on MC-38 tumor cells; D) fresh or cultured MC-38 TIL were co-cultured with MC-38 or irrelevant B16 tumor cells and incubated for 48 hours at 37°C. The number of IFN-γ producing cells in response to stimulation was evaluated in an ELISPOT assay. The number of spots was counted in triplicates and calculated using an automatic ELISPOT counter; E) TIL was cultured with 51Cr labeled MC-38 or B16 tumor cells at 50:1 and 25:1 effector to target ratios. After 5 hours, the percentage of specific 51Cr release was determined by the following equation: (experimental cpm − spontaneous cpm)/ (total cpm incorporated − spontaneous cpm) × 100. All determinations were done in triplicate, and the SE of all assays was calculated and was typically 5% of the mean or less.
TIL Expansion In Vitro
We next examined the feasibility of expanding TIL in vitro from B16 and MC-38 tumors. C57/BL6 mice were injected with B16 or MC-38 and tumors were collected on day 21. Tumor cell suspensions were prepared from tumors by enzymatic digestion as described in methods section. Cells were labeled with anti-CD90 microbeads and purified using an autoMACS. After autoMACS purification, TIL were cultured for 5 days in the presence of IL-2 (3000 IU/ml). T cell purity was checked by flow cytometry and > 95% cells were positive for CD3. On day 5, TIL were collected, counted and used for in vitro assays TIL from MC-38 tumor expanded two-fold after 5 days of culture in media containing IL-2 (data not shown). However, we were not able to expand B16 TIL in culture for 5 days. Hence we utilized MC-38 tumor model for our TIL studies. An ELISPOT assay was performed to determine the number of IFN-γ producing TIL in response to stimulation by the MC-38 or irrelevant tumor cells. We compared freshly isolated TIL and TIL that were cultured for 5 days in media containing 3000 IU/ml IL-2. As shown in Fig 2D, both fresh and cultured TIL secreted IFN-γ in response to MC-38 cells. Significantly lower secretion of IFN-γ was measured in response to irrelevant B16 cells. Higher production of IFN-γ was measured in TIL cultured in the presence of IL-2 compared to fresh TIL (p<0.05). Next, we evaluated the ability of expanded TIL to mediate specific cell lysis in vitro. TIL isolated from MC-38 tumor bearing mice were cultured in vitro for 5 days in the presence of IL-2. On day 5, TIL were co-cultured with 51Cr-labeled MC-38 or B16 cells at effector to target ratios of 50:1 and 25:1. TIL exhibited cytotoxicity against MC-38 tumor cells at a 50:1 and 25:1 ratio and were tumor-specific (Fig 2E, p<0.001 compared to killing of irrelevant B16 cells). This data shows that cultured TIL mediates specific cytotoxicity against MC-38 cells.
Inhibitory Immune Checkpoint Blockade Delays Tumor Growth in MC-38 Tumor Bearing Mice
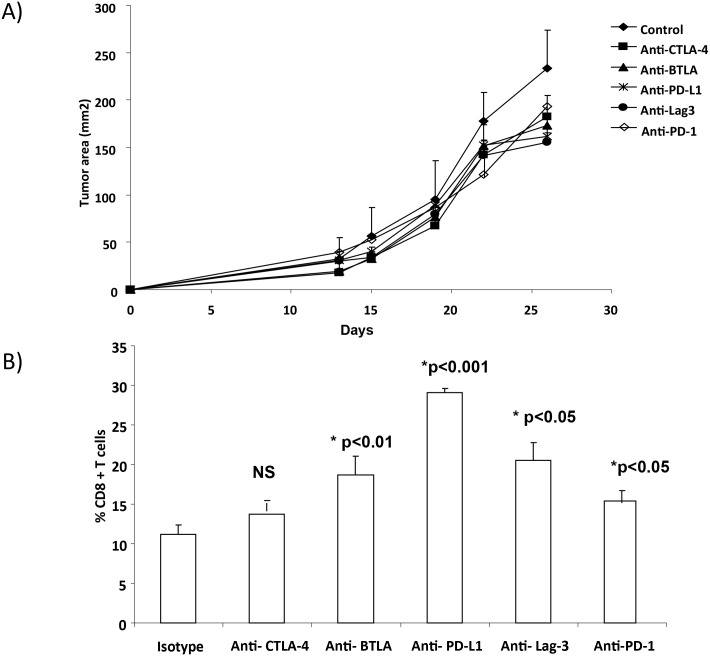
Based on the expression levels of inhibitory immune checkpoints, we examined whether blockade of these inhibitory immune checkpoints had an effect on TIL infiltration and tumor growth. As shown in Fig 3A, antibody treatment with anti-CTLA-4 (p<0.02), anti-BTLA (p<0.01), anti- PD-L1 (p<0.01), anti- Lag-3 (p<0.001) and anti- PD-1 antibodies (p<0.02) led to a modest but a significant delay in tumor growth in MC-38 bearing mice (p values compared to mice that received isotype control antibody). We next investigated whether blockade of individual co-inhibitory molecules had an effect on TIL infiltration that could contribute to the observed delay in MC-38 tumor growth. Tumors were collected on day 21 and CD8+ T cells within the TIL population were measured by flow cytometry. Treatment with anti-BTLA or anti-PD-L1, anti-Lag3 and anti-PD-1 led to a significant increase in CD8+ T cell infiltration compared to mice treated with isotype control antibody (Fig 3B). Tumor bearing mice that received anti-PD-L1 antibody demonstrated the most significant increase in T cell infiltration (p<0.001, compared isotype control). There was no significant increase in T cell infiltration in anti-CTLA-4 treated mice. Together, this data suggests that PD-L1 blockade is an effective strategy to increase CD8+ T cell infiltration in MC-38 tumors.
