Abstract
The determination of complex analytes, present at low concentrations, in biological fluids poses a diffcult challenge. This study relies on an optimized method of recovery, enzymatic treatment, and disaccharide analysis by liquid chromatography–tandem mass spectrometry to rapidly determine low concentrations of glycosaminoglycans in human urine. The approach utilizes multiple reaction monitoring (MRM) of glycosaminoglycan disaccharides obtained from treating urine samples with recombinant heparin lyases and chondroitin lyase. This rapid and sensitive method allows the analysis of glycosaminoglycan content and disaccharide composition in urine samples having concentrations 10-to 100-fold lower than those typically analyzed from patients with metabolic diseases, such as mucopolysaccharidosis. The current method facilitates the analysis low (ng/mL) levels of urinary glycosaminoglycans present in healthy individuals and in patients with pathological conditions, such as inflammation and cancers, that can subtly alter glycosaminoglycan content and composition.
Glycosaminoglycans (GAGs) are polydisperse, linear, anionic polysaccharides that are functionally important in the biology of cell–cell interaction, cellular communication, cell signaling, and are critical in the developmental biology of multicellular organisms.1–4 GAGs are biosynthesized either in the Golgi or at the cell membrane and are commonly secreted to the external cell surface or into the extracellular matrix.5–9 GAGs can be remodeled to shorter polysaccharides through the action of hydrolases10,11 or their functional groups (i.e., sulfates) can be removed through the action of sulfatases,12,13 modifying their biological activities. GAGs are rapidly turned over through lysosomal degradation and their local concentrations in a healthy organism are well-regulated.14
There are several major structural classes of GAGs, including hyaluronan, (→3)-β-D-GlcNAc(1 → 4)-β-D-GlcA(1→, chondroitin sulfates (→3)-β-D-GalNAc(1→4)-β-D-GlcA or α-L-IdoA(1→, and heparan sulfates (→4)-α-D-GlcNAc or α-D-GlcNS(1→4)-β-D-GlcA or α-L-IdoA (1→ (where GlcNAc is N-acetylglucosamine, GalNAc is N-acetylgalactosamine, GlcNS is N-sulfoglucosamine, GlcA is glucuronic acid, and IdoA is iduronic acid).4 While hyaluronan is unmodified, chondroitin sulfate can be modified with O-sulfo groups at the 4- or 6 positions of its GalNAc residue and the 2-positions of its uronic acid residues, and heparan sulfate can be modified with O-sulfo groups at the 3- or 6-positions of its GlcNAc or GlcNS residue and the 2-positions of its uronic acid residues.7 The fine structures, as well as the chain sizes, of GAGs have a profound impact on their biological activities.8,9,15–17 Thus, it is critically important to develop the means of GAG analysis, particularly in their natural environment, such as in tissues18,19and in biological fluids. Urine, while representing an easy to access biological fluid, presents a particular challenge to analysis, because normal urine contains variable and extremely low levels of excreted GAGs, in addition to other sulfated metabolites.
GAG analysis is still less developed that protein or nucleic acid analyses and often relies on three levels of structural determination, disaccharide compositional analysis, oligosaccharide and domain mapping, and finally sequence analysis.20–23 Disaccharide compositional analysis provides information on total GAG content, classes of GAG present, and structural characteristics, such as the position and level of substitution of sulfo groups, which can be informative about the subclass of GAG, i.e., chondroitin 4-sulfate or chondroitin 6-sulfate, heparan sulfate or heparin, etc. Such data can often serve as biomarkers for diseases such as cancer24,25 and metabolic disorders such as mucopolysaccharidoses (MPS)14,26 and lysosomal storage diseases related to a failure of GAG catabolism, resulting in the detrimental buildup of excessive levels of GAGs.27–29 In MPS, the concentration of urinary GAGs is suffciently high that colorimetric dye-binding analysis using Dimethylmethylene Blue (DMMB) is possible; unfortunately, this is a relatively low-sensitivity method, it is subject to various interferences, and it fails to detect unsulfated GAGs or small GAG-derived oligosaccharides formed through GAG catabolism. Moreover, the DMMB assay cannot distinguish between the class and subclass of urinary GAG present and, thus, cannot be used to diagnose the type of MPS. Most recently, LC-MS and LC-MS/MS methods have been intensively investigated for measuring urinary GAGs by first breaking these GAGs down to disaccharides through the use of enzymatic or chemical methods.30–32 Most of these methods focus on a group of patients or patients in different grades of the same MPS disease and require GAG concentrations quite elevated over normal urinary levels. None of these are capable of analyzing multiple GAG types, particularly those with very different concentrations. These LC-MS and LC-MS/MS methods rely on a variety of LC methods, such as hydrophilic liquid interaction (HILIC),33,34 graphitic carbon (Hyper-Card),35–38 and hydrophobic (C18)31,39 resins, and the reported methods can be complicated and the separations achieved are not particularly good.
