Abstract
Background
Postoperative delirium (POD) is a common complication in the elderly. This retrospective study investigated the effect of intraoperative hemodynamics on the incidence of POD in elderly patients after major surgery to explore ways to reduce the incidence of POD.
Material/Methods
Based on the incidence of POD, elderly patients (81±6 y) were assigned to a POD (n=137) or non-POD group (n=343) after elective surgery with total intravenous anesthesia. POD was diagnosed based on the guidelines of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), using the confusion assessment method. The hemodynamic parameters, such as mean arterial pressure, were monitored 10 min before anesthesia (baseline) and intraoperatively. The incidence of intraoperative hypertension, hypotension, tachycardia, and bradycardia were calculated.
Results
At 30 min and 60 min after the initiation of anesthesia and at the conclusion of surgery, the monitored hemodynamic parameter values of the POD group, but not those of the non-POD group, were significantly higher than at baseline. Multivariate logistic regression analysis showed that intraoperative hypertension and tachycardia were significantly associated with POD.
Conclusions
Intraoperative hypertension and tachycardia were significantly associated with POD. Maintaining intraoperative stable hemodynamics may reduce the incidence of POD in elderly patients undergoing surgery.
MeSH Keywords: Hemodynamics, Patient Care, Postoperative Period
Background
Delirium is a disturbance of consciousness and cognition, characterized by altered mental status, inattention, impaired cognition function, abnormal psychomotor behaviors, and disturbance of the sleep-wake cycle [1,2]. Postoperative delirium (POD) usually occurs early after surgery and anesthesia and lasts for a short time with a fluctuating course [3].The incidence of POD ranges from 9% to 87%, and increases with the age of the patient [4–8]. Patients who develop POD have a high risk of many complications such as myocardial infarction, pneumonia, and respiratory failure [9]. The development of POD is associated with higher mortality and morbidity, increased hospitalization, and greater cost [10]. Elderly patients who develop POD have a poor long-term outcome and have the higher risk of institutionalization [10,11]. Therefore, reducing the incidence of POD is important for improving quality of life in elderly patients, and it is important to identify risk factors during surgery and anesthesia to reduce the incidence of POD in elderly patients.
The causes of POD are unclear, but it is generally believed that there are many diverse contributing factors. Some risk factors for developing POD have been identified, including older age, mental disease, poor physical condition, trauma, nutritional deficiency, anxiety, and depression [12,13]. Older age has been identified as an independent risk factor for developing POD [14]. POD is also associated with many diseases such as cerebral hypoxia and ischemia, cardiovascular disease, cardiopulmonary bypass, infection, metabolic disorder, sleep disorders, and drug intoxication with the use of benzodiazepines or anticholinergic drugs [12,15,16]. However, little is known concerning the prevention and management of risk factors for POD in elderly patients undergoing surgery.
Although many treatment strategies have been implemented to reduce the incidence of POD, including perioperative management and pharmacological, psychological, or multicomponent interventions, these treatments have not resulted in satisfactory outcomes [15,17]. Recently, a meta-analysis indicated that lighter anesthesia may reduce the incidence of POD [16]. It is well known that intraoperative low blood pressure is a potential risk factor for developing POD [18]. However, it remains unclear whether other intraoperative hemodynamic parameters changes are associated with the occurrence of POD. In this retrospective study, we investigated the effect of intraoperative hemodynamic changes on POD in elderly patients following surgery to explore a way to reduce the incidence of POD
Material and Methods
Patients
All patients or their relatives provided written informed consent prior to inclusion in the study. This retrospective study included 480 elderly patients (233 men and 247 women) who underwent elective surgery under total intravenous anesthesia between January 2012 and March 2014. The incidence rate for delirium was 28.5%. Their average age was 81±6 y (range, 75–87 y) and their mean body weight was 71.5±17.5 kg (range, 54–89 kg). The inclusion criteria were age ≥75 y; American Society of Anesthesiology class II or III; mini-mental state examination (MMSE) score >23; and >9 y of education. The exclusion criteria were a history of drug abuse; history of neurological or psychiatric diseases or hyperthyroidism; electrolyte disturbance; severe liver or kidney insufficiency; severe visual or hearing impairment; and recent administration of sedatives, antidepressants, or analgesics.
