Abstract
Energetic responses of zooxanthellate reef corals along depth gradients have relevance to the refugia potential of mesophotic coral ecosystems (MCEs). Previous observations suggested that MCEs in the Caribbean are thermally buffered during the warmest parts of the year and occur within or just below the chlorophyll maximum, suggesting abundant trophic resources. However, it is not known if mesophotic corals can maintain constant energy needs throughout the year with changing environmental and biological conditions. The energetic content of tissues from the stony coral species Orbicella faveolata and Agaricia lamarcki was measured on the southern insular shelf of St. Thomas, US Virgin Islands (USVI), using micro-bomb calorimetry. Three sites for each species, at depths of 6m, 25m, 38m and 63m, were selected to capture energetic differences across the major vertical range extent of both species in the USVI—and sampled over five periods from April 2013 to April 2014. Mesophotic colonies of O. faveolata exhibited a significant reduction in energetic content during the month of September 2013 compared to mid-depth and shallow colonies (p = 0.032), whereas A. lamarcki experienced similar energetic variability, but with a significant reduction in energy content that occurred in July 2013 for colonies at sites deeper than 25m (p = 0.014). The results of calorimetric analyses indicate that O. faveolata may be at risk during late summer stress events, possibly due to the timing of reproductive activities. The low-point of A. lamarcki energy content, which may also coincide with reproduction, occurs prior to seasonal stress events, indicating contrasting, species-specific responses to environmental variability on MCEs.
Introduction
Dramatic changes in the physical parameters of the ocean are predicted to increase mortality of corals and organisms associated with coral reefs [1–3]. Many of the studies undertaken to elucidate the effects of myriad stressors—including increased temperatures, ocean acidification and anthropogenic disturbance—on corals have suggested that coral reefs are at great risk of loss and possible extinction in the future [4–6]. Recently, a somewhat more positive outlook on the future of coral reefs has been promoted by those investigating the refuge potential of deep, light-dependent coral habitats—referred to as mesophotic coral ecosystems (MCEs) [7–9]. MCEs are defined as reef ecosystems comprised of phototrophic and azooxanthellate scleractinian corals, sponges and macroalgae between 30m and the depth at which light in the water column is too low to sustain photoautotrophy, perhaps as deep as 150m depending on local light attenuation [10–12]. Depth generalist coral species inhabiting both shallow and mesophotic reefs may experience widely variable conditions dependent on location and season [12–13]. Often located offshore, MCEs may experience unique thermal, light, salinity and sedimentation regimes compared to their shallow, nearshore counterparts [11–12]. Deeper water may provide a protective buffer for corals against increased temperature, storm-induced wave action and UV radiation [14–16]. The “deep reef refugia” hypothesis suggests that MCEs sheltered from increased temperature and wave action have the potential to support healthy coral that can provide larvae for the repopulation of degraded shallow water coral ecosystems [7,17].
The potential for MCEs to serve as coral refugia in the face of climate change depends largely on the ability of corals beyond 30m to persist through increasingly prevalent stress events. It has been shown that coral colony energy content can play an important role in the ability of corals to survive and recover from intense stress events [18–24]. Energy content—in this case lipid content—has been used to accurately predict survivorship of laboratory colonies exposed to a range of temperature, light and sedimentation [20]. Not only does energy content at time of bleaching greatly influence the survivorship of corals, the ability of colonies to increase heterotrophic feeding post-bleaching has been shown to increase resilience in at least one coral species subjected to thermally induced bleaching [19, 24]. Corals that are able to supplement reduced autotrophic energy production by suspension feeding on particulate matter may be more likely to survive prolonged bleaching events [21–24]. Heterotrophic plasticity, however, is based on both the coral species in question and the presence of coral food sources in the water column [13, 25–27].
Several different techniques can be used to measure the physiological and energetic status of corals. Along with lipid content and isotopic analyses, measures of tissue biomass and zooxanthellae type and density are widely used to inform the energetic quality and stress susceptibility of corals [18,20,26,28–32]. Another technique not often utilized in modern reef study is coral calorimetry. First applied to corals by Richmond, calorimetry is a direct measure of the total energy within a coral holobiont [33]. The reductive nature of this methodology provides a single measure of energy content that can be easily compared through space and time as well as across species. The technique is limited, however, in that the energy content measured is that of the overall pool of energy in a colony, and does not provide information on the sources of incoming energy (i.e., heterotrophy versus autotrophy) or causes of energy loss. The energy available for growth, reproduction and physiological maintenance is contained within the overall measure of energetic content.