Recently, we demonstrated GAG disaccharide analysis that effciently separated 17 standard disaccharides in a 50 min LC run.40 In the current study, we now report a simplified method for the excellent recovery of GAGs from urine, their enzymatic conversion to GAG disaccharides, and the sensitive and rapid determination of GAG content and composition using LC-MS/MS, even at the normal low levels of urinary GAGs.
EXPERIMENTAL SECTION
Materials
CS and HS standard were purchased from Celsus Laboratories (Cincinnati, OH). CS, HS, and HA disaccharide standards (see Table 1 for structures) were purchased from Iduron (Manchester, U.K.). Normal human urine samples—one pooled, two from males, and two from females—were purchased from BioreclamationIVT (Westbury, NY). DMMB, 2-aminoacridone (AMAC), sodium cyanoborohydride (NaCNBH4), and acetic acid were purchased from Sigma’Aldrich (St. Louis, MO). Methanol (HPLC grade), ammonium acetate (HPLC grade), and dimethyl sulfoxide (DMSO) were purchased from Fisher Scientific (Springfield, NJ). E. coli expression and purification of the recombinant F. heparinum heparin lyase I, II, III (EC Nos. 4.2.2.7, 4.2.2.X, and 4.2.2.8, respectively) and P. vulgaris chondroitin lyase ABC (EC No. 4.2.2.20) were performed in our laboratory as described.41
Table 1.
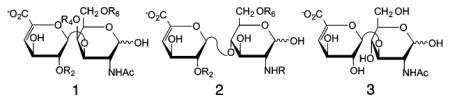
|
||||
|
| ||||
| CS disaccharides | structure 1 | R2 | R4 | R6 |
|
| ||||
| TriSCS | ΔUA2S(1,3)GalNAc4S6S | SO3− | SO3− | SO3− |
| 2S4SCS | ΔUA2S(1,3)GalNAc4S | SO3− | SO3− | H |
| 2S6SCS | ΔUA2S(1,3)GalNAc6S | SO3− | H | SO3− |
| 4S6SCS | ΔUA(1,3)GalNAc4S6S | H | SO3− | SO3− |
| 2SCS | ΔUA2S(1,3)GalNAc | SO3− | H | H |
| 4SCS | ΔUA(1,3)GalNAc4S | H | SO3− | H |
| 6SCS | ΔUA(1,3)GalNAc6S | H | H | SO3− |
| 0SCS | ΔUA(1,3)GalNAc | H | H | H |
|
| ||||
| HS disaccharides | structure 2 | R2 | NR | R6 |
|
| ||||
| TriSHS | ΔUA2S(1,4)GlcNS6S | SO3− | SO3− | SO3− |
| NS6SHS | ΔUA(1,4)GlcNS6S | H | SO3− | SO3− |
| NS2SHS | ΔUA2S(1,4)GlcNS | SO3− | SO3− | H |
| NSHS | ΔUA(1,4)GlcNS | H | SO3− | H |
| 2S6SHS | ΔUA2S(1,4)GlcNAc6S | SO3− | Ac | SO3− |
| 6SHS | ΔUA(1,4)GalNAc6S | H | Ac | SO3− |
| 2SHS | ΔUA2S(1,4)GlcNAc | SO3− | Ac | H |
| 0SHS | ΔUA(1,4)GlcNAc | H | Ac | H |
|
| ||||
| HA disaccharides | structure 3 | |||
|
| ||||
| 0SHA | ΔUA(1,3)GlcNAc | |||
Human Samples
Urine samples were obtained from a study of urinary biomarkers of critical illness (Clinicaltrials.gov No. NCT01900275). All human protocols were approved by the Colorado Multiple Institutions Review Board (No. 13-0425). Urine was collected from patients within 24 h of admission to the Denver Health Medical Center Surgical Intensive Care Unit (Denver, CO) for treatment of major trauma, as defined by an Injury Severity Score of >15. Collected urine (5 mL) was centrifuged at 2000 rpm, with the supernatant snap-frozen and stored at –80 °C for later analysis.
