Abstract
Previous studies have shown that thyroid hormone directly stimulates bone resorption in in vitro organ culture, and in adults excess thyroid hormone is associated with decreased bone mineral density. There are limited data in children regarding the effect of hyperthyroidism on bone metabolism and even fewer instances in the literature of hyperthyroidism presenting with bone demineralization and fracture. We report a case of an 11-year-old boy with undiagnosed hyperthyroidism presenting with fractures and osteoporosis. This case emphasizes the importance of maintaining a broad differential diagnosis when a patient presents with a pathologic fracture.
Graves’ disease is the most common cause of hyperthyroidism in children, and it results from autoantibodies against the thyroid-stimulating hormone (TSH) receptor, leading to increased synthesis and secretion of thyroid hormones.1 Previous studies in adult patients have shown that excess thyroid hormone is associated with decreased bone mineral density independent of BMI;2 however, few data exist on the effects of elevated thyroid hormone levels on bone resorption in pediatric patients. We describe a case of an 11-year-old, previously healthy boy who presented with a pathologic wrist fracture and was found to have decreased bone mineral density and multiple vertebral compression fractures on radiograph, prompting additional workup and ultimate diagnosis of Graves’ disease. Although decreased bone mineral density has been reported at diagnosis of hyperthyroidism in children,3 fracture itself is a rare presentation.4
Case Presentation
An 11-year-old previously healthy boy presented to the emergency department with left arm pain after a sports injury. The patient had been standing still when an opposing player ran into his left arm, hyperextending his wrist. On examination, the patient’s temperature was 36.5°C, heart rate 126 beats per minute, blood pressure 123/67 mm Hg, respiratory rate 20 breaths per minute, weight 33 kg (28th percentile for age), and height 154 cm (89th percentile for age). He appeared anxious, but his pain had resolved completely during initial examination, and he was answering questions appropriately. In addition to the tachycardia, his cardiac examination was significant for a hyperdynamic precordium and bounding pulses. No thyromegaly was appreciated on initial neck examination in the emergency department; however, mild diffuse thyroid enlargement was noted on later examination by endocrinology specialists, who also noted a fine resting hand tremor. There was mild swelling of his distal left forearm, with minimal point tenderness over the radius. There was no obvious proptosis, nystagmus, or blue sclerae. Joints were normal, with no hypermobility. His family history was significant for osteoporosis and scoliosis in adults but no childhood history of decreased bone density, hypermobility, or fractures. His father was diagnosed with Graves’ disease at age 30. His diet history included 3 cups of milk daily, with additional milkshakes, yogurt, and cheese. Review of systems was significant for poor weight gain over the past several months.
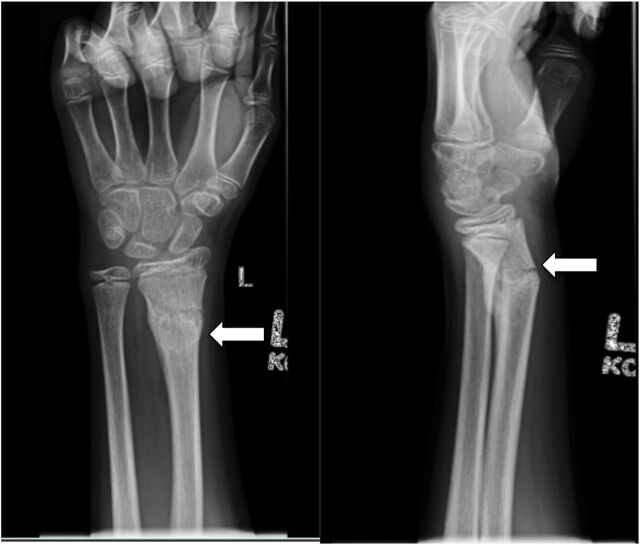
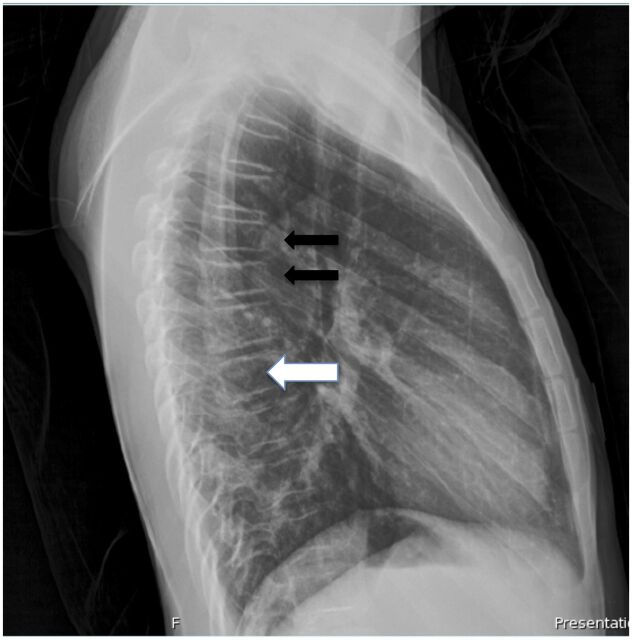
