Abstract
Aim
To evaluate the diagnostic accuracy and clinical utility of the fibrosis index based on the four factors (FIB-4), aspartate aminotransferase -to-platelet ratio index (APRI), and aspartate aminotransferase–alanine aminotransferase ratio index (AAR) for predicting liver fibrosis in patients with HBV infection.
Methods
From January 2006 to December 2010,a total of 1543 consecutive chronic hepatitis B(CHB) patients who underwent liver biopsies were enrolled. FIB-4,APRI, and AAR were calculated.The areas under the receiver-operating characteristic curves (AUROCs) were calculated to assess the diagnostic accuracy of these models.The AUROCs of these models were compared by DeLong’s test.For further comparisons in different studies,the AUROCs were adjusted to conduct Adjusted AUROCs(ADjAUROCs) according to the prevalence of fibrosis stages using the difference between advanced and nonadvanced fibrosis (DANA).
Results
For prediction of significant fibrosis,severe fibrosis,and cirrhosis,the AUROCs of FIB-4 were 0.646(ADjAUROC 0.717),0.670(ADjAUROC 0.741), and 0.715(ADjAUROC 0.786) respectively;whereas it were 0.656(ADjAUROC 0.727),0.653(ADjAUROC 0.724) and 0.639(ADjAUROC 0.710) for APRI, 0.498(ADjAUROC 0.569),0.548(ADjAUROC 0.619) and 0.573(ADjAUROC 0.644) for AAR. The further comparisons demonstrated that there were no significant differences of AUROCs between FIB-4 and APRI in predicting significant and severe fibrosis(P > 0.05),while FIB-4 was superior to APRI in predicting cirrhosis(P < 0.001). Further subgroup analysis demonstrated that the diagnostic accuracy of FIB-4 and APRI in patients with normal alanine aminotransferase(ALT) were higher than that in patients with elevated ALT.
Conclusions
The results demonstrated that FIB-4 and APRI are useful for diagnosis of fibrosis. FIB-4 and APRI have similar diagnostic accuracy in predicting significant and severe fibrosis,while FIB-4 is superior to APRI in predicting cirrhosis. The clinical utility of FIB-4 and APRI for fibrosis need further external validation in a large population before it was used for prediction of fibrosis in patients with HBV infection.
Introduction
Hepatitis B virus (HBV) infection affects 350 million individuals and there are almost one million people died for HBV-related liver diseases every year[1]. Liver biopsy is still the gold standard for assessing hepatic fibrosis in patients with HBV infection. However, liver biopsy is limited by invasiveness and susceptibility of this technique to sampling error[2,3]. Magnetic Resonance Imaging (MRI), Computed Tomography (CT),and transient elastography(TE) have a better diagnostic value in detecting of hepatic fibrosis. However,these imaging examinations are limited by the high cost and not readily available in most hospitals. From the perspective of cost-effectiveness and clinical practice, an ideal diagnostic method for assessment of liver fibrosis should be a simple, noninvasive,inexpensive, readily available, and easier practical test. Therefore, FIB-4,APRI,and AAR had been suggested to evaluate the liver fibrosis[4–6]. However,the conclusions of these previous studies were controversial and their clinical utility for fibrosis in patients with HBV infection were uncertain[7–9].
Therefore,we performed this retrospective study to evaluate diagnostic accuracy and clinical utility of FIB-4, APRI,and AAR for predicting liver fibrosis in hepatitis B virus-infected patients.
Materials and Methods
Patients
Between January 2006 to December 2010, 1620 consecutive patients who had been diagnosed with HBV infection and had undergone a liver biopsy in department of infectious diseases of Shunde First People’s Hospital. The Patients were enrolled based on the following criteria: chronic hepatitis B(CHB) defined as hepatitis B surface antigen (HBsAg) positivity for more than 6 months; detectable HBV-DNA with a level >103 copies/ml. The exclusion criteria were as follows: liver cancer or co-infection with hepatitis C virus, hepatitis D virus or human immunodeficiency virus; autoimmune liver diseases suah as autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis; alcohol ingestion in excess of 20 g/day;hereditary and metabolic liver diseases suah as Wilson’s disease, hemochromatosis, and α-1-antitrypsin deficiency.
Therefore, there were 77 patients excluded from the study according to above criteria. There were no significant differences in terms of demographic and clinical parameters between patients included and excluded (data not shown).Finally, a total of 1543 patients (1182 males and 361 females) were recruited into the study. The written consent was obtained from patients before inclusion.The study was approved by the ethics committee of the Shunde First People’s Hospital. All clinical investigation were conducted according to the principles expressed in the Declaration of Helsinki.
Liver biopsy
Liver biopsies were performed by two experienced physicians using a 16-gauge needle(16G biopsy Menghini’s needle, ShangHai). A minimum of 1.5 cm of liver tissue with at least 7 portal tracts was required for diagnosis.The specimens were fixed, paraffin-embedded and stained with haematoxylin and eosin (HE). Histological grading of necro-inflammation (G0–G4) and staging of the liver fibrosis (S0–S4) were carried out according to Scheuer classification [10] by one experienced pathologist blinded to the clinical data. In the study,Significant fibrosis was defined as fibrosis stage≥S2;Severe fibrosis was defined as fibrosis stage≥S3;Cirrhosis was defined as fibrosis stage = S4.
Serum markers and noninvasive models
All patients systematically underwent complete biochemical workups, ultrasonography and liver biopsy within 2 days.Blood samples of the subjects were obtained before LB. Biochemical tests were performed by commercial assays in our hospital laboratory for alanine aminotransferase(ALT,U/L), aspartate aminotransferase(AST,U/L), hemoglobin (HGB, g/L), uric acid (UA,μmol/L), Fasting plasma glucose(FPG, mmol/L),Total cholesterol (TC,mmol/L),and Glycerin three greases (TG,mmol/L), high-density lipoprotein (HDL, mmol/L); low-density lipoprotein (LDL, mmol/L). The serum HBV-DNA level was detected with a Real-Time polymerase chain reaction (PCR) System (ABI7700;Applied Shenzhen city Daeran Biological Engineering Co Ltd, Shenzhen, Guangdong,CHN). HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, anti-HCV were measured with CLIA systems(Abbott ARCHITECT i2000 SR system, Abbott Laboratories, Abbott Park, IL, USA).