Sample Preparation
Urine samples were defrosted at 4 °C and mixed well using a vortex mixer. A 400-μL aliquot of each sample was desalted by passing through a 3 kDa molecule weight cutoff (MWCO) spin column and washed twice with distilled water. A series of urine samples were also prepared for method validation by adding aqueous solutions containing varying amounts of standard CS and HS affording final concentrations of 50–500 ng/mL.
The casing tubes were replaced before 150 μL of digestion buffer (50 mM ammonium acetate containing 2 mM calcium chloride adjusted to pH 7.0) was added to the filter unit. Recombinant heparin lyase I, II, III (pH optima 7.0–7.5) and recombinant chondroitin lyase ABC (10 mU each, pH optimum 7.4) were added to each sample and mixed well. The samples were all placed in a water bath at 37 °C for 2 h, after which enzymatic digestion was terminated by removing the enzymes by centrifugation. Under these reaction conditions, these lyases could completely depolymerize their GAG substrates (in amounts of over 100 μg) into products containing each class of GAG disaccharides. The filter unit was washed twice with 100 μL distilled water and the filtrates, containing the disaccharide products, were dried via vacuum centrifuge and stored at –20 °C.
The dried samples were AMAC-labeled by adding 10 μL of 0.1 M AMAC in DMSO/acetic acid (17/3,V/V) incubating at room temperature for 10 min, followed by adding 10 μL of 1 M aqueous NaBH3CN and incubating for 1 h at 45 °C. A mixture containing all 17-disaccharide standards prepared at 1250 ng/mL was similarly AMAC-labeled and used for each run as an external standard. After the AMAC-labeling reaction, the samples were centrifuged and each supernatant was recovered and an equal volume of DMSO:acetic acid:distilled water (17:3:20) was added to each. Samples were stored in a light-resistant container at room temperature until analyzed via LC-MS/MS.
LC-MS/MS Analysis
LC was performed on an Agilent 1200 LC system at 45 °C using an Agilent Poroshell 120 EC-C18 (2.7 μm, 3.0 × 50 mm) column. Mobile phase A (MPA) was 50 mM ammonium acetate aqueous solution, and the mobile phase B (MPB) was methanol. The mobile phase passed through the column at a flow rate of 300 μL/min. The concentration of MPB increased from 5% to 45% during 10 min, then rose to 100% MPB in the following 0.2 min, and a 4 min flow of 100% MPB was applied to elute all compounds.
A triple quadrupole mass spectrometry system equipped with an ESI source (Thermo Fisher Scientific, San Jose, CA) was used a detector. The online MS analysis was at the Multiple Reaction Monitoring (MRM) mode. The conditions and collision energies for the all of the disaccharides MRM-transitions are listed in Table 2.
Table 2.
| disaccharide | precursor ion (m/z) |
production ion (m/z) |
collisional energy (V) |
tube len (V) |
retention time (min) |
limit of detection, LOD (ng/mL) |
limit of quantification, LOQ (ng/mL) |
|---|---|---|---|---|---|---|---|
| TriSHS | 770 | 690 | 20 | −70 | 4.83 | 2.53 | 8.42 |
| NS6SHS | 690 | 610 | 20 | −75 | 5.94 | 0.44 | 1.45 |
| NS2SHS | 690 | 610 | 20 | −70 | 6.67 | 0.41 | 1.35 |
| TriSCS | 405.5 | 365.5 | 20 | −70 | 7.18 | 0.72 | 2.40 |
| NSHS | 610 | 354 | 33 | −100 | 7.63 | 0.26 | 0.88 |
| 2S4SCS | 732 | 652 | 20 | −80 | 7.92 | 0.69 | 2.31 |
| 2S6SHS | 732 | 652 | 20 | −80 | 8.04 | 0.69 | 2.31 |
| 2S4SCS | 732 | 652 | 20 | −80 | 8.50 | 1.22 | 4.07 |
| 4S6SCS | 732 | 652 | 20 | −80 | 8.68 | 0.85 | 2.84 |
| 6SHS | 652 | 396 | 37 | −104 | 8.70 | 0.81 | 2.71 |
| 2SHS/2SCS | 652 | 157 | 29 | −106 | 9.24 | 0.59 | 1.96 |
| 4SCS | 652 | 396 | 40 | −110 | 9.45 | 0.93 | 3.09 |
| 6SCS | 652 | 396 | 31 | −110 | 9.86 | 0.73 | 2.43 |
| 0SHS | 572 | 396 | 28 | −90 | 9.95 | 0.26 | 0.85 |
| 0SHA | 572 | 396 | 26 | −80 | 10.29 | 0.65 | 2.17 |
| 0SCS | 572 | 396 | 28 | −90 | 10.60 | 0.26 | 0.86 |
Urinary Creatinine Analysis
To control for differences in urinary dilution,42,43 glycosaminoglycan values were normalized to urine creatinine (Beckman Coulter AU Analyzer), as measured in a blind fashion by the Colorado Clinical and Translational Sciences Institute (CCTSI) at the University of Colorado Hospital Core Laboratory (Aurora, CO).