A radiograph of the wrist was obtained (Fig 1), which demonstrated a healing transverse fracture through the distal radial diaphysis, with dorsal displacement. The bones were also demineralized. There was discordance between the history and radiograph, in that the patient experienced acute trauma with radiographic findings of a subacute fracture. At this point, the patient recalled another injury 2 weeks before this visit in which he injured his left arm sliding while playing baseball. Additional workup for the patient’s significant tachycardia in absence of pain was initiated to avoid subjecting the patient to the potential risks of ketamine sedation, as initially planned for fracture reduction. An electrocardiogram showed sinus tachycardia, with heart rate of 138 beats per minute. A chest radiograph showed normal heart size and clear lung fields but revealed demineralization of the bones, with multiple compression deformities of the thoracic spine of indeterminate age and etiology (Fig 2). Diseases to consider included metabolic bone disease or possible leukemia or juvenile osteoporosis. Given the pathologic fractures and tachycardia, laboratory work was performed to look for an endocrinopathy or malignancy as the underlying cause.
FIGURE 1.
Wrist radiograph, anteroposterior and lateral views. Note the transverse fracture through the distal left radial diaphysis with callus formation (white arrow).
FIGURE 2.
Chest radiograph of patient, lateral view. Note the multiple compression fractures of the caudal thoracic vertebral bodies (black arrows) and demineralization throughout the thoracic vertebrae (white arrow).
Serum laboratory work is reported in Table 1. Laboratory values were most notable for a TSH <0.01 μIU/mL (normal range 0.5–3.8 μIU/mL) and a thyroxine (T4) of 21.9 μg/dL (normal range 4.7–9.9 μg/dL). 25-Hydroxyvitamin D was normal at 30.5 ng/mL (optimum level 25–80 ng/mL). Complete blood cell count, complete metabolic panel, C-reactive protein, erythrocyte sedimentation rate, lactate dehydrogenase, and uric acid were all normal. The hematopathology department reviewed the peripheral blood smear, which showed mild microcytic anemia. Given the low TSH and elevated T4, the diagnosis of hyperthyroidism was made. The patient was admitted for additional management. Thyroid antibody testing performed on an inpatient basis (Table 1) confirmed the diagnosis of Graves’ disease, and the patient was started on methimazole 20 mg twice daily and atenolol 25 mg daily, and was ultimately discharged from the hospital. The atenolol has since been discontinued. His T4 level normalized ∼3 months after he started treatment, and his most recent free T4 level was 1.12 ng/dL (normal 0.93–1.60 ng/dL). He had a bone densitometry scan (dual-energy radiograph absorptiometry [DXA]) 2 months after diagnosis, which showed significantly low bone mineral density, but his thyroid disease was also suboptimally controlled at that time. A follow-up scan is scheduled but has not yet been performed. Based on the history, physical examination, and laboratory data available, common causes of low bone mineral density, including vitamin D deficiency (normal level), hypocalcemia (normal level with normal dietary intake), and inflammatory bowel disease (lack of abdominal pain and diarrhea, normal inflammatory markers) were either ruled out or determined to be unlikely etiologies of the patient’s fractures.
TABLE 1.