Of the 480 patients, 142 patients underwent radical resection for gastric cancer, 134 underwent radical resection for recto-colonic cancer, 137 underwent radical resection for lung cancer, and 67 underwent thoracic or lumbar discectomy. The patients were assigned to 2 groups based on the presence of POD: the POD group (n=137) and the non-POD group (n=343).
Cognitive function assessment
MMSE was administered as routine to assess cognitive functions after hospital admission. The assessed cognitive functions included time orientation (5 points, maximum) and spatial orientation (5 points); short-term memory (3 points) and delayed memory (3 points); language function, including naming (2 points), repeating (1 point), and writing (1 point); executive function (5 points); and calculation (5 points). The total maximum score was 30. A MMSE score >23 points was defined as normal cognition function [19]. Patients with MMSE score ≤23 were excluded from this study.
Anesthesia procedure
All patients were deprived of food for 8 h and water for 2 h before surgery, and did not receive any drugs before the procedure. Electrocardiography, heart rate (HR), blood pressure (BP), and blood oxygen saturation (SpO2) were monitored as routine in the operating room. Prior to anesthesia, the catheters (Becton Dickinson Critical Care Systems, Singapore) were implanted into the radial artery and internal jugular vein for monitoring the invasive arterial BP and central venous pressure (CVP), respectively.
All patients underwent total intravenous anesthesia after tracheal intubation. Anesthesia was induced by intravenous injections of fentanyl (4 μg/kg; Yichang Humanwell Pharmaceutical, China; Lot No. 2110606), propofol (2 mg/kg; Xian Libang Pharmaceutical, China; Lot No. 1203071), and cisatracurium (0.3 mg/kg; JiansuHengRui Medicine, China; Lot No. 12021917). The trachea was intubated under direct vision, and connected to a mechanical ventilator attached to an anesthesia machine. The ventilator settings were: frequency, 12–14 beats/min; inspiratory-to-expiratory ratio, 1: 2; tidal volume, 8–10 mL/kg; minute volume, 100–120 mL/kg; fraction of inspired oxygen, 40–60%; and end-tidal carbon dioxide partial pressure (PETCO2), 35–45 mmHg.
Fentanyl (2 μg/kg) was intravenously injected 5 min before the skin was incised. Anesthesia was maintained with continuous infusion of propofol (3–5 mg·kg−1·h−1), cisatracurium (1–3 μg·kg−1·min−1), and fentanyl (2 μg·kg−1·h−1). The continuous intravenous infusion rate of propofol and fentanyl was adjusted based on each patient’s vital signs and bispectral index (BIS). Blood pressure was controlled to ≤20% of baseline (10 min before the initiation of anesthesia), if BP was greater or less than 30% of baseline (10 min before the initiation of anesthesia), the vasoactive drugs (continuous infusion of nitroglycerin or methoxamine) were given to maintain the stability of circulation. The BIS value was maintained between 40 and 60 [20]. During surgery, sodium lactate Ringer’s solution and 6% hydroxyethyl starch (130/0.4 in 0.9% sodium chloride; Voluven; Fresenius KabiNorge A.S., Halden, Norway) were administered intravenously at an infusion rate of 10 mL·kg−1·h−1.
After surgery, all drugs were immediately stopped, and patients received patient-controlled intravenous analgesia (PCIA) using a PCIA pump (Zhuhai Fornia Medical, China) in the operating room. Patients were transferred to the Post Anesthesia Care Unit (PACU) and monitored until they had a BIS value >90, full awareness, recovery of muscle strength, normal SpO2, removal of tracheal intubation, stable vital signs, and Steward recovery score ≥4. The Steward recovery score was assessed for consciousness (0 for no consciousness, 1 for responding to stimuli, 2 for full consciousness); airway (0 for airway requiring maintenance, 1 for maintaining good airway, 2 for coughing on command); and movement (0 for no movement, 1 for no purposeful movement, 2 for purposeful movement) [21].