Fig 3. Inhibitory immune checkpoint blockade delays tumor growth in MC-38 tumor bearing mice.
A total of 1x105 MC-38 colon cancer tumor cells were injected subcutaneously (s.c.) in C57BL/6 mice. Three days later, mice were injected with 300 ug of monoclonal antibodies (isotype, anti-PD-1, anti-PD-L1, anti-Lag-3, anti-CTLA-4 or anti-BTLA) intraperitoneally (i.p.). Mice continued to receive this treatment every 3–4 days until the tumor reached a size of 400mm2. Tumor size was measured and recorded every two days. In another set of experiments, mice were euthanatized at day 21 after tumor injection. Tumors were harvested for in vitro assays A) Tumor growth B) Flow cytometry analysis of T cells infiltrating tumors after checkpoint blockade.
PD-L1 Blockade Improves Tumor-Specific Immune Responses of TIL
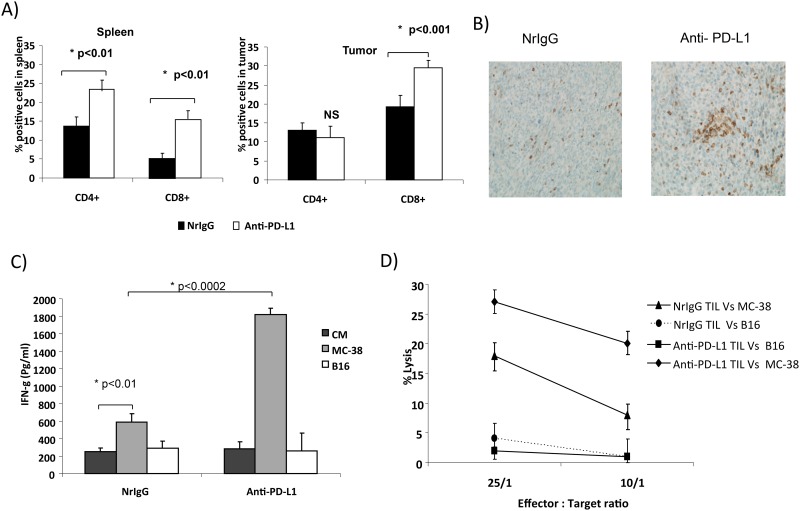
Since PD-L1 blockade led to a significant increase in T cell infiltration, we next examined whether PD-L1 blockade enhanced tumor-specific immune responses in MC-38 tumor bearing mice. Administration of anti-PD-L1 antibody enhanced the percentage of CD8+ T cells in the spleen (15.3%) and tumor (28.5%) compared to 5.6% of CD8+ T cells in the spleen and 17.6% of CD8+ T cells in tumor of mice treated with NrIgG (Fig 4A, n = 8). Similarly, anti-PD-L1 antibody treated mice had increased levels of CD4+ T cells in the spleen compared to NrIgG treated mice. No significant difference was measured in CD4+ T cell infiltration into tumors between mice treated with anti-PD-L1 antibody or NrIgG. Immunohistochemical staining revealed an increase in CD3+ T cell infiltration in the tumors of PD-L1 treated mice (Fig 4B, n = 8).
Fig 4. PD-L1 blockade increases T cell infiltration and enhances cytotoxic function of TIL.
A) Bar graph represents CD4 and CD8+ T cell infiltration in spleen and tumors (n = 8); B) CD3+ T cell infiltration in tumor (n = 8); C) measurement of IFN-γ by ELISA; D) Percent of 51Cr release by TIL.