DMMB Assay
DMMB reagent was prepared following standard protocol in which DMMB (16 mg), glycine (3.05 g), sodium chloride (1.6 g), and acetic acid (544 μL) were mixed with distilled water up to a volume of 1 L. Chondroitin sulfate A was used as a standard, and a calibration curve was generated at chondroitin sulfate concentrations ranging from 0 to 125 μg/mL. All urine samples were thawed at 4 °C. Standards and samples (8 μL of each) were transferred to a 96-well plate and 200 μL DMMB reagent was added to each well using a multichannel pipettor. The absorbance at 525 nm was immediately determined using a plate reader, and the GAG concentration in each urine sample was calculated based on the chondroitin sulfate calibration curve.
Data Analysis
The data analysis was performed on Thermo Xcalibur software. In addition, the disaccharides were quantified via comparison of the peak area to that of an external standard and normalized with urinary creatinine data.
RESULTS AND DISCUSSION
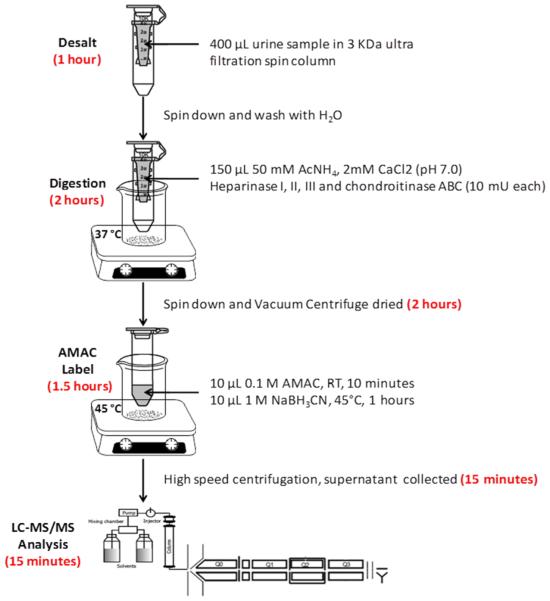
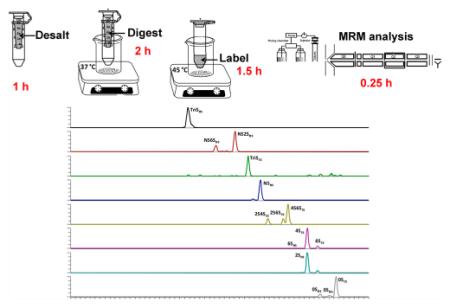
Urine samples were collected from critically ill trauma patients and were stored frozen until they were analyzed. In addition, frozen normal human urine samples, pooled and from individuals, were also defrosted immediately before analysis. The analytical workflow for determining GAG content and composition was optimized to minimize the number of analytical steps (Figure 1). In this approach, GAGs are desalted and enzymatically digested with heparin lyases I, II, III and chondroitin lyase ABC to obtain HS and CS disaccharides; these disaccharides were AMAC-labeled by reductive amination to improve analytical selectivity and separation effciency, and then the AMAC-labeled disaccharides were analyzed by reversed-phase LC-MS/MS. Urine sample preparation steps relied on a single tube for desalting and enzymatic treatment to decrease processing time, reduce sample loss (see Figure S1 in the Supporting Information) and improve digestion effciency. AMAC labeling conditions were optimized to reducing labeling time and improve the labeling effciency (see Figure S2 in the Supporting Information). The entire sample preparation process can be run in parallel on multiple samples and requires 4.75 h.
Figure 1.
Analytical workflow for determining GAG content and composition.