Anesthesia monitoring
The patients’ electrocardiography, mean arterial pressure (MAP), HR, SpO2, cardiac output (CO), stroke volume (SV), cardiac index (CI), and CVP were monitored using a Mindray Beneview T8 monitor (Shenzhen Mindray Bio-Medical Electronics, China) and a Flotrac Vigileo monitoring system (Edwards Life Sciences, U.S.A.). BIS was monitored using a HXD-1 multifunctional electroencephalogram monitor (Heilongjiang Huaxiang Technical Developing, Harbin, China). MAP, HR, rate pressure product (RPP; the product of HR and systolic blood pressure), CVP, SpO2, CO, SV, CI and BIS were recorded at 10 min before (baseline) and 10 min after the initiation of anesthesia, 30 min and 60 min after anesthesia, and the conclusion of surgery. The duration of surgery, recovery time from anesthesia, the incidence of intraoperative hypertension (BP was greater or less than 30% of baseline), hypotension (BP <30% of baseline), tachycardia (HR >100 bpm), and bradycardia (HR <60 bpm), and intraoperative dosages of fentanyl and propofol were calculated.
Postoperative analgesia management
After recovery of consciousness, all patients were provided with PCIA by fentanyl. The PCIA was programmed to deliver a loading dose of 0.3 μg/kg fentanyl, with a lockout of 15 min, background infusion of 0.1 μg·kg−1·h−1, and single dose of 0.1 μg/kg for 48 h.
Postoperative pain intensity and comfort level were evaluated 1, 6, 12, 18, 24, 36, and 48 h after surgery. Pain intensity was assessed using the visual analogue scale (VAS) [22], which ranged from 0 (no pain) to 10 (worst possible pain).
The comfort level was evaluated using the Bruggemann Comfort Scale (BCS) [23] as follows: 0 for persistent pain; 1 for no pain at rest but severe pain during deep breath or cough; 2 for no pain at rest but mild pain during deep breath or cough; 3 for no pain during deep breath; and 4 for no pain during cough.
POD assessment
Surgeons closely observed changes in patients’ condition within one week after surgery. When any suspicious symptoms of POD were observed, neurologists were immediately consulted to evaluate the condition of the patient 1–3 days after operation. POD was diagnosed with symptoms of both acute onset of altered and fluctuating mental status and inattention, and either disorganized thinking or altered level of consciousness, using the confusion assessment method [3,20].
Statistical analyses
Statistical analyses were performed using SPSS 15.0 software (SPSS, Chicago, IL, USA). Numerical values are presented as the mean and standard deviation. Repeated-measures analysis of variance (ANOVA) was used to compare differences within the same group. One-way ANOVA was used to compare differences between groups. Categorical data were compared using the chi-squared test. The multivariate logistic regression model was used to evaluate the association between POD and the incidence of intraoperative hypertension, hypotension, tachycardia, and bradycardia. P-values <0.05 were considered statistically significant.
Results
There were no significant differences in gender, age, body weight, or ASA score between the POD and non-POD groups (P>0.05; Table 1). The preoperative MMSE score in the POD group was not significantly different from that of the non-POD group (P>0.05). The percentage of complications such as hypertension and coronary disease, diabetes, and pulmonary disease did not differ significantly between the 2 groups (P>0.05).