This study assesses seasonal changes in adult coral energetic status and measures seasonal energy changes across the depth ranges of two threatened Caribbean scleractinian corals, Orbicella faveolata and Agaricia lamarcki. We asked if energy content varied between shallow and mesophotic zones and whether this might influence the potential tolerance of these coral species to stress events. Five coral collections were made between April 2013 and April 2014 across three depths representing the primary habitat range for each species– 6 to 38m for O. faveolata and 25 to 63m for A. lamarcki. Calorimetric values at each depth were compared to seasonal measures of environmental characteristics to describe the relationship between depth, light, chlorophyll-a fluorescence—as a proxy for heterotrophic potential—and the energy content of both species.
Materials and Methods
Site Selection
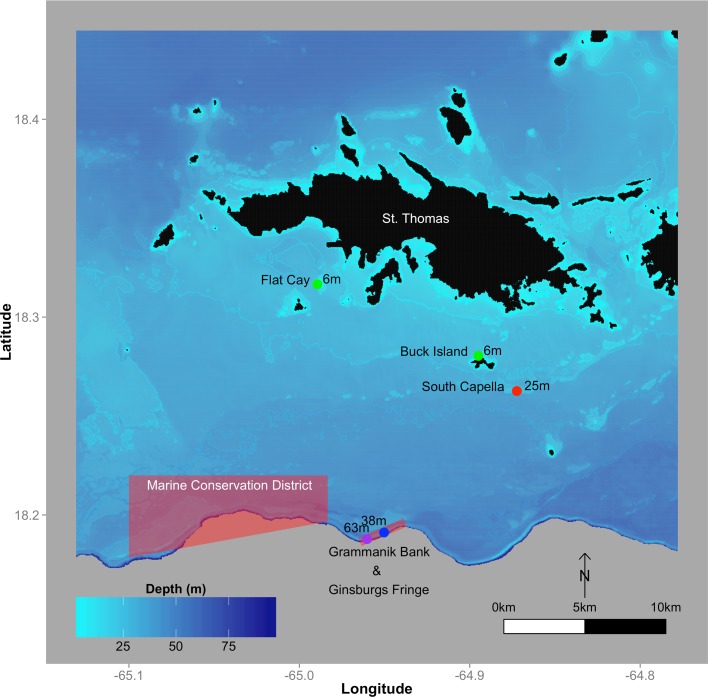
All field work was conducted under permit ##DFW14017T issued by the Virgin Islands Department of Planning and Natural Resources. Sampling locations were chosen to encompass the primary depth range for each species (Fig 1; see [34] for GPS). Colonies of O. faveolata were sampled at sites in approximately 6, 25, and 38m of depth, while A. lamarcki was sampled at 25, 38, and 63m depth (Table 1). Initial shallow samples of O. faveolata taken on May 1, 2013 were from an offshore site at Buck Island where colony density was found to be very low. Therefore, subsequent shallow O. faveolata sampling was conducted at another offshore island, Flat Cay—deemed analogous to Buck Island due to similar environmental histories and distances from shore [data in S1 Supporting Information]. All sites sampled are included in the annual Territorial Coral Reef Monitoring Program (TCRMP) for the US Virgin Islands, providing consistent historic datasets for temperature and coral health [34].
Fig 1. Study Area.
Sampling locations on insular shelf south of St. Thomas, USVI. Major offshore Marine Protected Areas indicated in red shading. Land masses are colored in black. Green dots are used to indicate 6m sites, red for 25m, blue for 38m and purple for 63m.