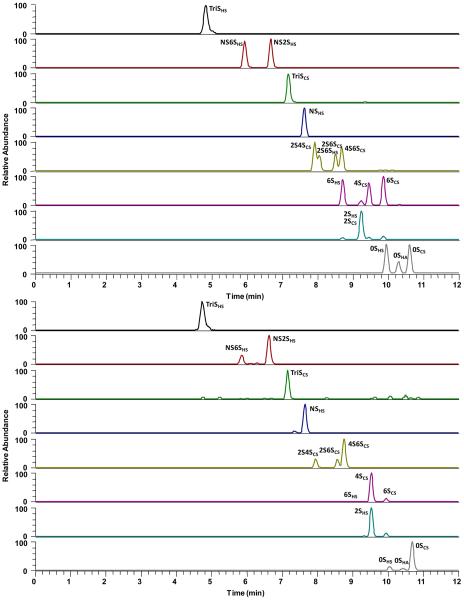
LC chromatography for the separation of AMAC-labeled disaccharides was improved, compared to our previous method,22,40 reducing the separation time from 50 min to 15 min. Thus, the analysis of 12 samples a day was possible, even when relying on serial analysis by LC-MS/MS. The extracted ion chromatograms (EICs) of AMAC-labeled disaccharide standards are shown in Figure 2A. LC separation of the AMAC-labeled HS, HA, and CS disaccharide standards, with the exception of 2SHS and 2SCS, was possible within 15 min (Figure 2A). Next, a urine sample was similarly treated and the EICs for the same AMAC-labeled disaccharides are shown in Figure 2B. A urine sample spiked with GAG standard was used to examine GAG recovery at low, medium, and high GAG concentrations. different amounts of urine were used to determine the GAG recovery linearity of the new sample preparation method, and different amounts of disaccharide standards were used to determine the limit of detection (LOD) and limit of quantification (LOQ for each disaccharide). The LOD and LOQ vary from 0.26 and 0.85 ng/mL, respectively, for the unsulfated disaccharides (0SHS and 0SCS) to 2.53 and 8.4 ng/mL, respectively, for TriSHS (see Table 2). This difference in LOD and LOQ primarily results from the reduced ionization effciency of more highly sulfated disaccharides.
Figure 2.
Extracted ion chromatograms (EICs) of AMAC-labeled disaccharides. (A) Analysis of AMAC-labeled disaccharide standards; the sixth and seventh EICs show small, unlabeled peaks, corresponding to minor fragments produced by monosulfated precursor ions. (B) Disaccharides formed from GAGs in a urine sample; the small, unlabeled peaks in the third EIC are noise resulting from the extremely low concentration of TriSCS. The seventh EIC shows a small, unlabeled peak corresponding to minor fragments produced by monosulfated precursor ions.
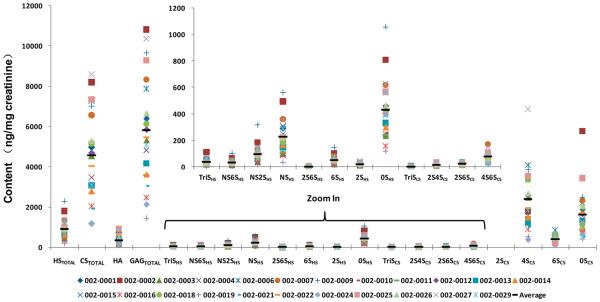
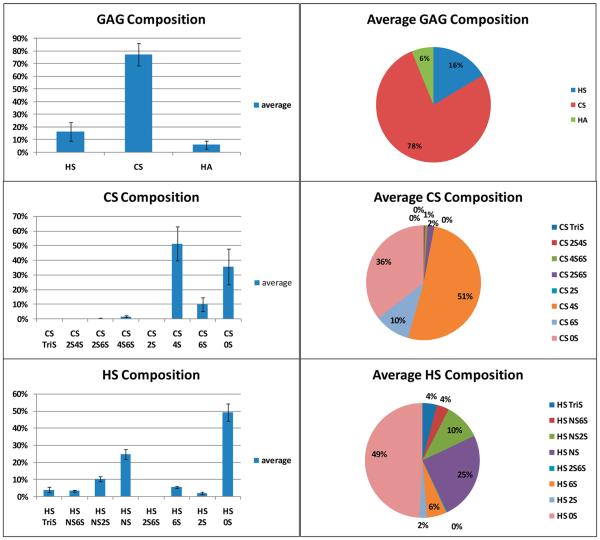
The GAG compositions on 5 commercially purchased normal human urine samples and 23 urine samples from trauma patients were examined next. These patients had been admitted to the hospital surgical ICU for severe trauma, including automobile accidents, skiing accidents, gunshot wounds, etc. Within 24 h of admission, urine samples were collected and frozen. LC-MS/MS analysis was performed on each sample and the total HS, CS, and HA content, as well as the HS and CS disaccharide composition (ng/mL) in urine, was determined (see Tables S1 and S4 in the Supporting Information). To control for urinary dilution (as a consequence of fluid resuscitation, diuresis, etc.), GAG content was normalized to urine creatinine. Normalization to urine creatinine is a standard approach to control for urinary dilution.42,43 The raw total GAG content was highly variable, ranging from 580 ng/mL to 11 198 ng/mL (average of 5149 ng/mL); normalization to urine creatinine decreased this range to 1430–10 808 ng/mg creatinine (average of 5818 ng/mg creatinine) (see Tables S2 and S5 in the Supporting Information). The creatinine-normalized data for each urine sample are shown in Figure 3. CS (4563 ng/mg creatinine) was the predominant GAG, followed by HS (901 ng/mg creatinine) and HA (354 ng/mg creatinine), with a total GAG content of 5818 ng/mg creatinine. Thus, the vast majority (78%) of the GAG in human urine was CS with much smaller quantities of HS (16%) and HA (6%) (Figure 4 and Figure S3 in the Supporting Information). Next, the disaccharide composition of urinary HS and CS were examined (Figures 3 and 4).