The formulas of FIB-4, APRI,and AAR were calculated as described in the original articles[4–6].FIB -4:(age [year]*AST [U/L]) / {(PLT [109/L])*(ALT [U/L])1/2};APRI:(AST/ [ULN]/PLT [109/L]) *100; AAR:AST(U/L)/ALT(U/L).
Standardisation of AUROC according to the prevalence of fibrosis stages
It had been found that the prevalence of liver fibrosis stages may be a major factor of variability in assessing the diagnostic accuracy of noninvasive model.Therefore, AUROC should be adjusted according to the prevalence of fibrosis stages using the Difference between advanced and nonadvanced fibrosis (DANA) [11].DANA was calculated according to the following formula:[(prevalence F2*2 + prevalence F3*3 + prevalence F4*4) ⁄ (prevalence F2 + prevalence F3 + prevalence F4)]–[prevalence F1⁄ (prevalence F0 + prevalence F1)]. The adjusted AUROCs (adjAUROCs) were calculated as follows:AdjAUROC = observed AUROC (obAUROC) +0.1056 *(2.5 –DANA).
Statistical analysis
Continuous data were expressed as mean±SD or median(quartile range)depending on the normality of the data. Continuous variables were compared with one-way ANOVA analysis of variance or Kruskal-Wallis H test, depending on the normality of the data; Categorical variables were expressed as proportions and compared with Chi-square test.
Receiver-operating characteristic (ROC) curves were constructed and the area under the ROC curve(AUROC) were calculated. The overall diagnostic accuracy of different models was evaluated by AUROC. The AUROC values of these models were compared by DeLong’s test[11].
The optimal cut off value was determined by maximal sum of sensitivity and specificity. To further evaluate the clinical utility,the sensitivity (Se), specificity(Sp), positive predictive value (PPV), and negative predictive value (NPV) were calculated using the ROC curve.
To validate diagnostic accuracy and clinical utility of three models, we conducted an internal validation test using bootstrap resampling method. This involved generating ROC curves by drawing 1543 new samples with replacements from the original samples. Then, the AUROCs,sensitivity, specificity, PPV, and NPV accord to the optimal cut off value were calculated in the validation group consisting of 1543 new samples again.
Statistical analyses were performed using SPSS 13.0(SPSS Inc., Chicago, IL).P < 0.05 was considered statistically significant.
Results
Baselines characteristics of Patients
A total of 1543 patients were recruited into the study with a mean age of 31.55±9.73 years. Of all subjects in the study,1182(76.60%) were male and 361(23.40%) were female, 1168(75.70%) were HBeAg positive and 375(24.30%) were HBeAg negative. The fibrosis stages were 267 (17.30%) in S1, 554 (35.90%) in S2, 423(27.41%) in S3 and 299 (19.38%) in S4. The inflammation grades were 76 (4.93%) in G1, 742 (48.09%) in G2, 527(34.15%) in G3 and 198 (12.83%) in G4. The baseline characteristics were summarized in Table 1. The mean values of FIB-4 and APRI were significantly higher for each successive fibrosis stage (P <0.05). There were no differences between successive fibrosis stages for AAR (p >0.05,except for S1 VS S2-4 P = 0.037).
Table 1. Baseline characteristics of 1543 patients with HBV infection.
| Parameters | Totel | S1 | S2 | S3 | S4 | Test | P |
|---|---|---|---|---|---|---|---|
| (n = 1543) | (n = 267) | (n = 554) | (n = 423) | (n = 299) | value | value | |