Table 1. Site Information.
| Site | Depth (m) | Species | Dates (N) |
|---|---|---|---|
| Buck Island | 4–7 | O. faveolata | 1-May-13 (5) |
| Flat Cay | 4–10 | O. faveolata | 11-Jul-13 (4), 13-Sep-13 (5), 19-Nov-13 (6), 2-Apr-14 (6) |
| South Capella | 23–28 | O. faveolata/A. lamarcki | 1-May-13 (5/5), 11-Jul-13 (4/5), 13-Sep-13 (5/4), 19-Nov-13 (5/6), 2-Apr-14 (6/7) |
| Grammanik Bank | 36–40 | O. faveolata/A. lamarcki | 26-Apr-13 (4/5). 5-Jul-13 (4/4), 18-Sep-13 (5/6), 14-Nov-13 (5/5), 4-Apr-14 (5/6) |
| Ginsburgs Fringe | 60–67 | A. lamarcki | 26-Apr-13 (5), 5-Jul-13 (5), 18-Sep-13 (6), 14-Nov-13 (5), 4-Apr-14 (6) |
Sampling sites with depth, species sampled, sampling dates and sample sizes.
Coral Collection
Coral samples were collected over five periods between April 2013 and April 2014 at approximately two-month intervals (Table 1). Divers haphazardly sampled seven 15-30cm2 replicate, independent sections of each species separated by five fin-kicks while maintaining consistent depth at each site—producing a total of 105 samples for O. faveolata and 101 for A. lamarcki (Table 1). Colonies were not resampled during multiple collection periods. Hammer and chisel were used to collect from the tops of O. faveolata colonies and the colony edges of A. lamarcki with a minimum radius of 25cm. Colonies of A. lamarcki were not sampled from the center as this caused fracturing of the entire colony; however attempts were made to include as much of the central portion of the colony as possible. Lastly, divers recorded the collection depths, and length, width and height of each sampled colony. At the surface, samples were transferred without seawater to pre-labeled whirl-packs and placed on ice for transport back to the laboratory. Time constraints related to post-processing limited sampling to two sites per field day—concurrent samplings were carried out no more than seven days apart.
Calorimetry
Coral samples were denuded with an airbrush according to the methods of Szmant and Gassman using ultra-pure 18mOHM water [35]. The blastate was homogenized and immediately frozen and stored at -20°C. Later, samples were partially thawed and transferred to lyophilization tubes before being re-frozen at -80°C for two hours. Samples were then freeze-dried for 24-36hrs at 220mbar and -105°C. Drying times were dependent on sample size and density—larger samples required longer drying times and in some cases re-freezing and a second round of lyophilization. Drying was deemed complete when samples could be easily powdered using a scapula without the presence of ice or liquid water. Powdered coral samples were stored in centrifuge tubes in a dehumidified cabinet set to 10% humidity.
Calorimetric analyses were carried out using a semi-microbomb calorimeter (Model 6725, Parr Instrument Company, Illinois, USA). Powdered coral samples weighing 8-24mg were pelletized and combined with a purified mineral oil spike of known energy density for combustion. Due to variable humidity in the laboratory it was difficult to consistently re-hydrate samples. The mineral oil spike ensured complete combustion of the coral powder and slowed the burn to an acceptable rate. Samples were loaded into the prepared microbomb and pressurized to 30atm with medical grade pure oxygen. Calorimetric analysis requires fifteen minutes per run and each sample was analyzed at least twice. Traditionally, relative standard deviation (RSD) between two or more calorimetry runs is used to ensure the accuracy of the final energetic content [36]. If the first two runs did not achieve an acceptable RSD, the sample was rerun until either an acceptable RSD was achieved or the sample was depleted. A minimum of 25mg freeze dried tissue was required for successful calorimetric analyses—74 samples of O. faveolata and 80 samples of A. lamarcki were sufficiently sized for calorimetric sampling.
Carbonate rich organisms present a unique problem in calorimetry due to the reduced combustion of calcium carbonate. Samples with >20% carbonate require a correction of 0.586 J/g carbonate [36–37]. 6-38mg of each sample was burned for 4 hours at 500C to ascertain carbonate percentage. In all cases, carbonate proportions were greater than 20% and required correction.