Table 1.
Demographic and clinical features patients in the POD and non-POD groups.
| POD | Non-POD | |
|---|---|---|
| Subjects | 137 | 343 |
| Genders, Male/female, n | 65/72 | 168/175 |
| Age, y | 81.5±5.5 | 80.5±5.5 |
| Body weight, kg | 71.5±17.5 | 72.3±15.5 |
| ASA, II/III, n | 97/40 | 244/99 |
| Preoperative MMSE score | 27.6±2.1 | 27.4±2.0 |
| Hypertension and coronary disease, % | 41.6 | 43.1 |
| Diabetes, n | 32.1 | 29.7 |
| Pulmonary disease, n | 26.3 | 25.9 |
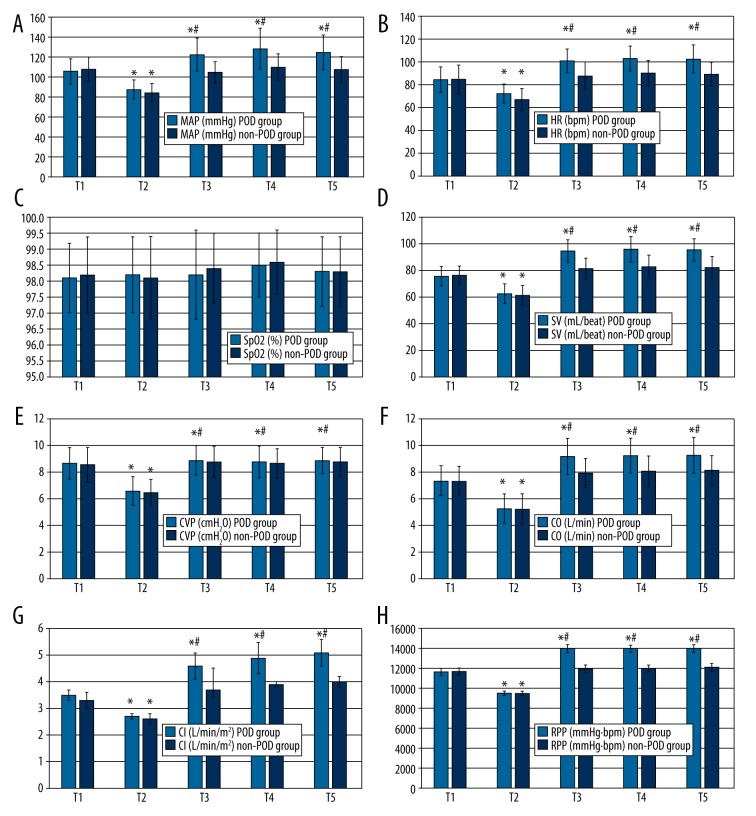
The hemodynamic parameters of patients in the POD group and non-POD groups were measured at 10 min before the initiation of anesthesia (baseline), at 10, 30, and 60 min after the initiation of anesthesia, and at the conclusion of surgery (Figure 1A–1H). For both groups, at 10 min after the initiation of anesthesia the MAP, HR, RPP, CVP, CO, SV, and CI values were significantly lower than at the baseline timepoint (P<0.05).
Figure 1.
MAP (A), HR (B), SpO2 (C), SV (D), CVP (E), CO (F), CI (G), and RPP (H) in patients in the POD and non-POD groups. Data are expressed as the mean ± standard error of the mean. n=137 for the POD group. n=343 for the non-POD group. * P<0.05 cf. T1; # P<0.05 cf. non-POD group. T1, 10 min before the initiation of anesthesia (baseline); T2, 10 min after the initiation of anesthesia; T3, 30 min after the initiation of anesthesia; T4, 60 min after the initiation of anesthesia; T5, conclusion of surgery.
In the POD group, 30 and 60 min after the initiation of anesthesia and at the conclusion of surgery, the MAP, HR, RPP, CVP, CO, SV, and CI values were significantly higher than the corresponding measurements at baseline (P<0.05). However, in the non-POD group, 30 min and 60 min after the initiation of anesthesia and at the conclusion of surgery, the MAP, HR, RPP, CO, SV, and CI values were not significantly different from the baseline readings (P>0.05).
Thirty minutes and 60 min after the initiation of anesthesia and at the conclusion of surgery, the MAP, HR, RPP, CO, SV, and CI values were significantly higher in the POD group than in the non-POD group (P<0.05). The SpO2, CVP, and PETCO2 values were within normal range, and BIS values were maintained between 40 and 60 for both groups during the surgeries.
There were no significant differences in the duration of surgeries, recovery time, BIS, intraoperative dosages of fentanyl and propofol, or postoperative dosage of fentanyl between the 2 groups (P>0.05; Table 2).
Table 2.
Operative time, recovery time, intraoperative dosage of anesthetics, and postoperative dosage of analgesics in the POD and non-POD groups.
| POD | Non-POD | P-value | |
|---|---|---|---|
| Subjects, n | 137 | 343 | |
| Operative time, min | 182.4±31.7 | 190.3±32.6 | 0.083 |
| Recovery time, min | 18.2±6.2 | 16.9±5.8 | 0.102 |
| Intraoperative fentanyl, mg | 0.51±0.07 | 0.53±0.05 | 0.135 |
| Propofol, mg | 1197.3±82.5 | 1209.6±84.2 | 0.073 |
| Postoperative fentanyl, mg | 0.66±0.05 | 0.64±0.03 | 0.131 |
| BIS | 55.5±3.4 | 56.2±3.1 | 0.079 |
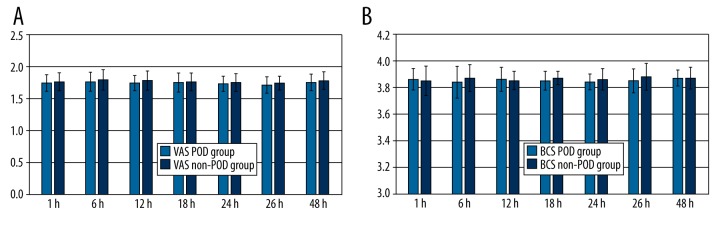
There were no significant differences in the VAS or BCS scores at any timepoint after surgery (1, 6, 12, 18, 24, 36, or 48 h) between the 2 groups (P>0.05; Figure 2A, 2B).
