Abstract
Background and Aims
Genetic defects in ATP8B1 or ABCB11 account for the majority of cholestasis with low GGT. But the ranges for GGT in patients with ATP8B1 or ABCB11 deficiency are unclear. This study tried to unravel the features of GGT in these patients that improve diagnostic efficiency.
Methods
This study enrolled 207 patients with chronic cholestasis who were ordered to test for ATP8B1 and/or ABCB11 from January 2012 to December 2015. Additional 17 patients with ATPB81 or ABCB11 deficiency diagnosed between January 2004 and December 2011 were also enrolled in this study. 600 population-matched children served as controls. Clinical data were obtained by retrospectively reviewing medical records.
Results
A total of 26 patients were diagnosed with ATP8B1 deficiency and 30 patients were diagnosed with ABCB11 deficiency. GGT levels were similar between the two disorders at any observed month of age, but varied with age. The peak GGT value was <70U/L in the 2nd~6th month of life, <60U/L in the 7th~12th month and <50U/L beyond one year. GGT levels in patients with a genetic diagnosis were different from that in patients without a genetic diagnosis and controls. Larger ranges for GGT were found in patients without a genetic diagnosis. Some controls had GGT≥70U/L in the 2nd~6th month. Of the 207 patients, 39 (18.8%) obtained a genetic diagnosis. 111 patients met the ranges described above, including all the 39 patients with ATP8B1 or ABCB11 deficiency. The sensitivity was 100.0%. The rate of a positive molecular diagnosis increased to 35.1% (39/111 vs. 39/207, X2 = 10.363, P = 0.001). The remaining 96 patients exceeded the ranges described above and failed to receive a genetic diagnosis. These patients accounted for 43.8% of sequencing cost.
Conclusions
GGT levels in patients with ATP8B1 or ABCB11 deficiency varied with age. The peak GGT value was <70U/L in the 2nd~6th month of life, <60U/L in the 7th~12th month and <50U/L beyond one year.
Introduction
Cholestasis is one of the most common manifestations of liver disease in infancy and affects about 1 in 2500–5000 live births. A range of hepatobiliary disorders, that involve the process of bile acid biosynthesis, bile secretion and excretion, can result in cholestasis [1]. The etiology includes infectious, obstructive, metabolic, toxic as well as other rare causes [2]. Due to the advancement in molecular diagnoses, multiple specific genetic causes were identified, including citrin deficiency, Alagille syndrome, progressive familial intrahepatic cholestasis, inborn errors of bile acid synthesis, cystic fibrosis and so on [3].
In clinical practice, cholestasis is usually divided into two categories depending on serum GGT levels: those with elevated GGT and those with low/normal GGT [4]. The latter includes at least two distinct subtypes: ATP8B1 deficiency and ABCB11 deficiency. ATP8B1 deficiency is caused by mutations in ATP8B1, and can present as either progressive familial intrahepatic cholestasistype 1 (PFIC1) or benign recurrent intrahepatic cholestasis type 1 (BRIC1) [5,6]. ABCB11 deficiency is caused by mutations in ABCB11. The ABCB11 gene encodes bile salt export pump (BSEP) that is responsible for bile acid (BA) secretion [7]. Mutations in ABCB11 can cause PFIC2 and BRIC2 [7,8]. Occasionally, BRIC can progress to PFIC [6,9]. Therefore, ATP8B1 or ABCB11 deficiency represents a spectrum of diseases, with PFIC as severe forms and BRIC as mild ones. Low serum GGT activity is the characteristic feature of these diseases.
GGT binds to the canalicular membrane by a glycosyl phosphatidyl inositol (GPI) anchor [10]. The detergent bile acid liberates GGT. In healthy infants, GGT activity varies with age [11–12]. The upper limit of GGT value could be 7 times of adult range in the first three months of life, then decreases gradually and reaches adult range at 6 months old [12–13]. It has been unclear whether GGT value in patients with ATP8B1 or ABCB11 deficiency varies as it does in healthy infants. The ranges for GGT in these patients were referred as normal, or low, or low for the degree of cholestasis [14–17]. The ambiguity might hinder the diagnosis. The aim of this study was to unravel the features of GGT in these patients that improve the efficiency of diagnosis.
Methods
Subjects
We retrospectively analyzed all patients who were ordered to screen for mutations in ATP8B1 and/or ABCB11 for chronic cholestasis between January 2012 and December 2015. 207 patients, including 112 boys and 95 girls, were enrolled in this study. Among them, 110 tested for both ATP8B1 and ABCB11, 38 tested for ATP8B1 and 59 tested for ABCB11 only. The patients were referred to the Center for Pediatric Liver Disease of the Children’s Hospital of Fudan University and/or the Pediatrics Department of Jinshan Hospital of Fudan University. Following an extensive workup as described previously [13,18–20], other causes of chronic cholestasis were excluded, including infections, drug-induced, metabolic and inborn errors in bile acid synthesis. Cytomegalovirus (CMV) infection was defined as a positive urinary CMV-DNA or pp65 antigenemia or serum immunoglobulin M (IgM) [21–22], and not excluded because of its high prevalence in Chinese infants [23]. To expand the patient number, 17 patients with ATPB81 or ABCB11 deficiency diagnosed between January 2004 and December 2011 were also enrolled in this study. 36 patients with positive diagnoses were partly reported previously [18–20]. The patients were treated with ursodesoxycholic acid (UDCA) and fat-soluble vitamins. Cholestyramine was given if patients had intractable pruritus.