Figure 3.
Composition of urinary GAGs, HS, CS, and HA and the disaccharide composition of urinary HS and CS. Each of 23 urine samples and the average value are provided in terms of ng/mg of creatinine. The inset is the enlargement, zooming in on the minor components in the main figure.
Figure 4.
Average percentage composition of urinary GAGs, HS, CS, and HA, and the disaccharide composition of urinary HS and CS.
CS was comprised primarily (51%) of the 4S, followed closely by the 0S (36%) and 6S (10%) disaccharides (Figure 4). Four of the five remaining disaccharides comprised 3% of urinary CS and no 2S was observed in any of the 23 urine samples (see Tables S1 and S2 in the Supporting Information). The absence of 2SCS disaccharide in urine, first demonstrated using a 50 min LC separation,40 allowed the use of a rapid (15 min) LC separation speeding sample analysis. It is noteworthy that bikunin, which is a CS proteoglycan drug prepared from normal human urine and is used to treat acute pancreatitis,44 contains a single CS chain comprised of 0S (~66%) and 4S (~33%) disaccharide units.17,23,45–47 These results suggest that bikunin comprises only a portion of the CS present in human urine.
HS was comprised primarily of 0S (49%), NS (25%), and NS2S (10%) disaccharides. All of the five remaining disaccharides could be detected and comprised the remaining 16% of the HS composition.
In the past, the normal levels of human urinary GAGs have been evaluated primarily by DMMB colorimetric assay. Examination of these samples by DMMB assay gave total urinary GAG levels that paralleled those obtained using LC-MS/MS analysis (see Tables S1–S5 in the Supporting Information). Differences between the absolute amounts obtained by the two methods arise from the insensitivity of DMMB assay to small or uncharged GAGs and the presence of interfering substances present in urine, such as sulfated metabolites (see Figure S4 in the Supporting Information). This study provides the first detailed look at the disaccharide composition and distribution of these GAGs. Most prior studies14,29,31,33,35 have only evaluated GAGs present at greatly elevated levels in patients suffering from MPS. Moreover, since <1 μg of GAG might be present in 1 mL of a normal urine sample, a more effcient method of GAG isolation, as well as a more sensitive method of GAG detection, than that used in previous studies of MPS patients with high urinary GAG levels was required. MRM detection of GAG disaccharides also affords the disaccharide compositional analysis of the HS and CS present in a urine sample. The current study offers a method that is suffciently sensitive to study changes in urinary GAG levels and compositions resulting from diseases outside of MPS such as inflammatory reactions48 and cancers.25 Future studies will be directed at applying the current method to such patients.
Supplementary Material
ACKNOWLEDGMENTS
The work was supported by Grants from the National Institutes of Health in the form of Grant Nos. HL125371, GM38060, GM090127, HL096972, and HL10172. Sample collection was funded through NIH/NCATS Colorado CTSI UL1 TR000154. X.S. was supported by the Shandong University Scholarship Program.