Serum Laboratory Results for Patient
| Laboratory (Serum) | Value | Normal Range |
|---|---|---|
| Calcium | 9.8 mg/dL | 8.8–10.1 mg/dL |
| Phosphorus | 6.1 mg/dL | 3.7–5.6 mg/dL |
| Alkaline phosphatase | 361 U/L | 135–530 U/L |
| Thyroid-stimulating hormone | <0.01 μIU/mL | 0.5–3.8 μIU/mL |
| Thyroxine | 21.9 μg/dL | 4.7–9.9 μg/dL |
| Free T4 | 3.7 ng/dL | 1–1.8 ng/dL |
| Parathyroid hormone | 67 pg/mL | 9–52 pg/mL |
| 25-hydroxy vitamin D | 30.5 ng/mL | 25–80 ng/mL |
| Erythrocyte sedimentation rate | 11 mm/h | 0–20 mm/h |
| Antithyroid peroxidase Ab | 749 IU/mL | 0–35 IU/mL |
| Antithyroglobulin Ab | 66.2 IU/mL | 0–40 IU/mL |
| Thyroid-stimulating Ig | 177% | 0%–122% |
Ab, antibody; Ig, immunoglobulin.
Discussion
Graves’ disease is the most common cause of hyperthyroidism in children and results from autoantibodies against the TSH receptor, leading to elevated synthesis and secretion of thyroid hormones.1 In children, early symptoms are often nonspecific and include irritability, fatigue, emotional lability, sleep disturbance, poor concentration, and decline in academic performance.1 More objective signs include weight plateau or loss (often with increased appetite), tachycardia, and increased blood pressure,1 as thyroid hormone causes an increase in the metabolic rate and cardiovascular activity.5 Presentations classic for adults, such as true exophthalmos and thyrotoxic crisis, are rare in children.1
It has been known for years that thyroid hormone directly stimulates bone resorption in in vitro organ culture.6 In adults, excess thyroid hormone is associated with decreased bone mineral density independent of BMI,2 and a history of hyperthyroidism increases risk for hip fractures,7 especially at diagnosis.8 A few recent studies discuss a novel pituitary–bone axis, wherein TSH itself has been proposed to have an anabolic or protective effect on bone.9 In older adults, subclinical hyperthyroidism, defined as normal thyroid hormone but low TSH, is associated with osteoporosis9 and elevated fracture rate.10–12 However, this association has not been shown in other subgroups.10 Additionally, the administration of recombinant human TSH to prevent bone loss has not produced convincing results.10 We hope future studies will clarify the role of the hypothalamus and pituitary, including TSH, in bone metabolism.
The literature is also limited regarding the effect of hyperthyroidism on bone health in children. Lucidarme et al3 prospectively evaluated bone mineral density at diagnosis and 1 and 2 years after initiation of medical treatment in 26 children with Graves’ disease. They found severe osteopenia at diagnosis, with preferential loss of cortical bone compared with trabecular bone. The osteopenia rapidly corrected after 1 and 2 years of treatment to a euthyroid state. Nubenjapon et al13 also studied the effect of elevated thyroid hormone on bone by using computed tomography to measure bone density. They found that cortical bone density was significantly lower in children and adolescents with hyperthyroidism compared with matched controls. Compared with pubertal and postpubertal patients with Graves’ disease, prepubertal children with Graves’ disease have accelerated linear growth and bone maturation and therefore an advanced bone age of 1.5 to 2.5 years.14
Despite these studies showing low bone mineral density and accelerated linear growth in untreated hyperthyroidism, fracture as the presenting finding in a child with hyperthyroidism, as in our case, is rare.3 One case report by Cheruvu et al4 describes a 3-year-old boy who presented with a right oblique femur fracture and was found to have Graves’ disease. Unlike our case, however, this patient had a more prominent goiter and significant proptosis. Both for our patient and in this case report, low bone density as measured by DXA, −4.3 (forearm) and −2.7 (spine), respectively, was consistent with osteoporosis.
Our patient had a mildly elevated parathyroid hormone (PTH) level with a slightly elevated phosphorus level but normal calcium, 25-hydroxyvitamin D, and alkaline phosphatase levels (see Table 1). Repeat PTH levels were within normal range after 1 month of treatment (40 pg/mL, range 9–52 pg/mL). Patients with Graves’ disease have altered mineral metabolism, including exaggerated calcium-stimulated PTH responses, and are at elevated risk for hypocalcemia during thyroidectomy.15 In Hashimoto’s thyroiditis, a related autoimmune disorder of the thyroid, the incidence of primary hyperparathyroidism was 6 times higher than in the general population.16
Our case is unique in that our patient had multiple pathologic fractures with less prominent classic symptoms of hyperthyroidism. His wrist fracture brought him to medical attention, where a more detailed examination revealed asymptomatic tachycardia, fine resting hand tremor, and a mildly enlarged thyroid. Imaging revealed asymptomatic vertebral compression fractures and decreased bone mineral density initially by radiograph and later by DXA. This finding emphasizes the importance of maintaining a broad differential diagnosis when a patient presents with a pathologic fracture, especially in the setting of abnormal vital signs, and to consider hyperthyroidism as a potential diagnosis when appropriate clinical features are present.