600 population-matched children severed as controls for analysis of GGT level variation in Chinese population. No liver disease history was found in these children. Liver function test was performed during airway infections course and revealed normal serum total bilirubin (TB) and normal alanine aminotransferase (ALT).
This study was approved by the ethics committee of the Children’s Hospital of Fudan University, and was dispensed from informed consent. Patients’ information was de-identified prior to analysis.
Genetic analysis
Genetic analysis was performed in the Translational Center of Children’s Hospital of Fudan University. Genomic DNA was extracted from EDTA treated peripheral blood (Tiangen Biotech, Shanghai, China). All coding exons and splice junctions of ATP8B1 (RefSeq NM_005603.3) and ABCB11 (RefSeq NM_003742.2) were amplified by polymerase chain reaction (PCR) and directly sequenced as described previously [18–19]. Frameshift, nonsense, canonical splice site variations and previously reported mutations were considered deleterious. Missense variations predicted to be damaging were considered as potentially deleterious mutations. Deleterious and potentially deleterious mutations were confirmed by directly sequencing affected exons in both parents.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as reported previously [20]. The pathologists were blinded to genotyping results at assessment. Absent canalicular BSEP expression was defined as the typical IHC pattern for ABCB11 deficiency. Specific canalicular GGT expression, that was absent in centrilobular but preserved in periportal areas, was defined as the typical IHC pattern for ATP8B1 deficiency.
Molecular diagnosis
Subjects were diagnosed with ATP8B1 or ABCB11 deficiency if deleterious or potentially deleterious mutations were detected in both alleles of ATP8B1 or ABCB11, or in whom though only one mutant allele was detected in ATP8B1 or ABCB11 but decreased/absent GGT or BSEP expression was demonstrated respectively by IHC; otherwise the subjects were defined as patients lacking a genetic diagnosis.
Clinical data and statistical analysis
The clinical data were obtained by reviewing medical records. GGT activities during disease course were used for statistical analysis, but those after liver transplantation, partial billiary diversion or liver failure were discarded. If GGT activities were tested several times within one month, the mean was used for comparison of GGT levels.
Statistic analysis was performed with the SPSS version 17.0 software (University of Chicago, Chicago, IL, United States). Data were expressed as median [P25, P75] for non-normality. Comparison of GGT levels between two groups was done by the nonparametric Mann-Whitney test. Comparison of GGT levels among three or more groups was performed by the nonparametric Kruskal-Wallis H test. The difference between two ratios was tested by Chi-square test. P<0.05 was considered significant.
Results
Molecular results
Mutations in ATP8B1
26 patients fulfilled the definition of ATP8B1 deficiency, including 1 heterozygote with weak canalicular GGT expression, 17 compound heterozygotes and 8 homozygotes (Table 1). 30 different mutations were identified, including eight novel mutations. 5 missense mutations were predicted to be damaging by Polyphen-2 and MutationTaster (S1 Table). c.1030-1G>A known to affect splicing. c.811A>T (p.R271X) and c.2013_2014delAA leaded to truncated protein.
Table 1. Clinic and genetic characteristics of 17 patients with ATP8B1 deficiency.
| Patient no. | Sex/Age at onset | Symptoms§ | Nucleotide change | Amino acid change | Origin | Ref |
|---|---|---|---|---|---|---|
| 1 | Male/1mo | J, P, H, S, FT | c.2081T>A/c.2081T>A | p.I694N/ p.I694N | F/M | Li et al [20] |
| 2 | Female/1mo | J, D, H | c.1367C>A/c.3292delG | p.T456K/p.V1098X | F/M | Li et al [20] |
| 3 | Female/4mo | J, P, H, S | c.886C>T/c.1675_1689delGTAAACGCTGCCAGG | p.R296C/p.V559_R563del | M/F | Li et al [20] |
| 4 | Male/14y | J, H | c.1982T>C/c.1982T>C | p.I661T/p.I661T | F/M | Present study |