Footnotes
Supporting Information
The GAG content and composition of the individual urine samples are presented as Tables S1–S5 and Figure S3 in the Supporting Information. Control experiments examining sample preparation and labeling methods are presented in Figures S1 and S2 in the Supporting Information. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.5b00913.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Iozzo RV, Schaefer L. Matrix Biol. 2015 doi: 10.1016/j.matbio.2015.02.003. DOI: 10.1016/j.matbio.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Rowlands D, Sugahara K, Kwok JC. Molecules. 2015;20:3527–3548. doi: 10.3390/molecules20033527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Stewart MD, Sanderson RD. Matrix Biol. 2014;35:56–59. doi: 10.1016/j.matbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Linhardt RJ, Toida T. Acc. Chem. Res. 2004;37:431–438. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- (5).Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A. Biochem. Biophys. Acta. 2014;1840:2452–2459. doi: 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- (6).Lindahl U. Matrix Biol. 2014;35:3–7. doi: 10.1016/j.matbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- (7).Mikami T, Kitagawa H. Biochem. Biophys. Acta. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- (8).Esko JD, Selleck SB. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- (9).Capila I, Linhardt RJ. Angew. Chem., Int. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- (10).Kaneiwa T, Mizumoto S, Sugahara K, Yamada S. Glycobiology. 2010;20:300–309. doi: 10.1093/glycob/cwp174. [DOI] [PubMed] [Google Scholar]
- (11).Peterson S, Liu JJ. Biol. Chem. 2012;287:34836–34843. doi: 10.1074/jbc.M112.390161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rosen SD, Lemjabbar-Alaoui H. Expert Opin. Ther. Targets. 2010;14:935–949. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Saad OM, Ebel H, Uchimura K, Rosen SD, Bertozzi CR, Leary JA. Glycobiology. 2005;15:818–826. doi: 10.1093/glycob/cwi064. [DOI] [PubMed] [Google Scholar]
- (14).Lawrence R, Brown JR, Lorey F, Dickson PI, Crawford BE, Esko JD. Mol. Genet. Metab. 2014;111:73–83. doi: 10.1016/j.ymgme.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rabenstein DL. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- (16).Feizi T, Mulloy B. Curr. Opin. Struct. Biol. 2003;3:602–604. doi: 10.1016/j.sbi.2003.09.001. [DOI] [PubMed] [Google Scholar]
- (17).Lamkin E, Cheng G, Calabro A, Hascall VC, Joo EJ, Li L, Linhardt RJ, Lauer ME. J. Biol. Chem. 2015;290:5156–5166. doi: 10.1074/jbc.M114.636258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Leymarie N, McComb ME, Naimy H, Staples GO, Zaia J. Int. J. Mass Spectrom. 2012;312:144–154. doi: 10.1016/j.ijms.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Shao C, Shi X, Phillips JJ, Zaia J. Anal. Chem. 2013;85:10984–10991. doi: 10.1021/ac402517s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang Z, Li D, Sun X, Bai X, Jin L, Chi L. Anal. Biochem. 2014;451:35–41. doi: 10.1016/j.ab.2014.02.005. [DOI] [PubMed] [Google Scholar]
- (21).Li D, Chi L, Jin L, Xu X, Du X, Ji S, Chi L. Carbohydr. Polym. 2014;99:339–344. doi: 10.1016/j.carbpol.2013.08.074. [DOI] [PubMed] [Google Scholar]
- (22).Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ. J. Chromatogr. A. 2012;225:91–98. doi: 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ly M, Laremore TN, Linhardt RJ. Omics. 2010;4:389–399. doi: 10.1089/omi.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Nat. Rev. Cancer. 2002;2:521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- (25).Weyers A, Yang B, Yoon DS, Park J-H, Zhang F, Lee KB, Linhardt RJ. Omics. 2012;16:79–89. doi: 10.1089/omi.2011.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bodamer OA, Giugliani R, Wood T. Mol. Genet. Metab. 2014;113:34–41. doi: 10.1016/j.ymgme.2014.07.013. [DOI] [PubMed] [Google Scholar]