| Male(n,%) | 1182(76.60) | 199(74.51) | 417(75.27) | 312(73.76) | 254(84.95) | 14.72 | <0.001 |
| Age(years) | 31.55±9.73 | 29.43±9.09 | 30.55±9.23 | 31.22±9.11 | 35.79±10.80 | 70.23 | 0.002 |
| ALT(U/L) | 85(48,166) | 69(41,128) | 90(50,162) | 97(48,188) | 73(45,167) | 21.457 | <0.001 |
| AST(U/L) | 60(42,97) | 52(37,71) | 58(41,92) | 72(48,110) | 60(44,108) | 54.978 | <0.001 |
| GGT(U/L) | 54(30,99) | 32(19,61) | 45(27,82) | 60(38,113) | 84(50,141) | 187.99 | <0.001 |
| ALB(G/L) | 44.24±5.42 | 45.77±4.15 | 45.16±5.35 | 43.78±5.62 | 41.85±5.4 | 123.94 | <0.001 |
| GLO(G/L) | 27.84±5.01 | 26.81±4.37 | 27.18±4.97 | 28.32±4.75 | 29.32±5.53 | 55.68 | <0.001 |
| TBil(umol/l) | 17.55±11.43 | 16.34±8.44 | 16.00±10.74 | 17.96±12.97 | 20.92±12.21 | 54.13 | <0.001 |
| DBil(umol/l) | 7.36±8.25 | 5.83±4.83 | 6.24±6.21 | 7.89±9.78 | 10.08±10.6 | 77.89 | <0.001 |
| PT(seconds) | 12.17±1.95 | 11.86±2.05 | 12.01±1.46 | 12.11±2.22 | 12.83±2.1 | 146.19 | <0.001 |
| WBC(G/L) | 5.81±1.65 | 6.03±1.63 | 5.83±1.59 | 5.79±1.67 | 5.6±1.73 | 9.46 | 0.024 |
| HGB(G/L) | 143.12±18.79 | 143.20±21.52 | 145.52±18.37 | 142.05±18.83 | 140.09±16.26 | 26.53 | <0.001 |
| PLT(G/L) | 188.68±56.87 | 203.75±52.84 | 200.44±56.16 | 186.88±52.71 | 155.99±54.03 | 155.18 | <0.001 |
| BUN(umol/l) | 4.22±1.26 | 4.37±1.29 | 4.23±1.24 | 4.11±1.22 | 4.24±1.32 | 8.29 | 0.04 |
| Cr(umol/l) | 79.15±23.77 | 78.78±19.96 | 78.14±20.02 | 78.41±18.33 | 82.93±36.55 | 2.35 | 0.503 |
| Glu(mmol/l) | 4.52±1.24 | 4.43±0.94 | 4.51±1.14 | 4.52±1.2 | 4.65±1.65 | 0.76 | 0.860 |
| TC(mmol/l) | 3.91±1.76 | 3.89±2.00 | 4.04±1.75 | 3.82±1.73 | 3.83±1.57 | 13.16 | 0.004 |
| TG(mmol/l) | 1.00±0.66 | 0.94±0.61 | 1.04±0.75 | 0.97±0.61 | 1.02±0.6 | 3.27 | 0.352 |
| LogDNA(copies/ml) | 5.87±1.40 | 6.12±1.43 | 5.97±1.4 | 5.77±1.39 | 5.6±1.36 | 29.87 | <0.001 |
| HBeAg+(n,%) | 1168(75.70) | 206(77.22) | 444(80.15) | 323(76.33) | 195(65.16) | 13.89 | <0.001 |
| Antiviral therapy | 169(10.95) | 39(14.6) | 61(11.1) | 38(9.0) | 31(10.4) | 5.44 | 0.142 |
| G1(n,%) | 76(4.93) | 52(19.48) | 23(4.15) | 0(0) | 1(0.33) | 181.6 | <0.001 |
| G2(n,%) | 742(48.09) | 207(77.53) | 417(75.27) | 104(24.59) | 14(4.68) | ||
| G3(n,%) | 527(34.15) | 7(2.62) | 111(20.04) | 284(67.14) | 125(41.81) | ||
| G4(n,%) | 198(12.83) | 1(0.37) | 3(0.54) | 35(8.27) | 159(53.18) | ||
| AAR | 0.70(0.49,1.09) | 0.69(0.50,1.03) | 0.65(0.45,1.0) | 0.69(0.49,1.12) | 0.79(0.57,1.25) | 20.444 | <0.001 |
| APRI | 0.87(0.56,1.42) | 0.63(0.44,0.97) | 0.78(0.50,1.27) | 1.01(0.65,1.59) | 1.09(0.73,1.89) | 129.68 | <0.001 |
| FIB-4 | 1.12(0.74,1.82) | 0.87(0.62,1.23) | 0.98(0.65,1.52) | 1.20(0.80,1.95) | 1.70(1.12,2.99) | 183.38 | <0.001 |
Footnotes:Hepatic steatosis were diagnosed by liver biopsy. Continuous data were expressed as mean±SD or median(quartile range)and compared with one-way ANOVA analysis of variance or Kruskal-Wallis H test, depending on the normality of the data. Categorical variables were expressed as proportions and compared with Chi-square test.ALT, Alanine aminotransferase;AST, Aspartate aminotransferase;γ-GT, γ-glutamyl transferase; hemoglobin,HGB;UA, uric acid;FPG, Fasting plasma glucose;TC, Total cholesterol;TG, Triglyceride;HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; FIB-4,the fibrosis index based on the four factors; APRI, aspartate aminotransferase -to-platelet ratio index;AAR,aspartate aminotransferase–alanine aminotransferase ratio index.