We next measured IFN-γ production in response to restimulation with tumor cells as a measure of T cell function. TIL isolated from tumors of NrIgG or anti-PD-L1 antibody treated mice were restimulated with MC-38 or irrelevant B16 cells for 48 hours. As shown in Fig 4C, TIL from anti-PD-L1 antibody treated tumor bearing mice demonstrated increased IFN-γ production in response to MC-38 cells (p<0.0002) compared to the TIL from NrIgG treated mice. This data suggests that PD-L1 blockade enhances TIL function in MC-38 tumor-bearing mice.
We next examined whether PD-L1 blockade improves the cytotoxic function of TIL. TIL isolated from tumors of anti-PD-L1 antibody or NrIgG treated mice were cultured in the presence of 3000IU/ml of IL-2. On day 5, TIL were analyzed for their cytotoxic function using a 51Cr release assay. Purified TIL from anti-PD-L1 antibody treated mice co-cultured with 51Cr-labeled MC-38 tumor cells had higher cytotoxicity (27%) at 25:1 and 10:1 (22%) ratios compared to the TIL from tumors of NrIgG treated mice (18% at 25:1 and 8%. at 10:1 ratio) (Fig 4D, p<0.01). No killing of irrelevant B16 cells was measured in either group. This data supports PD-L1 blockade to improve the cytotoxic function of TIL.
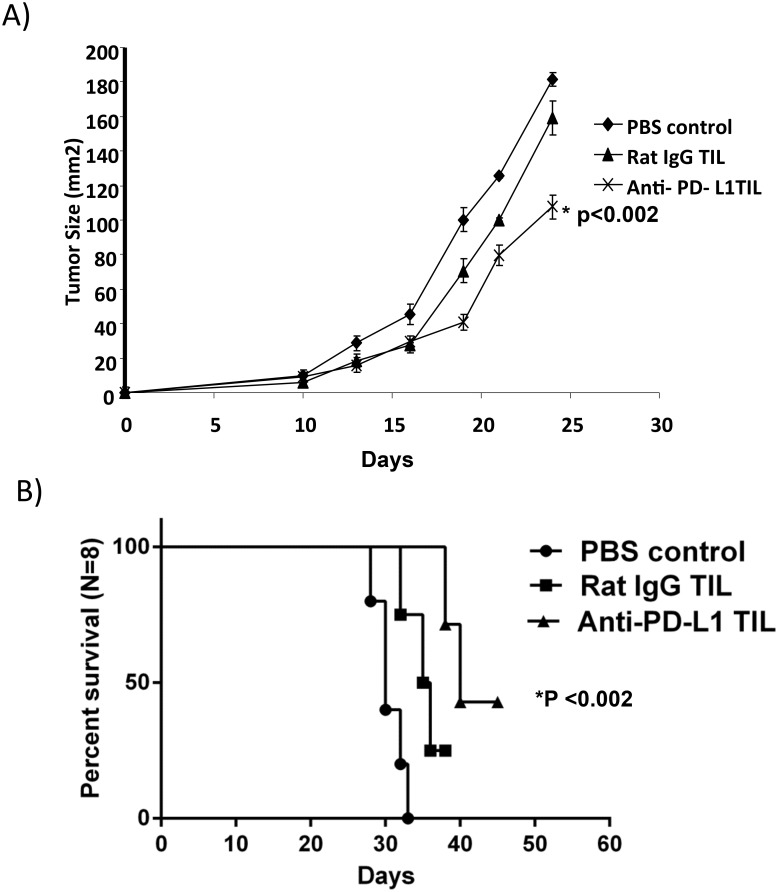
PD-L1 Blockade Improves Anti-Tumor Efficacy of TIL In Vivo
Next, we investigated PD-L1 blockade for the generation of TIL for adoptive cell therapy. Mice were injected s.c. with MC-38 tumor cells on day 0 followed by total body irradiation (TBI) with 600 rad on day 3. Cultured TIL from anti-PD-L1 or NrIgG antibody treated mice were adoptively transferred on day 4. As shown in Fig 5A, mice that received TIL derived from either NrIgG or anti-PD-L1 treated mice demonstrated a significant delay in tumor growth compared to the control mice that did not receive any TIL therapy (p<0.01). However, adoptive transfer of TIL from anti-PD-L1 antibody treated mice led to a significant delay in tumor growth and enhanced survival compared to mice that received TIL from NrIgG treated mice (Fig 5B, p<0.002). This data demonstrates that PD-L1 blockade improves TIL function that may enhance anti-tumor immune responses after adoptive transfer.