Figure 2.
VAS (A) and BCS (B) scores at 1, 6, 12, 18, 24, 36, or 48 h after surgery in patients in the POD and non-POD groups. Data are expressed the mean ± standard error of the mean. n=137 for the POD group, n=343 for the Non-POD group.
The incidence of intraoperative hypertension and tachycardia were significantly higher in the POD group than in the non-POD group (P<0.05; Table 3). We further used the multivariate logistic regression model to determine associations between POD and the incidence of intraoperative hypertension, hypotension, tachycardia, and bradycardia. Based on this analysis, the incidence of intraoperative hypertension and tachycardia was significantly associated with POD (P<0.05, Table 3).
Table 3.
Multivariate logistic regression analysis of the intraoperative hemodynamic risk factors for POD, n (%).
| POD | Non-POD | OR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Subjects | 137 | 343 | – | – | – |
| Hypertension | 75 (54.7) | 91 (26.5.) | 2.832 | 1.572–6.157 | 0.042 |
| Hypotension | 8 (5.8) | 21 (6.1) | 1.471 | 0.583–2.354 | 0.851 |
| Tachycardia | 42 (30.7) | 53 (14.3) | 2.735 | 1.546–6.131 | 0.045 |
| Bradycardia | 6 (4.4) | 20 (5.8) | 0.989 | 0.915–1.045 | 0.747 |
Discussion
POD commonly occurs in elderly patients after major surgery, and is associated with increased mortality and morbidity, longer hospitalization, and greater medical cost [9,24,25]. Although the causes of POD have not been clearly elucidated, it is believed that it may be the consequence of many predisposing and precipitating factors [26]. Older age is a well-known risk factor for developing POD [14], but since aging cannot be countered it is therefore important to identify controllable risk factors that can be controlled. Patients older than 70 years commonly have poor physiological vital organ reserve, are more sensitive to drugs, and are often complicated with many diseases such as hypertension, coronary heart disease, diabetes, and pulmonary disease, and thereby have lower tolerance to surgery and anesthesia. Stable control of intraoperative hemodynamics must be carefully maintained to meet myocardial oxygen supply and demand balance during surgery for elderly patients.
In this study, we investigated the effect of intraoperative changes in the hemodynamic parameters on the incidence of POD in 480 elderly patients, 137 of whom experienced POD. Multivariate logistic regression analysis showed that the incidence of intraoperative hypertension and tachycardia was significantly associated with the occurrence of POD, indicating that stable control of intraoperative hemodynamics may reduce the incidence of POD in elderly patients undergoing surgery. In addition, BP, HR, and myocardial oxygen consumption increased in response to stress responses caused by surgery and anesthesia, which may worsen heart diseases that often exist in elderly patients. In the present study, during surgery, blood pressure was controlled to ≤20% of baseline (10 min before the initiation of anesthesia). If BP was greater or less than 30% of baseline, vasoactive drugs were given to maintain the stability of circulation. Stable control of intraoperative hemodynamics can reduce the cardiovascular and cerebrovascular accident.
The mechanisms that underlie POD associated with hemodynamic instability remain unclear. It has been reported that the peripheral release of inflammatory cytokines such as interleukin-1β, tumor necrosis factor-α, and interleukin-6 in response to surgical trauma can pass the blood brain barrier. This can result in the activation of astrocytes and microglia in the brain, leading to further release of neurotoxic inflammatory mediators that can subsequently induce delirium [27,28]. Activation of the cholinergic anti-inflammatory pathway can inhibit the peripheral release of inflammatory cytokines and suppress the neuroinflammatory response [29]. In a surgical stress rat model, acetylcholinesterase inhibitors that increase acetylcholine levels inhibited the peripheral protein expression of interleukin-1β and tumor necrosis factor-α and reduced neuroinflammation and degeneration in the cortex and hippocampus [30].