Glossary
- DXA
dual-energy radiograph absorptiometry
- PTH
parathyroid hormone
- T4
thyroxine
- TSH
thyroid-stimulating hormone
Footnotes
Dr Sarezky drafted the initial manuscript and critically reviewed the manuscript; Drs Corwin, Harrison, and Jacobstein critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: No external funding.
References
- 1.Léger J, Kaguelidou F, Alberti C, Carel JC. Graves’ disease in children. Best Pract Res Clin Endocrinol Metab. 2014;28(2):233–243 [DOI] [PubMed] [Google Scholar]
- 2.van der Deure WM, Uitterlinden AG, Hofman A, et al. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam Study. Clin Endocrinol (Oxf). 2008;68(2):175–181 [DOI] [PubMed] [Google Scholar]
- 3.Lucidarme N, Ruiz JC, Czernichow P, Léger J. Reduced bone mineral density at diagnosis and bone mineral recovery during treatment in children with Graves’ disease. J Pediatr. 2000;137(1):56–62 [DOI] [PubMed] [Google Scholar]
- 4.Cheruvu S, Alverson BK, Quintos JB. Femoral fracture as a rare presentation of prepubertal Graves disease. J Pediatr. 2013;162(2):429–430 [DOI] [PubMed] [Google Scholar]
- 5.Reddy PA, Harinarayan CV, Sachan A, Suresh V, Rajagopal G. Bone disease in thyrotoxicosis. Indian J Med Res. 2012;135(1):277–286 [PMC free article] [PubMed] [Google Scholar]
- 6.Mundy GR, Shapiro JL, Bandelin JG, Canalis EM, Raisz LG. Direct stimulation of bone resorption by thyroid hormones. J Clin Invest. 1976;58(3):529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings SR, Nevitt MC, Browner WS, et al. ; Study of Osteoporotic Fractures Research Group . Risk factors for hip fracture in white women. N Engl J Med. 1995;332(12):767–773 [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid. 2002;12(5):411–419 [DOI] [PubMed] [Google Scholar]
- 9.Harinarayan CV. Thyroid bone disease. Indian J Med Res. 2012;135(1):9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholls JJ, Brassill MJ, Williams GR, Bassett JH. The skeletal consequences of thyrotoxicosis. J Endocrinol. 2012;213(3):209–221 [DOI] [PubMed] [Google Scholar]
- 11.Bauer DC, Ettinger B, Nevitt MC, Stone KL; Study of Osteoporotic Fractures Research Group . Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134(7):561–568 [DOI] [PubMed] [Google Scholar]
- 12.Morris MS. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone. 2007;40(4):1128–1134 [DOI] [PubMed] [Google Scholar]
- 13.Numbenjapon N, Costin G, Gilsanz V, Pitukcheewanont P. Low cortical bone density measured by computed tomography in children and adolescents with untreated hyperthyroidism. J Pediatr. 2007;150(5):527–530 [DOI] [PubMed] [Google Scholar]
- 14.Lazar L, Kalter-Leibovici O, Pertzelan A, Weintrob N, Josefsberg Z, Phillip M. Thyrotoxicosis in prepubertal children compared with pubertal and postpubertal patients. J Clin Endocrinol Metab. 2000;85(10):3678–3682 [DOI] [PubMed] [Google Scholar]
- 15.Annerbo M, Hultin H, Stålberg P, Hellman P. Left-shifted relation between calcium and parathyroid hormone in Graves’ disease. J Clin Endocrinol Metab. 2014;99(2):545–551 [DOI] [PubMed] [Google Scholar]
- 16.Ignjatovic VD, Matovic MD, Vukomanovic VR, Jankovic SM, Džodić RR. Is there a link between Hashimoto’s thyroiditis and primary hyperparathyroidism? A study of serum parathormone and anti-TPO antibodies in 2267 patients. Hell J Nucl Med. 2013;16(2):86–90 [PubMed] [Google Scholar]