Fig 5. PD-L1 blockade improves the anti-tumor efficacy of TIL in vivo.
A) Mice received MC-38 tumor cells on day 0 followed by 600 rad TBI on day 3. TIL isolated from NrIgG or anti-PD-L1 antibody treated mice (n = 8) were cultured with 3000IU/ml of IL-2 for 5 days and adoptively transferred on day 4 followed by 6 injections of 2.5e5 IU of IL-2 every 12 hours; B) survival curve (n = 8).
Discussion
While TIL is a prognostic factor in cancer and is associated with prolonged survival rates in cancer patients, the infiltrating T cells are unable to induce total tumor regressions [2]. Adoptive transfer of infiltrating T cells has been a promising approach but is unable to induce total tumor regressions. This is due to the exhaustion of TIL that has been correlated with the expression of various immune checkpoint receptors on T cells and ligands by tumor cells and this may prevent TIL from inducing strong anti-tumor immune responses. Increased expression of co-inhibitory molecules can down regulate T cell activity and lead to tumor evasion. The purpose of this study is to restore the function of TIL for the use of adoptive cell therapy. In this study, blockade of Lag-3, BLTA, PD-1 or PD-L1 enhanced T cell infiltration into MC-38 tumors. However, the most significant increase in CD8+ T cell infiltration was seen following treatment with PD-L1 blocking antibodies. MC-38 tumor cells expressed high levels of PD-L1 and several reports support that PD-L1 expression on tumor cells can inhibit cytotoxic T lymphocytes through its interaction with the PD-1 receptor expressed by activated T cells [24,25]. The role of PD-1 and PD-L1 interactions in T cell exhaustion has been studied extensively. Expression of PD-1 on tumor-specific T cells leads to a profound impairment in the production of IL-2 that compromises their CTL function [26]. Blockade of the PD-1/PD-L1 interaction can reverse the T cell exhaustion and restore anti-tumor immunity [1,27]. A recent study has shown that anti-PD-L1 antibody treatment in combination with IL-15 reduces PD-1 expression on CD8+ T cells in murine metastatic colon cancer resulting in enhanced IFN-γ secretion and prolonged survival of tumor bearing animals [27]. Our laboratory has shown the therapeutic effect of PD-L1 blockade in improving DC vaccination and adoptive T cell transfer in the B16 melanoma model [23]. In this study, T cells isolated from MC-38 tumors after PD-L1 blockade produced IFN-γ in response to MC-38 cells and demonstrated increased CTL responses compared to the TIL from NrIgG-treated control mice. Our results show that blocking PD-L1 could restore the functional ability of TIL in inducing an anti-tumor immune response.
Although blockade of CTLA-4 delayed tumor growth in our model, we did not observe an increase in T cell infiltration into MC-38 tumor. CLTA-4 blockade has been shown to enhance anti-tumor immunity in tumor models when combined with other immunotherapies [28,29]. Dual blockade of PD-1 and CTLA-4 combined with a tumor vaccine restored T cell activity [30]. Hence, combination immunotherapies that include the blockade of CTLA-4 may improve T cell infiltration into tumors.
Among the immune checkpoint molecules measured on T cells, we observed low levels of Lag-3 expression on TIL compared to other co-inhibitory molecules. However, blockade of Lag-3 led to delayed growth of MC-38 tumors. While Lag-3 is expressed on CD4+ and CD8+ T cells, Lag-3 expression is well defined on an active CD4+CD25+Foxp3+ regulatory T cell subset. These cells have been shown to be highly suppressive and expanded in tumor sites [31]. A recent study has shown that pDCs also express high levels of Lag-3. Lag-3-mediated activation of pDCs within tumors may be in part responsible for directing an immune-suppressive environment [15]. It is possible that blockade of Lag-3 led to decreased suppression within the tumor that resulted in delayed tumor growth in the MC-38 model.
TIL-based therapies have been shown to induce effective anti-tumor immunity and tumor regression in various cancers [3,4,32,33]. TIL therapy depends on the expansion of tumor-specific T cells from tumor fragments. Strategies to improve reactive T cell infiltration and activation within tumors may increase the probability of expanding tumor-specific T cells for infusion. We show that blockade of PD-L1 led to improved infiltration and activation of anti-tumor T cells within tumors. Expansion in vitro and adoptive transfer of these T cells resulted in improved anti-tumor immunity. Although, we observed only a moderate delay in tumor growth in mice that received TIL from anti-PD-L1 treated mice, we believe that the treatment with anti-PD-L1 or other co-inhibitory antibodies after TIL transfer will enhance T cell persistence and efficacy. These studies are ongoing. Together, our results support co-inhibitory blockade prior to collection of tumor to generate TIL for adoptive cell therapy protocols for the treatment of cancer in clinical settings.