Diagnostic accuracy of noninvasive models for prediction of fibrosis
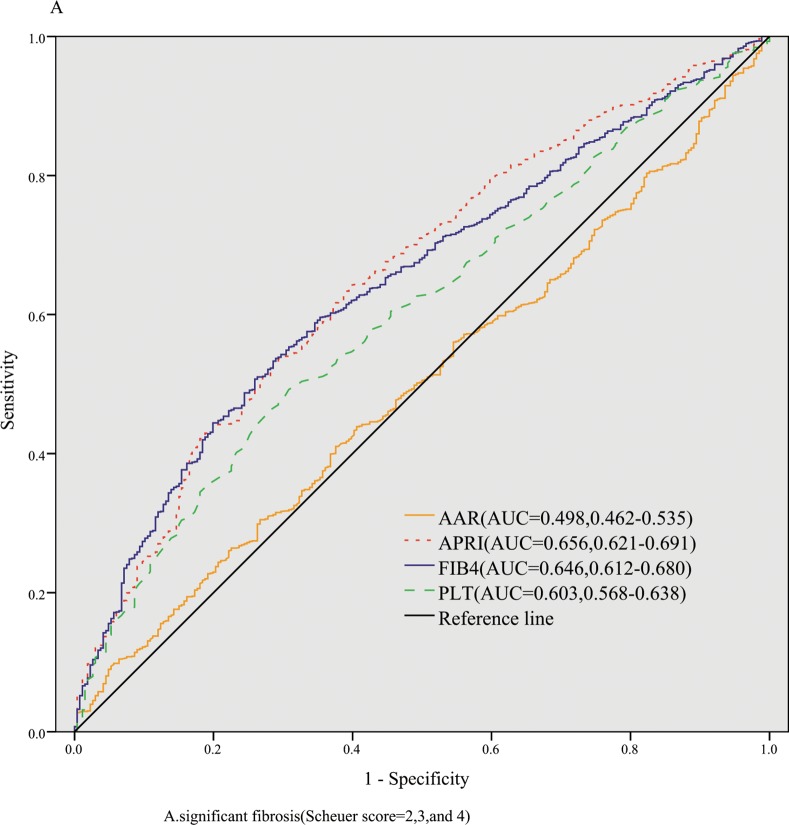
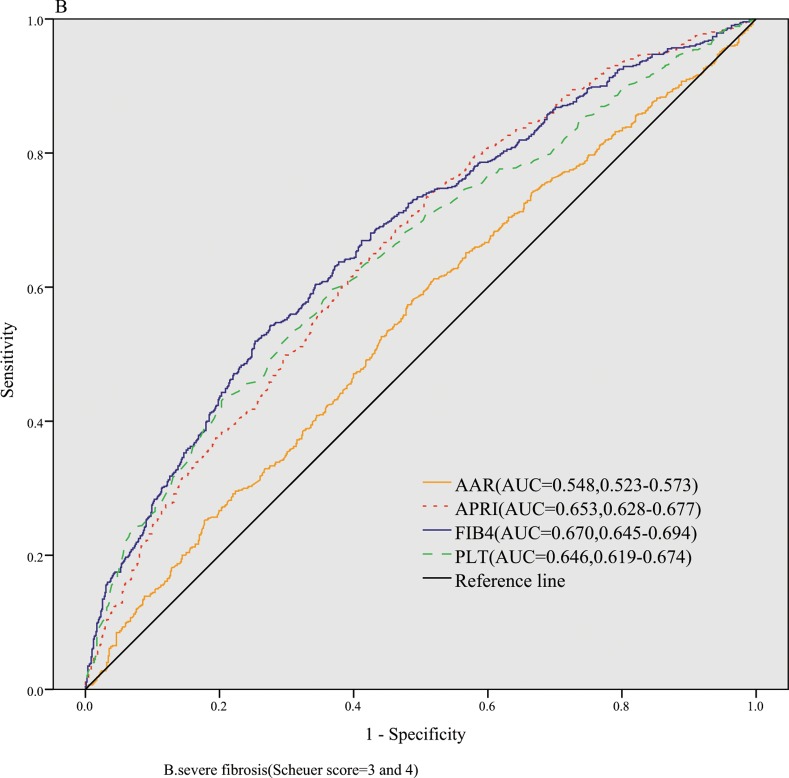
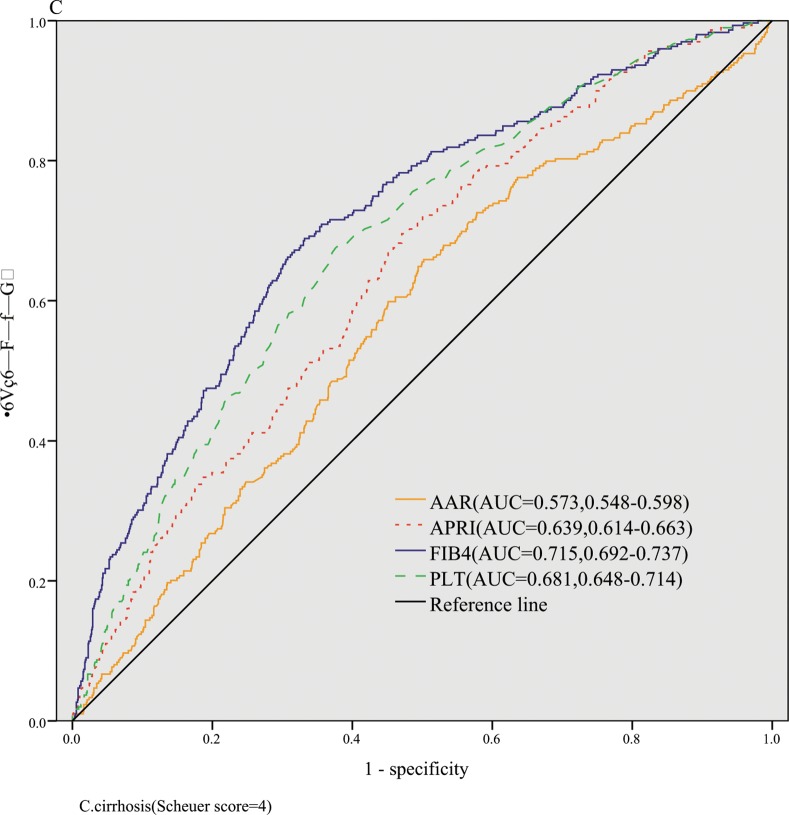
The AUROCs of FIB-4, APRI, AAR,and PLT for identification of significant fibrosis were 0.646(ADjAUROC 0.717,95%CI: 0.612–0.680),0.656(ADjAUROC 0.727,95%CI: 0.621–0.691), 0.498(ADjAUROC 0.569,95%CI: 0.462–0.535),and 0.603(ADjAUROC 0.674,95%CI: 0.568–0.638), respectively (Fig 1). The AUROCs of FIB-4, APRI, AAR,and PLT for severe fibrosis were 0.670(ADjAUROC 0.741,95%CI: 0.646–0.694), 0.653(ADjAUROC 0.724,95%CI: 0.628–0.677), 0.548(ADjAUROC 0.619,95%CI: 0.523–0.573), and 0.646(ADjAUROC 0.717,95%CI: 0.619–0.674), respectively (Fig 2). The AUROCs of FIB-4, APRI, AAR,and PLT for cirrhosis were 0.715(ADjAUROC 0.786,95%CI: 0.692–0.737), 0.639(ADjAUROC 0.710,95%CI: 0.614–0.663), 0.573(ADjAUROC 0.644,95%CI: 0.548–0.598),and 0.681(ADjAUROC 0.752,95%CI: 0.648–0.714), respectively (Fig 3).
Fig 1. The diagnostic accuracy of noninvasive models for predicting significant fibrosis.
Fig 2. The diagnostic accuracy of noninvasive models for predicting severe fibrosis.
Fig 3. The diagnostic accuracy of noninvasive models for predicting cirrhosis.
Then we conducted the comparisons of AUROCs among different tests by DeLong’s test[12]. There were no significant differences of AUROCs between FIB-4 and APRI (P = 0.505) for predicting significant fibrosis,which were both superior to AAR (all P < 0.01). To predict severe fibrosis, FIB-4 and APRI had same diagnostic accuracy (P = 0.170), while the AUROCs of FIB-4 and APRI were better than that of AAR(all P < 0.001). FIB-4 was superior to APRI (P < 0.001) and APRI was superior to AAR(P = 0.008) in predicting cirrhosis.
Clinical utility of FIB-4,APRI and AAR for prediction of fibrosis
To explore the clinical utility of these models for prediction of fibrosis, The optimal cut off value was determined by maximal sum of sensitivity and specificity. The sensitivity, specificity, PPV, and NPV were summarized in Table 2.
Table 2. Clinical utility of three models for prediction of fibrosis.
| model | Cut-off | sensitivity | 95%CI | specificity | 95%CI | PPV | 95%CI | NPV | 95%CI |
|---|---|---|---|---|---|---|---|---|---|
| value | % | % | % | % | |||||
| S1 vs S2-4 | |||||||||
| FIB-4 | 1.13 | 53.3 | 50.5–56.1 | 71.4 | 65.6–76.8 | 89.9 | 87.5–92.0 | 24.2 | 21.3–27.4 |
| APRI | 0.72 | 63.8 | 61.0–66.4 | 60.5 | 54.4–66.4 | 88.5 | 86.3–90.5 | 25.9 | 22.5–29.5 |
| AAR | 0.41 | 17.1 | 15.1–19.3 | 88.0 | 83.5–91.7 | 87.2 | 82.4–91.1 | 18.2 | 16.1–20.4 |
| S1-2 vs S3-4 | |||||||||
| FIB-4 | 1.32 | 54.3 | 50.6–58.0 | 72.4 | 69.2–75.4 | 63.4 | 59.4–67.2 | 64.3 | 61.1–67.4 |
| APRI | 0.69 | 74 | 70.7–77.2 | 48.4 | 44.9–51.9 | 55.8 | 52.6–59.0 | 67.9 | 64.0–71.7 |
| AAR | 0.66 | 58.7 | 55.0–62.3 | 50.1 | 46.6–53.6 | 50.9 | 47.4–54.3 | 58.0 | 54.2–61.6 |
| S1-3 vs S4 | |||||||||
| FIB-4 | 1.35 | 68.9 | 63.3–74.1 | 66.8 | 64.1–69.4 | 33.4 | 29.7–37.3 | 89.9 | 87.8–91.8 |
| APRI | 0.84 | 69.6 | 64.0–74.7 | 52.8 | 50.0–55.6 | 26.2 | 23.2–29.4 | 87.8 | 85.2–90.1 |
| AAR | 0.66 | 65.9 | 60.2–71.2 | 48.8 | 46.0–51.7 | 23.6 | 20.8–26.7 | 85.6 | 82.8–88.1 |
Footnotes: Cut-off value was determined by maximal sum of sensitivity and specificity.PPV,positive predictive value;NPV,negative predictive value;CI, confidence interval.