Environmental Characterization
Continuous in situ records of temperature were recorded with sensors affixed to the substrate (Hobo Water Temperature Pro v2 U22, Onset Computer Corporation, Massachusetts, USA). Paired instruments at each site and at the coral sampling depths provided continuous temperature records at fifteen-minute intervals over the course of the study. Temperature probes were calibration checked pre- and post-deployment in a freshwater ice bath and ambient temperature bath, and probes were not deployed if their temperature deviated more than 0.3°C from that recorded with a bulb thermometer.
Vertical profiles of water column temperature, PAR and chl-a fluorescence were sampled within one month of coral collection dates using a Seabird 25 Conductivity-Temperature-Depth multi-sensor (Seabird Scientific, Washington, USA) equipped with an ECO-AFL/FL fluorometer (Wetlabs, Oregon, USA) sampling at a frequency of 8 Hz. Water column cross sections were taken at each sampling site as part of ongoing monitoring efforts. Additionally, in situ benthic chl-a fluorescence was sampled for one minute, every hour using an ECO-FLSB fluorometer (Wetlabs, Oregon, USA) at each of the mesophotic sites from September 21, 2013 to November 19, 2013.
Analysis
Site specific measures of PAR, chl-a fluorescence and temperature were created for each sampling event by averaging CTD measurements within one meter of the coral sampling depth at each site. At South Capella the CTD sampling depths did not always reach the coral sampling depth at approx. 25m (3 of 5 measurements). In order to increase the sample size of physical variables we used a CTD sampling depth of 19m. Available data for two casts that retrieved data to 23m showed that the difference in physical variables between the depths for the same cast was small and well within the differences between casts at different sites. (mean differences in 23m to 19m depths July and November 2013, ΔPAR = -28.78 μmol s-1 m-2, Δchl-a = 0.11mg m-3, Δtemperature = -0.028°C). Multidimensional results were first visualized using a principal component analysis (PCA) followed by ANOVA comparison of each environmental characteristic to site. Additionally, seasonal water column stratification was investigated using CTD casts from the 63m Ginsburgs Fringe site.
Plotted benthic temperature records were condensed to daily means for each site and compared using repeated measures ANOVA (rm-ANOVA). In addition, the potential thermal stress experienced for a given site was calculated as the Degree Heating Week metric (DHW) [38]. Site-specific DHW calculations were based on derived bleaching thresholds for Flat Cay, South Capella, and Grammanik Bank [39]. No specific bleaching threshold is available for the deepest site, Ginsburgs Fringe (63m). A hypothetical bleaching threshold of 28.4°C was developed based on a relationship of bleaching threshold with depth from 24 sites of the Territorial Coral Reef Monitoring Program (Bleaching Threshold = 30.03°C—0.025°C * Depth in meters).
The change in energy content over the sampling periods was tested separately for O. faveolata and A. lamarcki. The independent nature of individual coral samples through time allowed for the application of a two-way ANOVA. Sampling Period and Site were used as factors and Tukey’s HSD post-hoc analysis was used to compare means when significant effects of the main factors were found. Regression analyses indicated no significant relationship between colony surface area and energy status for either species. Therefore, colony size was not considered in further statistical analyses.
Results
Calorimetry
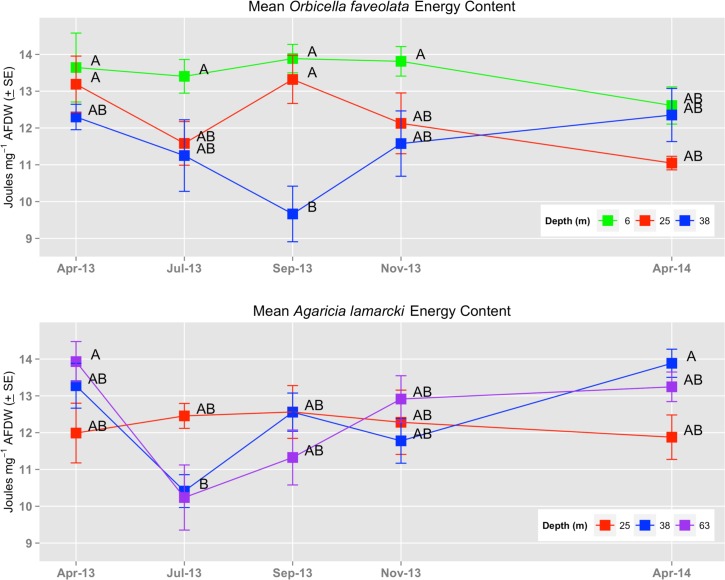
The energetic content of O. faveolata showed stability over time at 6m, varying by only 10.1% (Fig 2). In contrast, both the mid-depth and mesophotic sites exhibited considerable variability, 25m colonies varied by 20.5% and 38m colonies by 27.8% throughout the sampling period. Two-way ANOVA analysis resulted in a significant interaction between Site and Sampling Period (p = 0.032) (Table 2). Tukey’s HSD post-hoc analysis of the interaction indicated that the September 38m data point was significantly lower than a number of other data points, including four of five shallow sampling periods (Fig 2). The energy density of individual O. faveolata colonies varied two-fold, from a minimum of 7.995 J mg-1 ash-free dry weight (AFDW) at the 38m site to a high of 15.859 J mg-1 AFDW at the shallowest 6m site—with an overall mean of 12.402 + 0.205 J mg-1 AFDW (+ SE).