In the present study, increased MAP, HR, RPP, CO, SV, and CI found in the POD patients may have led to sympathetic activation and parasympathetic inhibition that subsequently resulted in a decreased release of acetylcholine. Decreased release of acetylcholine may weaken cholinergic anti-inflammation activity and result in increased peripheral release of inflammatory cytokines, enhanced neuroinflammation, and subsequent delirium. In addition, Longas et al. [31] reported that general anesthesia increased the blood levels of IL-6. Hadimioglu et al. [32] reported that the TNF-α levels were significantly less after general anesthesia and epidural anesthesia compared with the baseline, suggesting that the depth of general anesthesia may protect against inflammatory factors that contribute to POD. Therefore, too-light anesthesia may contribute to POD. We could not exclude the possibility that light anesthesia may have contributed to the POD in elderly patients in the present study.
In the present study, the SpO2, CVP, and PETCO2 values were within normal range and the BIS value was maintained between 40 and 60 for both groups during the surgeries. We excluded patients with preoperative or intraoperative use of benzodiazepines or anticholinergic drugs, thus reducing the possibility that these precipitating factors contributed to the development of POD [16,26,33,34].
In the present study, we found no significant differences between the POD and non-POD groups with regard to gender, age, body weight, ASA scores, preoperative MMSE scores, preexisting diseases, operative time, recovery time from anesthesia, or intraoperative dosages of propofol. These findings suggest that these factors did not significantly contribute to the occurrence of POD. In addition, we found no significant differences in intraoperative dosages of fentanyl and postoperative dosage of fentanyl between the POD and non-POD groups, suggesting that fentanyl did not significantly contribute to the occurrence of POD. However, Tokita et al. [35] reported that fentanyl produced a good analgesic effect and reduced the incidence of POD. The difference may be due to the different dosage of fentanyl used between the 2 studies.
Vaurio et al. [36] reported that postoperative pain was a risk factor for developing POD, and effective control of pain reduced the incidence of POD. In the present study, all patients achieved satisfactory analgesic outcomes, and there were no significant differences in the VAS and BCS scores at any timepoint between the POD and non-POD groups, suggesting that postoperative pain may not be a major contributor to the incidence of POD in elderly patients undergoing major surgery. Sieber et al. [13] reported that light sedation decreased the prevalence of postoperative delirium by 50% compared with deep sedation (BIS, approximately 50), indicating that deep sedation was a risk factor for developing POD. In the present study, the BIS values in the POD group and non-POD groups were 55.5±3.4 and 56.2±3.1, respectively. There was no significant difference in the BIS values between the 2 groups, suggesting that sedation (BIS >50) may not be a major contributor to the incidence of POD in elderly patients undergoing major surgery.
The incidence of POD varies greatly among reports in the literature, ranging from 9% to 87%, and increases with the age of the patient [4–8]. In this study, the incidence of POD was 28.5%. Since neurologists were only called if patients were suspected of having POD, the incidence of POD may be higher among the patients examined. In addition, in the majority of patients with delirium, the condition was hypoactive, and thus there was a higher risk of it going undiagnosed. Therefore, some patients with hypoactive delirium may have been missed.
Conclusions
We investigated the effect of intraoperative hemodynamics on the incidence of POD in elderly patients undergoing major surgery, and found that the MAP, HR, RPP, CO, SV, and CI values were significantly higher in POD patients than in non-POD patients. Multivariate logistic regression analysis found that the incidence of intraoperative hypertension and tachycardia was significantly associated with POD in elderly patients. Our study supports that maintaining intraoperative stable hemodynamics may reduce the incidence of POD in elderly patients undergoing surgery.