Validation of models using bootstrap resampling method
To validate the diagnostic accuracy and clinical utility of these noninvasive models for prediction of fibrosis, we conducted an internal validation test using bootstrap resampling method.
There was a good agreement in diagnostic accuracy and clinical utility between the results obtained from the original samples and the bootstrap samples(Table 3). In validation group,there was no significant difference of AUROCs between FIB-4 and APRI (P = 0.841) for predicting significant fibrosis,which were both superior to AAR (all P < 0.01). To predict severe fibrosis, FIB-4 and APRI had same diagnostic accuracy (P = 0.283), while the AUROCs of FIB-4 and APRI were better than that of AAR(all P < 0.001). FIB-4 was superior to APRI (P < 0.001) and APRI was superior to AAR(P = 0.007) in predicting cirrhosis.
Table 3. Diagnostic accuracy and clinical utility for prediction of fibrosis in validation group.
| model | AUROC | 95%CI | ADj | Cut-off | sensitivity | 95%CI | specificity | 95%CI | PPV | 95%CI | NPV | 95%CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUROC | value | % | % | % | % | % | % | % | % | |||
| S1 vs S2-4 | ||||||||||||
| FIB-4 | 0.627 | 0.603–0.652 | 0.698 | 1.13 | 52.0 | 48.8–54.4 | 69.5 | 63.9–74.7 | 87.9 | 85.3–90.1 | 25.2 | 22.2–28.3 |
| APRI | 0.631 | 0.606–0.655 | 0.702 | 0.72 | 63.7 | 61.0–66.4 | 57.9 | 52.0–63.6 | 86.6 | 84.2–88.8 | 27.2 | 23.7–30.9 |
| AAR | 0.502 | 0.477–0.527 | 0.573 | 0.41 | 84.1 | 81.9–86.1 | 12.3 | 8.8–16.7 | 80.4 | 78.2–82.5 | 15.3 | 11.0–20.6 |
| S1-2 vs S3-4 | ||||||||||||
| FIB-4 | 0.665 | 0.641–0.689 | 0.736 | 1.32 | 53.2 | 49.4–57.0 | 72.9 | 69.8–75.9 | 61.3 | 57.3–65.2 | 65.9 | 62.8–68.9 |
| APRI | 0.652 | 0.627–0.676 | 0.723 | 0.69 | 74.6 | 71.1–77.8 | 48.3 | 44.9–51.7 | 53.8 | 50.5–57.0 | 70.2 | 66.3–73.9 |
| AAR | 0.540 | 0.515–0.565 | 0.611 | 0.66 | 56.0 | 52.2–59.7 | 51.5 | 48.1–54.9 | 48.1 | 44.6–51.7 | 59.2 | 55.6–62.8 |
| S1-3 vs S4 | ||||||||||||
| FIB-4 | 0.720 | 0.696–0.742 | 0.791 | 1.35 | 67.3 | 61.5–72.6 | 69.2 | 66.6–71.8 | 33.3 | 29.5–37.3 | 90.2 | 88.2–92.0 |
| APRI | 0.640 | 0.616–0.664 | 0.711 | 0.84 | 67.6 | 61.8–73.0 | 54.0 | 51.2–56.8 | 25.2 | 22.1–28.4 | 87.9 | 85.4–90.1 |
| AAR | 0.572 | 0.547–0.597 | 0.643 | 0.66 | 63.1 | 57.2–68.7 | 50.7 | 47.9–53.5 | 22.6 | 19.8–25.7 | 85.7 | 83.0–88.2 |
Footnotes: Cut-off value was determined by maximal sum of sensitivity and specificity.PPV,positive predictive value;NPV,negative predictive value;CI,confidence interval.
Subgroup analysis of diagnostic accuracy for patients with normal ALT and elevated ALT
To assess the diagnostic accuracy and clinical utility of these noninvasive models for patients with normal ALTand elevated ALT,the datum of patients were separated by normal ALT(defined as ALT<40U/L) and elevated ALT(defined as ALT≥40U/L).The baseline characteristics of patients with normal ALT and elevated ALT were summarized in Table 4.
Table 4. Baseline characteristics of patients with normal ALT and elevated ALT.