| 5 | Female/1mo | J, P, H, S | c.625C>A+c.627+5G>T/c.2081T>A | p.P209T/p.I694N | M/F | Li et al [20] |
| 6 | Female/2mo | J, D, H | c.922G>A/c.2081T>A | p.G308S/p.I694N | F/M | Li et al [20] |
| 7 | Male/8y | J, D, P | c.602G>A/c.1587_1589delCTT | p.R201H/p.F527- | ND | Present study |
| 8 | Female/1mo | J, P, D, H | c.1367C>T/c.1587_1589delCTT | p.T456M/p.F527- | ND | Present study |
| 9 | Male/1mo | J, H | c.2821C>T/c.2821C>T | p.R941X/p.R941X | F/M | Li et al [20] |
| 10 | Female/1mo | J, D, H, S, FT | c.1336G>A/c.1587_1589delCTT | p.G446R/p.F527- | M/F | Li et al [20] |
| 11 | Female/4mo | J, P, H, S, FT | c.886C>T/c.2081T>A | p.R296C/p.I694N | ND | Li et al [20] |
| 12 | Male/1mo | J, H | c.2081T>A/c.2788C>T | p.I694N/p.R930X | ND | Li et al [20] |
| 13 | Male/15mo | J, P, H | c.920A>T/c.3401-2A>G | p.H307L/- | F/M | Li et al [20] |
| 14 | Male/1mo | J, H | c.1030-1G>A/c.2013_2014delAA | -/p.K672Vfs*17 | ND | Present study |
| 15 | Female/3mo | J, P, D, H,S | c.1799G>A/c.1799G>A | p.R600Q/p.R600Q | F/M | Li et al [20] |
| 16 | Male/3mo | J, H | c.1799G>A/c.1799G>A | p.R600Q/p.R600Q | F/M | Present study |
| 17 | Female/1mo | J, H | c.614dupA/c.2532delT | p.N205KfsX2/p.K845RfsX36 | ND | Present study |
| 18 | Male/2mo | J, H | c.625C>A+c.627+5G>T/ c.625C>A+c.627+5G>T | p.P209T/p.P209T | ND | Liu et al [19] |
| 19 | Male/1mo | J, P, H | c.1429+1G>A/c.1429+1G>A | -/- | ND | Liu et al [19] |
| 20 | Male/1mo | J, H | c.625C>A+c.627+5G>T/c.2081T>A | p.P209T/p.I694N | ND | Liu et al [19] |
| 21 | Female/1mo | J, D, H | c.625C>A+c.627+5G>T/ c.625C>A+c.627+5G>T | p.P209T/p.P209T | ND | Liu et al [19] |
| 22 | Male/1mo | J, H, S | c.614dupA/c.2532delT | p.N205KfsX2/p.K845RfsX36 | ND | Liu et al [19] |
| 23 | Male/1mo | J, P, H, S | c.625C>A+c.627+5G>T/c.2854C>T | p.P209T/p.R952X | ND | Liu et al [19] |
| 24 | Male/2mo | J, H | c.1264G>C/c.2734G>A | p.D422H/p.G912R | F/M | Li et al [20] |
| 25 | Female/14y | J, P, D,H | c.811A>T/c.1882C>T | p.R271X/p.R628W | ND | Present study |
| 26 ‡ | Female/1mo | J, P, D, H, FT | c.1661A>C+c.1741G>A/- | p.D554A+p.E581K/- | M/- | Present study |
J, jaundice; P, pruritus; D, diarrhea; H, hepatomegaly; S, splenomegaly; FT, failure to thrive; ND = not done; F, father; M, mother.
Novel mutations are shown in bold.
Patient 15 and 16, 17 and 22 are siblings.
§ Symptoms: the major symptoms when the patients were first referred to our hospital.
‡ Patient harboring the heterozygous ATP8B1 mutation and having decreased GGT expression.
Mutations in ABCB11
30 patients were diagnosed with ABCB11 deficiency, including 4 homozygotes, 24 compound heterozygotes and 2 heterozygotes with absent BSEP expression (Table 2). Sequence analysis revealed 54 distinct mutations including 13 novel mutations. The 13 mutations included one nonsense mutation, two 1-base deletions leading to truncated protein, one splice site mutation known to affect splicing, and 9 missense mutations predicted to be damaging (S1 Table).
Table 2. Clinic and genetic characteristics of 18 patients with ABCB11 deficiency.
| Patient no. | Sex/Age at onset | Symptoms§ | Nucleotide change | Amino acid change | Origin | Ref |
|---|---|---|---|---|---|---|
| 1 | Female/1mo | J, H | c.1460G>A/c.3169C>T | p.R487H/p.R1057X | M/F | Present study |
| 2 | Female/1mo | J, P, H, S | c.1197+1G>T/c.1197+1G>T | -/- | F/M | Li et al [20] |
| 3 | Female/1mo | J, H, S, FT | c.2935A>G/c.3746T>G | p.N979D/p.L1249X | ND | Li et al [20] |
| 4 | Female/3mo | J, P, H, S | c.1415A>G/c.3392A>T | p.Y472C/p.D1131V | F/M | Present study |
| 5 | Male/1mo | J, H | c.634G>A+c.849A>C/c.1638G>T | p.A212T+p.E283D/p.Q546H | M/de novo | Li et al [20] |
| 6 | Female/1mo | J, H | c.1493T>C/c.1493T>C | p.I498T/p.I498T | F/M | Li et al [20] |
| 7 | Male/1mo | J, D, H, S | c.212T>A/c.677C>T | p.L71H/p.S226L | ND | Li et al [20] |
| 8 | Male/1mo | J, H | c.2782C>T/c.3593A>G | p.R928X/p.H1198R | M/F | Li et al [20] |
| 9 | Male/1mo | J, H | c.542G>T/c.1370_1372dupGTG | p.R181I/p.-458G | M/F | Li et al [20] |
| 10 | Female/1mo | J, H | c.3457C>T/c.3623A>G | p.R1153C/p.Y1208C | F/M | Li et al [20] |