Fig 2. Coral Energy Content.
Mean energetic content of Orbicella faveolata and Agaricia lamarcki subsamples between April 2013 and 2014 from three site and depth levels. Letters adjacent to means values indicate results of a Tukeys HSD post-hoc analysis of the overall interaction between site and sampling period.
Table 2. Statistical Analyses.
| Species | Site p (Fdf) | Period p (Fdf) | Site*Period p (Fdf) |
|---|---|---|---|
| Orbicella faveolata | 0.00003 (12.432,59) | 0.287 (1.284,59) | 0.032 (2.308,59) |
| Agaricia lamarcki | 0.722 (0.332,65) | 0.003 (4.384,65) | 0.014 (2.658,65) |
Results of Two-Way ANOVA analyses comparing the energy content of coral tissue in Orbicella faveolata and Agaricia lamarcki across sites and sampling periods.
Shallow colonies of A. lamarcki (25m) exhibited greater stability through time relative to deeper samples, varying by only 5.8%. Conversely, the 38m and 63m sites varied over the sampling periods by 33.3% and 36.1%, respectively, and had a very similar pattern over time. Two-way ANOVA analysis indicated a significant interaction between Sampling Period and Site (p = 0.014) (Table 2). Tukey’s HSD post-hoc analysis of the interaction effect indicated that energetic content of A. lamarcki at both mesophotic sites in July 2013 were significantly less than the 63m site in April, 2013 and the 38m site in April 2014. The energy density of individual A. lamarcki colonies varied two-fold, from a minimum of 8.035 J mg-1 AFDW to a maximum of 15.514 J mg-1 AFDW, with both extremes occurring at the 63m site. Mean energetic content was 12.346 + 0.189 J mg-1 AFDW (+ SE).
Environmental Characterization
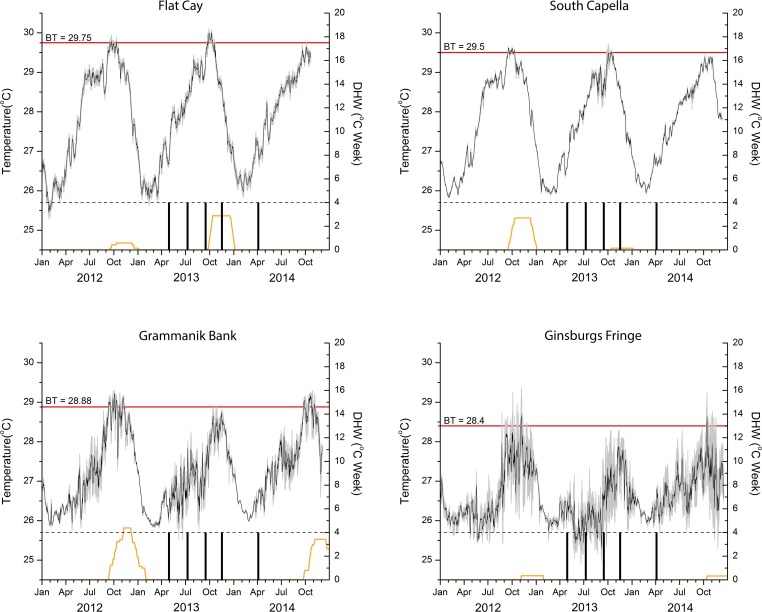
Temperature trends varied significantly with time and site (p<0.001; Table 3; Fig 3). Flat Cay (6m) and South Capella (25m) exhibited similar temporal trends for both 2013 and 2014. South Capella, however, experienced reduced thermal peaks when compared to Flat Cay, between the third and fourth sampling events. In 2012, prior to coral colony sampling, South Capella accumulated about 3 DHW of thermal stress, whereas Flat Cay showed almost no thermal stress. The pattern was reversed in 2013, when Flat Cay accumulated about 3 DHW during project sampling and South Capella experienced almost no thermal stress. During this period, temperatures at Flat Cay peaked to roughly 0.5°C higher than at South Capella. However, bleaching at Flat Cay in October was mild (9.8% prevalence) and not very different from other non-bleaching years (10.6% prevalence; mean of years 2009, 2011, and 2012 during the thermal maximum) [34].