Acknowledgements
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Footnotes
Source of support: Departmental sources
References
- 1.Diagnostic and statistical manual of mental disorders. 4th Edition. 1994. (DSM-IV) [Google Scholar]
- 2.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–78. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 3.Diagnostic and statisticalmanual of mental disorders IV-TR edition 2000
- 4.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 5.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–59. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011;77:448–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Guenther U, Radtke FM. Delirium in the postanaesthesia period. Curr Opin Anaesthesiol. 2011;24:670–75. doi: 10.1097/ACO.0b013e32834c7b44. [DOI] [PubMed] [Google Scholar]
- 8.Krenk L, Rasmussen LS. Postoperative delirium and postoperative cognitive dysfunction in the elderly – what are the differences? Minerva Anestesiol. 2011;77:742–49. [PubMed] [Google Scholar]
- 9.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54:1578–89. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 10.Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26:26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 11.Kat MG, Vreeswijk R, de Jonghe JF, et al. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord. 2008;26:1–8. doi: 10.1159/000140611. [DOI] [PubMed] [Google Scholar]
- 12.Noimark D. Predicting the onset of delirium in the post-operative patient. Age Ageing. 2009;38:368–73. doi: 10.1093/ageing/afp024. [DOI] [PubMed] [Google Scholar]
- 13.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HB, Mears SC, Rosenberg PB, et al. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59:2306–13. doi: 10.1111/j.1532-5415.2011.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies for delirium in the intensive care unit. Psychosomatics. 2012;53:203–11. doi: 10.1016/j.psym.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Moyce Z, Rodseth RN, Biccard BM. The efficacy of peri-operative interventions to decrease postoperative delirium in non-cardiac surgery: a systematic review and meta-analysis. Anaesthesia. 2014;69:259–69. doi: 10.1111/anae.12539. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Lu Y, Liu M, et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17:R47. doi: 10.1186/cc12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tognoni P, Simonato A, Robutti N, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch Gerontol Geriatr. 2011;52:e166–69. doi: 10.1016/j.archger.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Anthony JC, LeResche L, Niaz U, et al. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol Med. 1982;12:397–408. doi: 10.1017/s0033291700046730. [DOI] [PubMed] [Google Scholar]
- 20.Wahab AA, Janahi IA, Marafia MM. Pseudo-Bartter’s syndrome in an Egyptian infant with cystic fibrosis mutation N1303K. J Trop Pediatr. 2004;50:242–44. [PubMed] [Google Scholar]
- 21.Steward DL. Pantoprazole for sleepiness associated with acid reflux and obstructive sleep disordered breathing. Laryngoscope. 2004;114:1525–28. doi: 10.1097/00005537-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rauh KH, Andersen RS, Rosenberg J. [Visual analogue scale for measuring post-operative pain]. Ugeskr Laeger. 2013;175:1712–16. [in Danish] [PubMed] [Google Scholar]
- 23.Vasseur E, Gibbons J, Rushen J, de Passille AM. Development and implementation of a training program to ensure high repeatability of body condition scoring of dairy cows. J Dairy Sci. 2013;96:4725–37. doi: 10.3168/jds.2012-6359. [DOI] [PubMed] [Google Scholar]
- 24.Veiga D, Luis C, Parente D, et al. Postoperative delirium in intensive care patients: risk factors and outcome. Rev Bras Anestesiol. 2012;62:469–83. doi: 10.1016/S0034-7094(12)70146-0. [DOI] [PubMed] [Google Scholar]
- 25.Brooks P, Spillane JJ, Dick K, Stuart-Shor E. Developing a strategy to identify and treat older patients with postoperative delirium. AORN J. 2014;99:257–73. doi: 10.1016/j.aorn.2013.12.009. quiz 274–76. [DOI] [PubMed] [Google Scholar]
- 26.Steiner LA. Postoperative delirium. Part 1: pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28:628–36. doi: 10.1097/EJA.0b013e328349b7f5. [DOI] [PubMed] [Google Scholar]
- 27.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–75. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 28.Cerejeira J, Firmino H, Vaz-Serra A, et al. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–54. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 29.Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem Soc Trans. 2006;34:1037–40. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 30.Kalb A, von Haefen C, Sifringer M, et al. Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS One. 2013;8:e62679. doi: 10.1371/journal.pone.0062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longas Valien J, Guerrero Pardos LM, et al. Comparison of 4 techniques for general anesthesia for carotid endarterectomy: inflammatory response, cardiocirculatory complications, and postoperative analgesia. Rev Esp Anestesiol Reanim. 2004;51:568–75. [PubMed] [Google Scholar]
- 32.Hadimioglu N, Ulugol H, Akbas H, et al. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transplant Proc. 2012;44:2949–54. doi: 10.1016/j.transproceed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 34.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 35.Tokita K, Tanaka H, Kawamoto M, Yuge O. Patient-controlled epidural analgesia with bupivacaine and fentanyl suppresses postoperative delirium following hepatectomy. Masui. 2001;50:742–46. [PubMed] [Google Scholar]
- 36.Vaurio LE, Sands LP, Wang Y, et al. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102:1267–73. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]