| 11 | Female/5mo | J, P, H, S | c.2474A>G/c.2623C>T | p.E825G/p.Q875X | ND | Present study |
| 12 | Male/3y | J, P, H, S | c.1685G>A/c.1847G>A | p.G562S/p.R616H | F/M | Present study |
| 13 | Female/1mo | J, H, S | c.113delA/c.2702G>T | p.K38RfsX24/p.S901I | ND | Present study |
| 14 | Female/3y | J, P, H | c.229C>G/c.1880T>C | p.P77A/p.I627T | ND | Present study |
| 15 | Female/1mo | J, H, S | c.2484_2488delA/c.2542G>A | p.R830GfsX28 /p.D848N | ND | Present study |
| 16 | Female/2mo | J, P, H, S | c.197G>A/c.555G>A | p.S66N/p.M185I | ND | Present study |
| 17 | Female/1mo | J, P, H | c.909-2A>G/c.1550G>A | -/p.R517H | F/M | Present study |
| 18 | Female/1mo | J, P, H | c.1409G>A/c.1762G>C | p.R470Q/p.A588P | F/M | Present study |
| 19 | Female/1mo | J, H | c.2603T>A/c.3213+1G>T | p.V868D/- | M/F | Present study |
| 20 | Male/2mo | J, D, H, S | c.3457C>T/c.3623A>G | p.R1153C/p.Y1208C | F/M | Present study |
| 21 ‡ | Female/1mo | J, H, S | c.145C>T/- | p.Q49X/- | ND | Li et al [20] |
| 22 ‡ | Male/2mo | J, P, H, FT | c.612-2_4CTA>TT/- | -/- | M/- | Li et al [20] |
| 23 | Male/1mo | J, H, S | c.409G>A/c.2216delC | p.E137K/p.P740QfsX6 | de novo/F | Li et al [20] |
| 24 | Male/1mo | J, H, S | c.1760C>G/c.3677G>C | p.S587X/p.R1226P | M/F | Li et al [20] |
| 25 | Male/2mo | J, D, H, FT | c.1583T>C/c.1583T>C | p.I528T/p.I528T | ND | Li et al [20] |
| 26 | Male/1mo | J, P, H | c.499G>A/c.499G>A | p.A167T/p.A167T | F/M | Liu et al [18] |
| 27 | Male/1mo | J, P, H, S | c.562G>T/c.2814+3A>T | p.G188W/- | ND | Liu et al [18] |
| 28 | Female/3mo | J, P, H | c.1496G>A/c.2606A>C | p.G499E/p.Q869P | F/M | Liu et al [18] |
| 29 | Male/1mo | J, P, H, S | c.319T>C/c.3172C>T | p.C107R/p.Q1058X | M/F | Liu et al [18] |
| 30 | Male/1mo | J, H, S | c.1243C>T/c.3875G>T | p.R415X/p.G1292V | F/M | Liu et al [18] |
J, jaundice; P, pruritus; D, diarrhea; H, hepatomegaly; S, splenomegaly; FT, failure to thrive; ND = not done; F, father; M, mother.
Novel mutations are shown in bold.
Patient 10 and 20 are siblings.
§ Symptoms: the major symptoms when the patients were first referred to our hospital.
‡ Patient harboring the heterozygous ABCB11 mutation and having absent BSEP expression.
Laboratory evaluations
Only serum ALT and GGT activities in patients without a genetic diagnosis were different from that in the ATP8B1 deficiency and the ABCB11 deficiency simultaneously when liver function test results at presentation were compared among the three groups (Table 3). However, serum ALT activities were similar between patients with and without a genetic diagnosis (93.0[50.0, 187.5] vs. 100.0[41.7, 223.7], Z = -0.228, P = 0.819). GGT levels in patients without a genetic diagnosis were higher than that in patients with a genetic diagnosis (44.0[29.0, 65.5] vs. 29.0[19.0, 37.5], Z = -4.374, P = 0.000). In addition, no significant difference was found when the rates of CMV infection were compared between patients with and without a genetic diagnosis (17.9% vs. 23.8%, X2 = 0.858, P = 0.354).
Table 3. Biochemical features at presentation (median [P25, P75]).
| n | TB | ALT | ALP | GGT | BA | Albumin | Cholesterol | AFP | |
|---|---|---|---|---|---|---|---|---|---|
| P without | 168 | 150.2[94.6, 252.2] | 93.0[50.0, 187.5] | 542.5[372.7, 728.2] | 44.0[29.0, 65.5] | 171.8[101.1, 277.4] | 40.2[36.6, 43.0] | 4.5[3.3, 5.4] | 2408.0[14.5, 91900.0] |
| P ATP8B1 | 26 | 195.8[161.0, 259.8] | 46.0 [33.0, 87.0] * | 588.5[396.0, 728.0] | 27.0[18.0, 36.0] * | 216.5[167.7, 287.9] | 40.0[38.4, 41.5] | 3.4[2.9, 4.2] * | 33.7[4.5, 161.1] * |
| P ABCB11 | 30 | 124.7[101.3, 162.7] | 186.0[109.5, 273.5] *^ | 386.5[250.5, 530.2] *^ | 32.0 [23.5, 38.5] * | 294.1[205.2, 368.3] *^ | 43.9[40.0, 45.8] *^ | 4.4[3.0, 5.4] ^ | 1096.0[4.4, 23880.0] ^ |
| X2 | 5.427 | 21.479 | 8.240 | 20.114 | 14.611 | 13.955 | 8.864 | 10.642 | |
| P | 0.066 | 0.000 | 0.016 | 0.000 | 0.001 | 0.001 | 0.011 | 0.005 |
PATP8B1, patients with ATP8B1 deficiency; PABCB11, patients with ABCB11 deficiency; Pwithout, patients without a genetic diagnosis; ALP, alkaline phosphatase; AFP, alfafetoprotein.