- (27).Beasley GTN, Wraith JE. Curr. Paediat. 1997;7:128–134. [Google Scholar]
- (28).de Jong JGN, Wevers RA, Laarakkers C, Poorthuls BJHM. Clin. Chem. 1989;25:1472–1477. [PubMed] [Google Scholar]
- (29).Zhang H, Wood T, Young SP, Millington DS. Mol. Genet. Metab. 2015;114:123–128. doi: 10.1016/j.ymgme.2014.09.009. [DOI] [PubMed] [Google Scholar]
- (30).Przybylski C, Gonnet F, Buchmann W, Daniel J. Mass Spectrom. 2012;47:1047–58. doi: 10.1002/jms.3052. [DOI] [PubMed] [Google Scholar]
- (31).Chuang CK, Lin HY, Wang TJ, Tsai CC, Liu HL, Lin SP. Orphanet J. Rare Dis. 2014;9:135. doi: 10.1186/s13023-014-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zhang H, Young SP, Millington DS. Curr. Protoc. Hum. Genet. 2013;17:17.12. doi: 10.1002/0471142905.hg1712s76. [DOI] [PubMed] [Google Scholar]
- (33).Auray-Blais C, Bhérer P, Gagnon R, Young SP, Zhang HH, An Y, Clarke JT, Millington DS. Mol. Genet. Metab. 2011;102:49–56. doi: 10.1016/j.ymgme.2010.09.003. [DOI] [PubMed] [Google Scholar]
- (34).Auray-Blais C, Lavoie P, Zhang H, Gagnon R, Clarke JT, Maranda B, Young SP, An Y, Millington DS. Clin. Chim. Acta. 2012;413:771–778. doi: 10.1016/j.cca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- (35).Tomatsu S, Kubaski F, Sawamoto K, Mason RW, Yasuda E, Shimada T, Montaño AM, Yamaguchi S, Suzuki Y, Orii T. Nihon Masu Sukuriningu Gakkai Shi. 2014;24:19–37. [PMC free article] [PubMed] [Google Scholar]
- (36).Tomatsu S, Montaño AM, Oguma T, Chi Dung V, Oikawa H, Gutiérrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Barrera LA, Kida K, Kubota M, Orii T. Mol. Genet. Metab. 2010;99:124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- (37).Tomatsu S, Shimada T, Mason RW, Kelly J, LaMarr WA, Yasuda E, Shibata Y, Futatsumori H, Montaño AM, Yamaguchi S, Suzuki Y, Orii TJ. Anal. Bioanal. Technol. 2014:006. doi: 10.4172/2155-9872.S2-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).deRu MH, van der Tol L, van Vlies N, Bigger BW, Hollak CEM, lst LIJ, Kulik W, van Lenthe H, Saif MA, Wagemans T, van der Wal WM, Wanders RJ, Wijburg FAJ. Inherited Metab. Dis. 2013;36:247–255. doi: 10.1007/s10545-012-9538-2. [DOI] [PubMed] [Google Scholar]
- (39).Nielsen TC, Rozek T, Hopwood JJ, Fuller M. Anal. Biochem. 2010;402:113–120. doi: 10.1016/j.ab.2010.04.002. [DOI] [PubMed] [Google Scholar]
- (40).Li G, Li L, Tian F, Zhang L, Xue C, Linhardt RJ. ACS Chem. Biol. 2015;10:1303–1310. doi: 10.1021/acschembio.5b00011. [DOI] [PubMed] [Google Scholar]
- (41).Su H, Blain F, Musil RA, Zimmermann JJ, Gu K, Bennett DC. Appl. Environ. Microbiol. 1996;62:2723–2734. doi: 10.1128/aem.62.8.2723-2734.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Nathan DM, Rosenbaum C, Protasowicki VD. Diabetes Care. 1987;10:414–418. doi: 10.2337/diacare.10.4.414. [DOI] [PubMed] [Google Scholar]
- (43).Ginsberg JM, Chang BS, Matarese RA, Garella SN. Engl. J. Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- (44).Michalski C, Piva F, Balduyck M, Mizon C, Burnouf T, Huart J-J, Mizon J. Vox Sang. 1994;67:329–336. doi: 10.1111/j.1423-0410.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- (45).Chi l., Wolff JJ, Laremore TN, Restaino OF, Xie J, Schiraldi C, Toida T, Amster IJ, Linhardt RJJ. Am. Chem. Soc. 2008;130:2617–2625. doi: 10.1021/ja0778500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Enghild JJ, Thøgersen IB, Cheng F, Fransson LÅ, Roepstorff P, Rahbek-Nielsen H. Biochemistry. 1999;38:11804–11813. doi: 10.1021/bi9908540. [DOI] [PubMed] [Google Scholar]
- (47).Lord MS, Day AJ, Youssef P, Zhuo L, Watanabe H, Caterson B, Whitelock JMJ. Biol. Chem. 2013;288:22930–22941. doi: 10.1074/jbc.M112.404186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, Douglas IS, Linhardt RJJ. Biol. Chem. 2014;289:8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.