Acknowledgments
We thank the Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center and Research Institute for its contribution to this work.
Abbreviations
- TIL
Tumor infiltrating lymphocytes
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the research grant to Krithika Kodumudi by a Phi Beta Psi sorority award and in part by the Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center & Research Institute; an NCI designated Comprehensive Cancer Center (P30-CA076292). This work was also supported, in part, by a NCI-K23 award (Amod Sarnaik, K23 CA178083–01) and Miles for Moffitt award to Shari Pilon-Thomas. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wu X, Zhang H, Xing Q, Cui J, Li J, Li Y, et al. PD-1(+) CD8(+) T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer 111: 1391–1399. 10.1038/bjc.2014.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, et al. (2009) T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol 16: 2524–2530. 10.1245/s10434-009-0585-3 [DOI] [PubMed] [Google Scholar]
- 3.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, et al. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother 35: 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turcotte S, Gros A, Hogan K, Tran E, Hinrichs CS, Wunderlich JR, et al. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J Immunol 191: 2217–2225. 10.4049/jimmunol.1300538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26: 5233–5239. 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA (2003) Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 26: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 6: e19499 10.1371/journal.pone.0019499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 124: 2246–2259. 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocellin S, Nitti D CTLA-4 blockade and the renaissance of cancer immunotherapy. Biochim Biophys Acta 1836: 187–196. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, et al. (2003) BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4: 670–679. [DOI] [PubMed] [Google Scholar]
- 11.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72: 917–927. 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J, Hamid O, Amin A, O'Day S, Masson E, Goldberg SM, et al. Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma. Cancer Immun 13: 7 [PMC free article] [PubMed] [Google Scholar]
- 13.Triebel F (2003) LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol 24: 619–622. [DOI] [PubMed] [Google Scholar]
- 14.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. (2004) Role of LAG-3 in regulatory T cells. Immunity 21: 503–513. [DOI] [PubMed] [Google Scholar]
- 15.Camisaschi C, De Filippo A, Beretta V, Vergani B, Villa A, Vergani E, et al. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: involvement of LAG-3. J Invest Dermatol 134: 1893–1902. 10.1038/jid.2014.29 [DOI] [PubMed] [Google Scholar]
- 16.Demeure CE, Wolfers J, Martin-Garcia N, Gaulard P, Triebel F (2001) T Lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): role of LAG-3/MHC class II interactions in cell-cell contacts. Eur J Cancer 37: 1709–1718. [DOI] [PubMed] [Google Scholar]
- 17.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. (2007) LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 117: 3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serriari NE, Gondois-Rey F, Guillaume Y, Remmerswaal EB, Pastor S, Messal N, et al. (2010) B and T lymphocyte attenuator is highly expressed on CMV-specific T cells during infection and regulates their function. J Immunol 185: 3140–3148. 10.4049/jimmunol.0902487 [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 19: 462–468. 10.1158/1078-0432.CCR-12-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167–3175. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol 184: 3442–3449. 10.4049/jimmunol.0904114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maier H, Isogawa M, Freeman GJ, Chisari FV (2007) PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol 178: 2714–2720. [DOI] [PubMed] [Google Scholar]
- 25.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res 19: 1363–1374. 10.1158/1078-0432.CCR-12-2199 [DOI] [PubMed] [Google Scholar]
- 26.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207: 2187–2194. 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 16: 6019–6028. 10.1158/1078-0432.CCR-10-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocak E, Lute K, Chang X, May KF Jr., Exten KR, Zhang H, et al. (2006) Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res 66: 7276–7284. [DOI] [PubMed] [Google Scholar]
- 29.Son CH, Bae JH, Shin DY, Lee HR, Choi YJ, Jo WS, et al. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J Immunother 37: 1–7. [DOI] [PubMed] [Google Scholar]
- 30.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 73: 3591–3603. 10.1158/0008-5472.CAN-12-4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, et al. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol 184: 6545–6551. 10.4049/jimmunol.0903879 [DOI] [PubMed] [Google Scholar]
- 32.Chacon JA, Pilon-Thomas S, Sarnaik AA, Radvanyi LG Continuous 4-1BB co-stimulatory signals for the optimal expansion of tumor-infiltrating lymphocytes for adoptive T-cell therapy. Oncoimmunology 2: e25581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turcotte S, Gros A, Tran E, Lee CC, Wunderlich JR, Robbins PF, et al. Tumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapy. Clin Cancer Res 20: 331–343. 10.1158/1078-0432.CCR-13-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.