* compared with P without, P<0.05;
^ compared with P ATP8B1, P<0.05.
GGT levels in patients with or without a genetic diagnosis and controls
A comparison of GGT levels between the ATP8B1 deficiency and the ABCB11 deficiency revealed that GGT levels were similar between the two disorders at any observed month of age (Table 4). However, GGT levels in patients with a genetic diagnosis were different from that in patients without a genetic diagnosis and controls. Compared to controls, patients with a genetic diagnosis, including the ATP8B1 deficiency and the ABCB11 deficiency, had lower GGT levels at the second month of life but higher GGT levels beyond 4 months. In addition, GGT levels in patients with a genetic diagnosis were lower than that in patients without a genetic diagnosis at first several months of life.
Table 4. GGT levels in patients with or without a genetic diagnosis and controls (median [P25, P75], U/L).
| Age | Controls | P without | P ATP8B1 | P ABCB11 | X2 | P |
|---|---|---|---|---|---|---|
| 2nd | 86.0[48.7, 123.0] | 56.2[44.9, 69.1] * | 34.4[21.1, 50.8] *^ | 42.0[36.0, 50.0] *^ | 24.302 | 0.000 |
| 3rd | 43.5.0[29.7, 65.0] | 55.3[37.9, 67.8] | 36.0[29.3, 49.1] ^ | 38.0[32.5, 51.5] ^ | 9.160 | 0.027 |
| 4th | 32.0[24.0, 43.0] | 50.2[32.0, 78.8] * | 32.0[22.0, 42.5] ^ | 33.3[22.0, 40.4] ^ | 17.501 | 0.001 |
| 5th | 16.5[12.0, 25.0] | 46.5[30.4, 77.9] * | 28.7[20.0, 30.0] *^ | 36.0[24.4, 41.8] *^ | 45.240 | 0.000 |
| 6th | 20.0[14.0, 24.2] | 38.4[22.6, 64.0] * | 22.0[14.0, 40.0] ^ | 30.2[19.0, 36.8] * | 25.812 | 0.000 |
| 7th | 13.5[10.0, 18.3] | 32.5[21.0, 70.0] * | 25.4[15.0, 37.3] * | 32.0[15.5, 37.0] * | 31.922 | 0.000 |
| 8th | 14.0[10.8, 18.0] | 22.0[15.5, 28.9] * | 20.0[13.5, 24.0] * | 22.0[16.0, 30.0] * | 18.882 | 0.000 |
| 9th | 12.0[10.0, 17.0] | 19.0[14.0, 29.0] * | 21.0[12.0, 23.0] * | 31.9[18.0, 37.0] * | 22.544 | 0.000 |
| 10th | 11.0[9.0, 14.0] | 18.0[11.8, 38.9] * | 19.3[13.0, 24.0] * | 35.0[18.0, 41.3] * | 17.133 | 0.001 |
| 11th | 10.5[8.8, 15.3] | 25.0[11.1, 43.7] * | 17.0[10.0, 28.3] * | 31.0[24.0, 35.0] * | 23.085 | 0.000 |
| 12th | 10.0[8.0, 13.3] | 15.0[12.5, 27.0] * | 20.0[16.8, 21.0] * | 29.5[20.0, 32.0] *^ | 33.072 | 0.000 |
| 13th ~ | 10.0[8.0, 14.0] | 25.0[14.0, 49.0] * | 14.8[13.0, 22.0] *^ | 21.0[15.0, 28.0] *^ | 77.903 | 0.000 |
PATP8B1, patients with ATP8B1 deficiency; PABCB11, patients with ABCB11 deficiency;. Pwithout, patients without a genetic diagnosis.
* compared with controls, P<0.05;
^ compared with P without, P<0.05.
GGT levels varied with age in patients with a genetic diagnosis
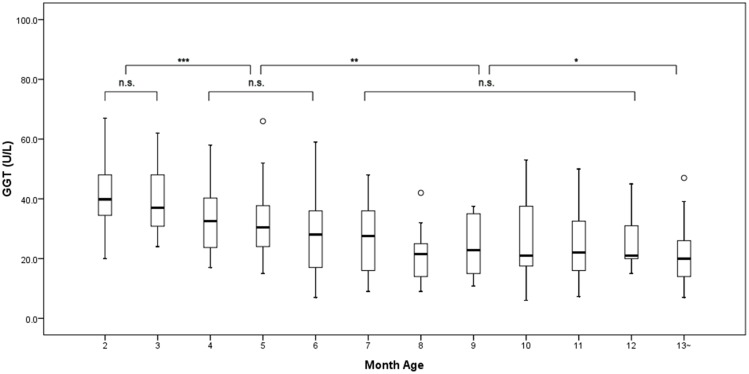
Significant difference was observed when GGT levels were compared among different ages by month in patients with a genetic diagnosis (X2 = 78.430, P = 0.000; Fig 1). GGT levels in the 2nd month of life were similar to that in the 3rd month. But GGT levels in the 2nd~3rd month were higher than that in the 4th~6th month, while they were similar among the 4th~6th month. When GGT levels were compared among the 7th~12th month, the difference was not significant. GGT levels in the 7th~12th month were higher than that beyond 1 year, but were lower than that in the 2nd~3rd month and the 4th~6th month.