| ALT<40U/L | ALT≥40U/L | Test value | P value | |
|---|---|---|---|---|
| n | 280 | 1263 | ||
| Male(n,%) | 195(69.60) | 987(78.15) | 9.25 | 0.002 |
| Age(years) | 32.11±10.79 | 31.43±9.48 | 0.981 | 0.327 |
| ALT(U/L) | 26(19,33) | 157(65,188) | -28.18 | <0.001 |
| AST(U/L) | 43(28,49) | 98(48,108) | -16.29 | <0.001 |
| GGT(U/L) | 47(18,56) | 84(34,109) | -9.93 | <0.001 |
| ALB(G/L) | 44.22±5.04 | 44.24±5.50 | -0.76 | 0.939 |
| GLO(G/L) | 27.03±5.02 | 28.02±4.99 | -2.99 | 0.003 |
| TBil(umol/l) | 16.17±10.25 | 17.85±11.66 | -2.23 | 0.026 |
| DBil(umol/l) | 6.20±5.45 | 8.73±7.62 | -3.478 | <0.001 |
| PT(seconds) | 11.92±2.37 | 12.22±1.84 | -2.41 | 0.016 |
| WBC(G/L) | 5.77±1.75 | 5.82±1.63 | -0.487 | 0.627 |
| HGB(G/L) | 139.6±22.63 | 143.9±17.75 | -2.976 | 0.003 |
| PLT(G/L) | 180.4±57.78 | 190.5±56.53 | -2.699 | 0.007 |
| BUN(umol/l) | 4.33±1.27 | 4.20±1.26 | 1.576 | 0.115 |
| Cr(umol/l) | 77.60±19.20 | 79.50±24.66 | -1.206 | 0.228 |
| TC(mmol/l) | 3.83±1.85 | 3.93±1.74 | -0.908 | 0.364 |
| TG(mmol/l) | 0.92±0.63 | 1.02±0.67 | -2.158 | 0.031 |
| LogDNA(copies/ml) | 5.42±1.62 | 5.97±1.33 | -5.244 | <0.001 |
| HBeAg+(n,%) | 200(71.43) | 931(73.71) | 0.61 | 0.434 |
| G1(n,%) | 34(12.14) | 42(3.33) | 44.42 | <0.001 |
| G2(n,%) | 143(51.07) | 599(47.43) | ||
| G3(n,%) | 74(26.43) | 453(35.87) | ||
| G4(n,%) | 29(10.36) | 169(13.38) | ||
| S1(n,%) | 62(22.14) | 205(16.23) | 7.26 | 0.064 |
| S2(n,%) | 101(36.07) | 453(35.87) | ||
| S3(n,%) | 64(22.86) | 359(28.42) | ||
| S4(n,%) | 53(18.930 | 246(19.48) | ||
| AAR | 2.04(1.03,2.20) | 0.77(0.45,0.84) | 8.487 | <0.001 |
| APRI | 0.66(0.37,.0.80) | 1.51(0.62,1.53) | -8.974 | <0.001 |
| FIB-4 | 1.79(0.85,2.14) | 1.56(0.73,1.76) | 1.724 | 0.085 |
The AUROCs of FIB-4 for patients with normal ALT and elevated ALT were 0.698 and 0.642 for significant fibrosis, 0.702 and 0.670 for severe fibrosis,0.772 and 0.704 for cirrhosis respectively. The AUROCs of APRI for patients with normal ALT and elevated ALT were 0.679 and 0.646 for significant fibrosis, 0.713 and 0.645 for severe fibrosis,0.744 and 0.630 for cirrhosis respectively (Table 5).
Table 5. AUROC and ADjAUROC for patients with normal ALT and elevated ALT.
| ALT<40U/L | ALT≥40U/L | |||||
|---|---|---|---|---|---|---|
| AUROC | 95%CI | ADj AUROC | AUROC | 95%CI | ADj AUROC | |
| n | 280 | 1263 | ||||
| S1 vs S2-4 | ||||||
| FIB-4 | 0.698 | 0.628–0.767 | 0.769 | 0.642 | 0.603–0.681 | 0.713 |
| APRI | 0.679 | 0.609–0.749 | 0.75 | 0.646 | 0.606–0.685 | 0.717 |
| AAR | 0.580 | 0.500–0.661 | 0.651 | 0.512 | 0.472–0.552 | 0.583 |
| PLT | 0.621 | 0.547–0.694 | 0.692 | 0.603 | 0.563–0.643 | 0.674 |
| S1-2 vs S3-4 | ||||||
| FIB-4 | 0.702 | 0.640–0.765 | 0.773 | 0.670 | 0.640–0.699 | 0.741 |
| APRI | 0.713 | 0.651–0.775 | 0.784 | 0.645 | 0.614–0.675 | 0.716 |
| AAR | 0.556 | 0.488–0.624 | 0.627 | 0.573 | 0.542–0.605 | 0.644 |
| PLT | 0.656 | 0.590–0.722 | 0.727 | 0.648 | 0.617–0.678 | 0.719 |
| S1-3 vs S4 | ||||||
| FIB-4 | 0.772 | 0.709–0.836 | 0.843 | 0.704 | 0.667–0.740 | 0.775 |
| APRI | 0.744 | 0.678–0.811 | 0.815 | 0.630 | 0.592–0.669 | 0.701 |
| AAR | 0.614 | 0.536–0.692 | 0.685 | 0.588 | 0.547–0.628 | 0.659 |
| PLT | 0.752 | 0.684–0.820 | 0.823 | 0.676 | 0.639–0.713 | 0.747 |
Discussion
The results of the present study showed that the AUROCs of FIB-4 were 0.646,0.670 and 0.715 for prediction of significant fibrosis,severe fibrosis,and cirrhosis,while it were 0.656,0.653 and 0.639 for APRI respceively. After standardisation according to the prevalence of fibrosis stages, ADjAUROCs of FIB-4 were 0.717,0.741 and 0.786 for prediction of significant fibrosis,severe fibrosis,and cirrhosis,while it were 0.727,0.724 and 0.710 for APRI respceively.The comparisons of AUROCs in the original group and validation group confirmed that FIB-4 and APRI had similar diagnostic accuracy in predicting significant and severe fibrosis,while FIB-4 was superior to APRI in predicting cirrhosis. Subgroup analysis demonstrated that the diagnostic accuracy of FIB-4 and APRI in patients with normal ALT were higher than that in patients with elevated ALT.
The major conclusions of our study were consistent with that of three previous meta analysis studies. Xu et al. reported that the areas under the SROC curve of FIB-4 and APRI were 0.75 and 0.77 for significant fibrosis, while it were 0.87 and 0.75 for cirrhosis, respectively[13].Li et al. reported that AUROCs of FIB-4 and APRI were 0.78 and0.79 for significant fibrosis, while it were 0.89 and 0.75 for cirrhosis, respectively[14]. Xiao et al. reported that the summary AUROC values of FIB-4 and APRI were 0.78 and 0.74 for significant fibrosis, 0.82 and 0.73 for severe fibrosis, 0.84 and 0.73 for cirrhosis respectively[15].These results demonstrated that the diagnostic accuracy of FIB-4 was similar to that of APRI for significant fibrosis while FIB-4 was superior to APRI in predicting cirrhosis.