Fig 1. The variation of GGT levels in patients with ATP8B1 or ABCB11 deficiency.
*P<0.05; **P<0.01; ***P<0.001; n.s., not significant.
The ranges for GGT in patients with a genetic diagnosis
Similar ranges for GGT were found in patients with a genetic diagnosis in the 2nd~3rd month and the 4th~6th month (Table 5). Serum GGT activity was tested 182 times in 36 patients with a genetic diagnosis in the 2nd~6th month, the peak GGT value was lower than 70U/L (range: 7.0~67.0U/L). But relatively larger ranges were found in patients without a genetic diagnosis and controls. Serum GGT activities in controls reached adult range (<50U/L) in the 7th month of life. However, one patient with ATP8B1 deficiency and two patients with ABCB11 deficiency had GGT≥50U/L in the 7th~12th month. Serum GGT activities of 117 tests revealed that the peak GGT value in patients with a genetic diagnosis was lower than 60U/L in the 7th~12th month. All patients with a genetic diagnosis had GGT<50U/L beyond one year, while 37.2% of patients lacking a genetic diagnosis still had GGT≥50U/L.
Table 5. The ranges for GGT in patients with or without a genetic diagnosis and controls.
| Age | P with | P without | Controls | |||
|---|---|---|---|---|---|---|
| n ‡ | GGT (min-max) | n ‡ | GGT (min-max) | n ‡ | GGT (min-max) | |
| In the 1st year | 299 | 5.0–67.0U/L | 613 | 5.0–434.2U/L | 550 | 2.0–273.0U/L |
| 2nd-3rd month | 76 | 12.0–67.0U/L | 236 | 15.0–434.2U/L | 100 | 15.0–273.0U/L |
| 4th-6th month | 106 | 7.0–66.0U/L | 199 | 9.0–364.0U/L | 150 | 4.0–100.0U/L |
| 7th-12th month | 117 | 5.0–58.0U/L | 178 | 5.0–257.0U/L | 300 | 2.0–41.0U/L |
| >1 year old | 123 | 5.3–47.0U/L | 155 | 8.0–356.0U/L | 50 | 4.0–22.0U/L |
Pwith, patients with a genetic diagnosis; Pwithout, patients without a genetic diagnosis.
‡ n, the times of serum GGT activities tests.
The ranges for GGT and the diagnostic efficiency
39 of the 207 patients, who were ordered to sequence ATP8B1 and/or ABCB11 between January 2012 and December 2015, received a molecular diagnosis. The overall rate of a positive molecular diagnosis was 18.8%. GGT levels of 111 (53.6%) patients were found to meet the ranges described in patients with a genetic diagnosis. All the 39 patients with ATP8B1 or ABCB11 deficiency were included in the 111 patients. The sensitivity was 100.0%. The rate of a positive molecular diagnosis increased to 35.1% (35.1% vs. 18.8%, X2 = 10.363, P = 0.001). 67 (60.4%) of the 111 patients were ordered to test for both genes, 21 (31.3%) obtained a molecular diagnosis. The 21 patients included 3 patients whose causal gene was identified when the second gene was tested. 44 of the 111 patients were ordered to test for ATP8B1 or ABCB11 only, 18 (40.9%) received a molecular diagnosis (18/44 vs. 21/67, X2 = 1.066, P = 0.301). GGT activities of the remaining 96 (57.1% of subjects lacking a genetic diagnosis and 46.4% of the 207 patients) patients exceeded the ranges described above. These patients failed to fulfill the definition of ATP8B1 or ABCB11 deficiency, and accounted for 43.8% of sequencing cost (Table 6).
Table 6. Information of genetic tests and sequencing cost.
| Within the ranges § | Over the ranges § | |||
|---|---|---|---|---|
| Case | Cost ‡ | Case | Cost ‡ | |
| Patients with diagnoses | 39 | 60T | 0 | 0 |
| Test for both genes | 21 | 42T | 0 | 0 |
| Test for one gene | 18 | 18T | 0 | 0 |
| Patients without diagnoses | 72 | 118T | 96 | 139T |
| Test for both genes | 46 | 92T | 43 | 86T |
| Test for one gene | 26 | 26T | 53 | 53T |
§ the ranges, the ranges described in patients with ATP8B1 or ABCB11 deficiency. The peak GGT value was <70U/L in the 2nd~6th month, <60U/L in the 7th~12th month and <50U/L beyond one year.
‡ Cost, the sequencing cost. The sequencing cost of ATP8B1 was equal to that of ABCB11. T was defined as the sequencing cost of one gene.
Discussion
ATP8B1 deficiency and ABCB11 deficiency are the most common causes of chronic cholestasis with low serum GGT activity. They were widely reported around the world including mainland China and Taiwan [16,18–20]. Nevertheless, the ranges for GGT in these patients were unclear. In this study, we found that GGT levels and ranges in patients with ATP8B1 or ABCB11 deficiency were different from that in patients without a genetic diagnosis and controls. GGT levels in these patients varied with age. The peak GGT value was <70U/L in the 2nd~6th month, <60U/L in the 7th~12th month and <50U/L beyond one year. These features of GGT could greatly improve the diagnostic efficiency.
Mutations in ATP8B1 or ABCB11 could result in dysfunction of BSEP, and impaired BA secretion [11]. The reduced bile acids in bile preserved GGT localisation at the canalicular membrane, and resulted in a phenotype with low GGT [10]. GGT levels were found to be similar at presentation or at time of diagnosis between the ATP8B1 deficiency and the ABCB11 deficiency [11,15]. We found that GGT levels in these patients were similar at any observed month of age. It was possible that mutations in ATP8B1 and ABCB11 had similar effect on BA secretion. However, higher serum BA and more vitamin deficiency were found in patients with ABCB11 deficiency [11,15]. This indicated that more severe impairment on BA secretion was caused by ABCB11 defect than ATP8B1 defect. It was found that GGT expression at canalicular membrane was more severely impaired in ATP8B1 deficiency patients than ABCB11 deficiency patients [24–25]. Therefore, the impairment on GGT expression and BA secretion might account for the similar GGT levels found in patients with ATP8B1 or ABCB11 deficiency.
Similar to healthy infants [11–12], GGT levels in patients with ATP8B1 or ABCB11 deficiency also varied with age. This might attribute to that GGT expression continued to decline until about 6 months old [13]. However, GGT levels in these patients were different from that in patients without a genetic diagnosis and controls. Larger ranges were found in patients without a genetic diagnosis. The peak GGT value in patients with a genetic diagnosis was lower than 70U/L (1.4 times of the normal adult range) in the 2nd~6th month of life. It was lower than that in healthy infants reported previously [12–13] and controls reported in this study. Some controls had GGT≥70U/L in the 2nd~6th month, especially at the second month of life. Three patients with a genetic diagnosis had GGT≥50U/L at the 7th~12th month of life, while controls reached the adult range (GGT<50U/L) at 6 months old. Therefore, we concluded that GGT<70U/L in the 2nd~6th month, <60U/L in the 7th~12th month and <50U/L beyond one year were important features of patients with ATP8B1 or ABCB11 deficiency.
It was known that ATP8B1 deficiency and ABCB11 deficiency accounted for the majority of patients with low GGT. These explained why 60.4% of patients whose GGT levels met the ranges described above were ordered to test for both ATP8B1 and ABCB11. However, the rates of a positive molecular diagnosis were similar between patients testing for both genes and patients testing for one gene (P = 0.301). This might attribute to clinic and laboratory differences between the two disorders as reported previously [11,15]. We proposed that genetic tests should be ordered according to the differences. As some patients obtained a molecular diagnosis from the second gene test, the second gene should be ordered if the first test revealed negativity. However, other genetic defects (eg, TJP2 [26]) also caused cholestasis with low GGT. Multi-gene panel [27] or whole exons sequencing [28] might be more effective for these patients.
In fact, the ranges for GGT in patients with ATP8B1 or ABCB11 deficiency were unclear when genetic tests were ordered. This caused some patients with elevated GGT were ordered to test for the two genes and resulted in a low rate of positive molecular diagnosis. According to our data, only 18.8% of patients who tested for ATP8B1 and/or ABCB11 could be assigned to a molecular diagnosis. 46.4% of patients had GGT activities over the ranges described above. Of these patients, other genetic defects should be taken into consideration. If these patients were waived from testing for these genes, the rate of positive molecular diagnosis would increase greatly. Meanwhile, 43.8% of the sequencing cost would have been saved.
Conclusion
GGT levels in the ATP8B1 deficiency were similar to that in the ABCB11 deficiency at any observed month of age. Similar to healthy infants, GGT levels in these patients also varied with age. But GGT levels in these patients were different from that in patients without a genetic diagnosis and controls. The peak GGT value was <70U/L in the 2nd~6th month, <60U/L in the 7th~12th month and <50U/L beyond one year of age. If only patients whose GGT levels met the ranges were ordered to test for these genes, the overall rate of a positive molecular diagnosis would have increased greatly. This also resulted in approximately 1/2 of sequencing cost saving.