The original AUROCs of FIB-4 and APRI in our study seemed to be lower than that of some previous studies, whereas the ADjAUROCs of FIB-4 and APRI in our study were similar to that of previous studies. Omer Basar et al. found that AUROCs of FIB-4 and APRI were 0.741 and 0.669 for significant fibrosis, 0.738 and 0.681 for severe fibrosis, 0.768 and 0.741 for cirrhosis[16]. V. MALLET et al. showed that AUROCs were 0.810 and 0.730 for FIB-4 and APRI in predicting fibrosis [17]. Fatma Ucar et al. reported that AUROCs were 0.687 and 0.662 for FIB-4 and APRI to predict fibrosis[18]. H. Wang et al. found that AUROCs of FIB-4 and APRI were 0.770 and 0.770 in predicting significant fibrosis, 0.810 and 0.770 for severe fibrosis [19]. Beom Kyung Kim et al. reported that AUROCs of FIB-4 and APRI were 0.910 and 0.702 for severe fibrosis, 0.926 and 0.731 in predicting cirrhosis [20]. Jing Ma et al. reported that AUROCs of FIB-4 and APRI were 0.789 and 0.731 for predicting severe fibrosis, 0.804 and 0.740 to predict cirrhosis[21].
On the other hand, similar results to our study were observed in some previous studies,showing lower diagnostic accuracy of FIB-4 and APRI for fibrosis[22–25].In the original study, Sterling et al.reported that the AUROC of FIB-4 in the training and validation cohorts were 0.711 and 0.688 for significant fibrosis, 0.737 and 0.765 for advanced fibrosis[22].Sebastiani et al. found that AUROCs of APRI and FIB-4 were 0.68(0.62–0.74) and 0.66(0.61–0.71) for significant fibrosis in 2411 patients with chronic liver disease;further analysis showed that AUROC s of APRI was 0.64(0.58–0.70) for significant fibrosis and 0.61(0.55–0.66) for cirrhosis in HBV patient[8].Wai et al.reported that the AUROC of APRI were 0.63(0.55–0.71) for significant fibrosis and 0.64(0.54–0.71) for cirrhosis[23].Bonnard P et al.reported that the AUROC of APRI and FIB-4 were 0.61(0.46–0.76) and 0.71(0.57–0.84) for significant fibrosis,0.50(0.32–0.68) and 0.74(0.60–0.87) for cirrhosis [24].
The disagreement between our study and previous studies may be correlated to several potential reasons. First, the heterogeneity may affect the results in different studies. Xu et al. found that the heterogeneity of APRI for detecting significant fibrosis was affected by median age, and for cirrhosis was affected by etiology[13]. Li et al.found that the potential influential factors of heterogeneity were mean age of subjects, prevalence of fibrosis stages, disease spectrum, a consecutive or random sample enrollment, interval between noninvasive model and liver biopsy, the liver blinded biopsy interpretation and a predefined cutoff value[14].Second, it had been found that the prevalence of liver fibrosis stages may be a major factor of variability and a cause of unsatisfactory results in assessing the diagnostic accuracy of noninvasive model.Therefore, the original AUROC should be adjusted according to the prevalence of fibrosis stages for further comparisons [11]. After calibration for prevalence of fibrosis stages,the ADjAUROCs of FIB-4 and APRI in our study were similar to that of previous studies. Third,the mean age of patients in our study was 31.55 years,which was younger than that of most previous studies and may impacted the results of the current study.Fourth, scoring systems of liver pathological diagnosis were different in these studies,affecting directly the results of the studies. The effect of different scoring system must be take into account while preforming comparisons of diagnostic accuracy between different studies. Fifth, sample size was important to construct a convincing conclusion for assessment of diagnostic accuracy. Some previous studies performed analysis base on a relatively small sample size,which might reduce the convince of the conclusions
The results of subgroup analysis showed that diagnostic accuracy of FIB-4 and APRI in patients with normal ALT were higher than that in patients with elevated ALT.Wang et al. reported that the AUROCs for patients with normal ALT was 0.81 for FIB-4 and 0.80 for APRI, compared with 0.71 for FIB-4 and 0.72 for APRI in patients with mildly elevated ALT level[19]. Poynard et al. reported that performance of non-invasive biomarkers was in line with that in patients with elevated ALT[25].On the other hand,some studies reported that performance of non-invasive biomarkers may be somewhat reduced in patients with normal ALT[26–29]. Consequently,further research is needed to determine the clinical utility of FIB-4 and APRI in patients with normal ALT.
There were several advantages in the present study. First,this study had a large sample size,which could reduce the sampling error and conduct a more convincing conclusion. Second, to enhance the credibility of results, we performed an internal validation to confirm the results of the present study by means of bootstrap resampling analysis with replacement.This method was proposed for internal validation of surgical regression models[30].The main advantage of this method is that the original samples can be used to build a more robust model,which can be used to assess the diagnostic accuracy[31].Third,the previous studies assessed the diagnostic accuracy of the FIB-4,APRI,and AAR for significant fibrosis and cirrhosis,but few studies evaluated and compared the diagnostic accuracy for severe fibrosis. For a more comprehensive understanding of the diagnostic accuracy for fibrosis,we attempted to explore the diagnostic accuracy and clinical utility for significant fibrosis,severe fibrosis,and cirrhosis.
There were two limitations in our study.First,all patients in this study were recruited from department of infectious diseases of The Shunde First People’s Hospital,which may reduce the representative of the study population.We recommend that future clinical studies should base on a large scale multi-center population to further compare the diagnostic accuracy and clinical utility of these models for hepatic steatosis in patients with HBV infection.Second,as a retrospective study, some important indicators such asα2-macroglobulin and ferritin could not obtain in the study.
In conclusion,the current study showed that FIB-4 and APRI have similar diagnostic accuracy in predicting significant fibrosis and severe fibrosis,while FIB-4 is superior to APRI for prediction of cirrhosis. The clinical utility of FIB-4 need further external validation in larger population before it was used in predicting fibrosis in patients with HBV infection.
Acknowledgments
The authors would like to acknowledge the following individuals who contributed to the design and execution of this study:Jing Li, Tingshan He, Yanyue Yu, Qingmei Liu,Yanling Ouyang,Yiyan Huang, Xiaoqiao Chen, Guotao Lv,Lang Ming, Chong Zheng,Yuewu Chen,Qiuli Xie,Jiexiong He,Yong Huang, Langsi Luo,Xinteng Chen,Minyi He.
Data Availability
All files are available from the Figshare database (accession number(s) https://figshare.com/s/20544de9547aa82bb367).
Funding Statement
This study was funded by Guangdong Provincial Health Department (No: A2013695). The funding account was RMB 10000. The funding was given by Finance Department of Guangdong Province. Peng Wang received the funding. The funders had no role in study design, data collection and analysis, decision to publish, preparation, or writing of the manuscript. The URL of Guangdong Provincial Health Department is http://www.gdwst.gov.cn/.