Supporting Information
(DOC)
(DOC)
Acknowledgments
Great thanks to those families who participated in this study, to Prof. J Lin and RX Wang for the revision of the manuscript, to the National Natural Science Foundation of China for financial support (No. 81070281, NO. 81361128006).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research work was supported by the National Natural Science Foundation of China (No. 81070281 and No. 81361128006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1–26. 10.1016/j.cld.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 2.Stormon MO, Dorney SF, Kamath KR, O'Loughlin EV, Gaskin KJ. The changing pattern of diagnosis of infantile cholestasis. J Paediatr Child Health. 2001;37:47–50. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfield GM, Chapman RW, Karlsen TH, Lammert F, Lazaridis KN, Mason AL. The genetics of complex cholestatic disorders. Gastroenterology. 2013;144:1357–1374. 10.1053/j.gastro.2013.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang LJ, Wang XH, Knisely AS, Yu H, Lu Y, Liu LY, et al. Chinese children with chronic intrahepatic cholestasis and high γ-glutamyl transpeptidase: clinical features and association with ABCB4 mutations. J Pediatr Gastroenterol Nutr. 2012;55:150–156. 10.1097/MPG.0b013e31824ef36f [DOI] [PubMed] [Google Scholar]
- 5.Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–224. [DOI] [PubMed] [Google Scholar]
- 6.van Mil SW, Klomp LW, Bull LN, Houwen RH. FIC1 disease: a spectrum of intrahepatic cholestatic disorders. Semin Liver Dis. 2001;21:535–544. [DOI] [PubMed] [Google Scholar]
- 7.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20: 233–238. [DOI] [PubMed] [Google Scholar]
- 8.van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127:379–384. [DOI] [PubMed] [Google Scholar]
- 9.van Ooteghem NA, Klomp LW, Berge-Henegouwen GP, Houwen RH. Benign recurrent intrahepatic cholestasis progressing to progressive familial intrahepatic cholestasis: low GGT cholestasis is a clinical continuum. J Hepatol. 2002;36:439–443. [DOI] [PubMed] [Google Scholar]
- 10.van Mil SWC, Houwen RHJ, Klomp LWJ. Genetics of familial intrahepatic cholestasis syndromes. J Med Genet. 2005;42:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–1655. 10.1002/hep.23539 [DOI] [PubMed] [Google Scholar]
- 12.Cabrera-Abreu JC, Green A. Gamma-glutamyltransferase: value of its measurement in pediatrics. Ann Clin Biochem. 2002;39:22–25. [DOI] [PubMed] [Google Scholar]
- 13.Wang JS, Tan N, Dhawan A. Significance of low or normal serum gamma glutamyl transferase level in infants with idiopathic neonatal hepatitis. Eur J Pediatr. 2006;165:795–801. [DOI] [PubMed] [Google Scholar]
- 14.van der Woerd WL, van Mil SW, Stapelbroek JM, Klomp LW, van de Graaf SF, Houwen RH. Familial cholestasis: Progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis and intrahepatic cholestasis of pregnancy. Best Pract Res Clin Gastroenterol. 2010;24:541–553. 10.1016/j.bpg.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 15.Pawlikowska L, Strautnieks S, Jankowska I, Czubkowski P, Emerick K, Antoniou A, et al. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J Hepatol. 2010;53:170–178. 10.1016/j.jhep.2010.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HL, Chang PS, Hsu HC, Ni YH, Hsu HY, Lee JH, et al. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low gamma-glutamyl transpeptidase levels. J Pediatr. 2002;140:119–124. [DOI] [PubMed] [Google Scholar]
- 17.Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerová D, Rayner A, Dutton L, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology 2008;134:1203–14. 10.1053/j.gastro.2008.01.038 [DOI] [PubMed] [Google Scholar]
- 18.Liu LY, Wang ZL, Wang XH, Zhu QR, Wang JS. ABCB11 gene mutations in Chinese children with progressive intrahepatic cholestasis and low gamma glutamyltransferase. Liver Int. 2010;30:809–815. 10.1111/j.1478-3231.2009.02112.x [DOI] [PubMed] [Google Scholar]
- 19.Liu LY, Wang XH, Wang ZL, Zhu QR, Wang JS. Characterization of ATP8B1 gene mutations and a hot-linked mutation found in Chinese children with progressive intrahepatic cholestasis and low GGT. J Pediatr Gastroenterol Nutr. 2010;50:179–183. 10.1097/MPG.0b013e3181c1b368 [DOI] [PubMed] [Google Scholar]
- 20.Li LT, Deheragoda M, Lu Y, Gong JY, Wang JS. Hypothyroidism Associated with ATP8B1 Deficiency. J Pediatr. 2015;167:1334–1339. 10.1016/j.jpeds.2015.08.037 [DOI] [PubMed] [Google Scholar]
- 21.Azevedo LS, Pierrotti LC, Abdala E, Costa SF, Strabelli TM, Campos SV, et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jim WT, Chiu NC, Ho CS, Shu CH, Chang JH, Hung HY, Kao HA, Chang HY, et al. Outcome of preterm infants with postnatal cytomegalovirus infection via breast milk: a two-year prospective follow-up study. Medicine (Baltimore). 2015;94:e1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang MH, Hsu HC, Lee CY, Wang TR, Kao CL. Neonatal hepatitis: A follow-up study. J Pediatr Gastroenterol Nutr. 1987;6:203–207. [DOI] [PubMed] [Google Scholar]
- 24.Liu LU, Qin L, Knisely AS. A patient with persistent pruritus. Semin Liver Dis. 2010;30:205–209. 10.1055/s-0030-1253523 [DOI] [PubMed] [Google Scholar]
- 25.Morotti RA, Suchy FJ, Magid MS. Progressive Familial Intrahepatic Cholestasis (PFIC) Type 1, 2, and 3: A Review of the Liver Pathology Findings. Semin Liver Dis. 2011;31:3–10. 10.1055/s-0031-1272831 [DOI] [PubMed] [Google Scholar]
- 26.Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014. April;46(4):326–8. 10.1038/ng.2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst SM, Schirmer S, Posovszky C, Jochum F, Rödl T, Schroeder JA, et al. Taking the next step forward-Diagnosing inherited infantile cholestatic disorders with next generation sequencing. Mol Cell Probes. 2015;29:291–298. 10.1016/j.mcp.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013;369:1502–1511. 10.1056/NEJMoa1306555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.