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med 1997; 337: 1733–1745. [DOI] [PubMed] [Google Scholar]
- 2.Shackel NA, McCaughan GW. Liver biopsy: is it still relevant. Intern Med J 2006;36:689–691. [DOI] [PubMed] [Google Scholar]
- 3.Emanuele E. Is biopsy always necessary? Toward a clinico-laboratory approach for diagnosing nonalcoholic steatohepatitis in obesity. Hepatology 2008;48:2086–2087. 10.1002/hep.22622 [DOI] [PubMed] [Google Scholar]
- 4.Valetpichard A,Mallet V,Nalpas B, Verkarre V,Nalpas A,Dhalluinvenier V,et al. FIB-4:an inexpensive and accurate marker of fibrosis in HCV infection.comparison with liver biopsy and Fibrotest.Hepatology 2007;46(1):32–36. [DOI] [PubMed] [Google Scholar]
- 5.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD,Marrero JA,Conjeevaram HS,et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518–526. [DOI] [PubMed] [Google Scholar]
- 6.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988;95(3):734–739. [DOI] [PubMed] [Google Scholar]
- 7.Lieber CS,Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol 2006; 101:1500–1508. [DOI] [PubMed] [Google Scholar]
- 8.Sebstiani G, Castera L, Halfon P,Pol S,Mangia A,Dimarco V, et al. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of noninvasive fibrosis biomarkers: an international study of 2411 cases.Aliment Pharmacol Ther 2011; 34:1202–1216. 10.1111/j.1365-2036.2011.04861.x [DOI] [PubMed] [Google Scholar]
- 9.Teshale E,Lu M,Rupp LB,Holmberg SD, Moorman AC,Spradling P,et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat.2014VN. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the study of the liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242. 10.1016/j.jhep.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 11.Poynard T, Halfon P, Castera L, Munteanu M,Imbertbismut F,Ratziu V,et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem 2007; 53: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarkepearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 13.Xu XY,Kong H,Song RX,Zhai YH,Wu XF,Ai WS, et al. The effectiveness of noninvasive biomarkers to predict hepatitis b-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One 2014;9(6):e100182 10.1371/journal.pone.0100182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y,Chen Y,Zhao Y. The diagnostic value of the fib-4 index for staging hepatitis b-related fibrosis: a meta-analysis. PLoS One 2014;9(8):e105728 10.1371/journal.pone.0105728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao G,ang J,Yan L.Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis b virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292–302. 10.1002/hep.27382 [DOI] [PubMed] [Google Scholar]
- 16.Basar O,Yimaz B,Ekiz F,Ginis Z,Altinbas A,Aktas B,et al. Non-invasive tests in prediction of liver fibrosis in chronic hepatitis B and comparison with post-antiviral treatment results. Clin Res Hepatol Gastroenterol 2013;37:152–158. 10.1016/j.clinre.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 17.Mallet V,Dhalluinvenier V,Roussin C,Bourliere M,Pettinelli ME,Giry C,et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2009;29(4): 409–415. 10.1111/j.1365-2036.2008.03895.x [DOI] [PubMed] [Google Scholar]
- 18.Ucar F,Sezer S,Ginis Z,Ozturk G,Albayrak A,Basar O,et al. APRI, the FIB-4 score, and Forn’s index have noninvasive diagnostic value for liver fibrosis in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol 2013;25(9):1076–1081. 10.1097/MEG.0b013e32835fd699 [DOI] [PubMed] [Google Scholar]
- 19.Wang H,Xue L,Yan R,Zhou Y,Wang MS,Cheng MJ, et al. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT. J Viral Hepat 2013; 20:e3–e10. 10.1111/jvh.12010 [DOI] [PubMed] [Google Scholar]
- 20.Kim BK,Kimdo Y,Park JY,Ahn SH,Chon CY,Kim JK,et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30(4):546–553. 10.1111/j.1478-3231.2009.02192.x [DOI] [PubMed] [Google Scholar]
- 21.Ma J,Jiang Y,Gong G. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol 2013;25(4): 428–434. 10.1097/MEG.0b013e32835cb5dd [DOI] [PubMed] [Google Scholar]
- 22.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J,et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepotology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 23.Wai CT,Cheng CL,Wee A,Dan YY,Chan E,Chua W,et al. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int 2006;26:666–672. [DOI] [PubMed] [Google Scholar]
- 24.Bonnard P, Sombie R, Lescure FX, Bougouma A, Guiardschmid JB, Poynard T,et al. Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)-infected patients in Burkina Faso. Am J Trop Med Hyg 2010; 82: 454–458. 10.4269/ajtmh.2010.09-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poynard T,Munteanu M,Ngo Y,Torres M,Benhamou Y,Thabut D,et al. Diagnostic value of FibroTest with normal serum aminotransferases. Hepatology 2006; 43: 374–375. [DOI] [PubMed] [Google Scholar]
- 26.Alberti A, Noventa F, Benvegnu L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection.Ann Intern Med 2002; 137: 961–964. [DOI] [PubMed] [Google Scholar]
- 27.Liu CH,Lin JW,Tsai FC,Yang PM,Lai MY,Chen JH,et al. Noninvasive tests for the prediction of significant hepatic fibrosis in hepatitis C virus carriers with persistently normal alanine aminotransferases. Liver Int 2006; 26:1087–1094. [DOI] [PubMed] [Google Scholar]
- 28.Fabris C,Smirne C,Toniutto P,Colletta C,Rapetti R,Minisini R, et al. Assessment of liver fibrosis progression in patients with chronic hepatitis C and normal alanine aminotransferase values: the role of AST to the platelet ratio index. Clin Biochem 2006; 39:339–343. [DOI] [PubMed] [Google Scholar]
- 29.Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases.J Viral Hepat 2008; 15: 212–218. [DOI] [PubMed] [Google Scholar]
- 30.Blackstone EH.Breaking down barriers: Helpful breakthrough statistical methods you need to understand better. J Thorac Cardiovasc Surg 2001; 122: 430–439. [DOI] [PubMed] [Google Scholar]
- 31.Grunkemeier GL, Wu Y. Bootstrap resampling methods:something for nothing.Ann Thorac Surg 2004; 77: 1142–1144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All files are available from the Figshare database (accession number(s) https://figshare.com/s/20544de9547aa82bb367).