Table 3. RM-ANOVA Results for Benthic Temperature.
| Df | Sum Sq | Mean Sq | F-value | p-value | ||
| Date | Site | 3 | 79 | 26.376 | 8.934 | <0.0001 |
| Residuals | 1076 | 3177 | 2.952 | |||
| Df | Sum Sq | Mean Sq | F-value | p-value | ||
| Date:Site | Site | 3 | 955.0 | 318.3 | 1166 | <0.0001 |
| Residuals | 3021 | 824.7 | 0.3 |
Repeated measures ANOVA output comparing benthic temperature records at the sampling sites
Fig 3. Annual Temperature Records.
Mean daily temperature and diel standard deviation (gray shading around mean line). Red line indicates bleaching threshold (BT) as calculated for each site and the BT value (°C) indicated. Yellow lines are calculated degree heating week (DHW) accumulation. Hatched black line indicates the 4 DHW level, suggested as the thermal stress level where bleaching is initiated in coral communities. Vertical black lines denote sampling periods.
The mesophotic sites at Grammanik Bank (38m) and Ginsburgs Fringe (63m) showed greater diel temperature variability than their shallow counterparts as well as reduced temperatures throughout both sampling years. In the year prior to sampling (2012), there was over 4 DHW of thermal stress recorded at Grammanik Bank, and this resulted in moderate bleaching (34.8% prevalence) compared to other non-bleaching years (12.0% prevalence, mean of years 2009, 2010, and 2011 during the thermal maximum) [34, 39]. Over the period of coral sampling in 2013 and 2014 there was little or no thermal stress recorded at Grammanik Bank or Ginsburgs Fringe.
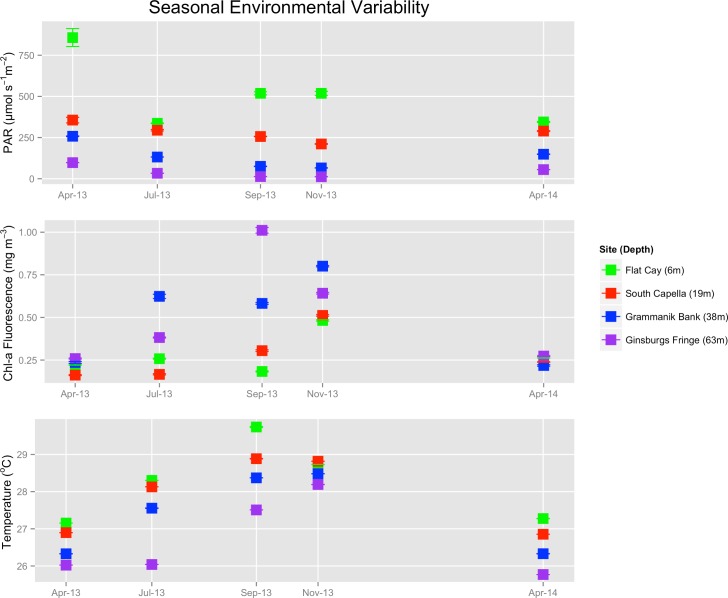
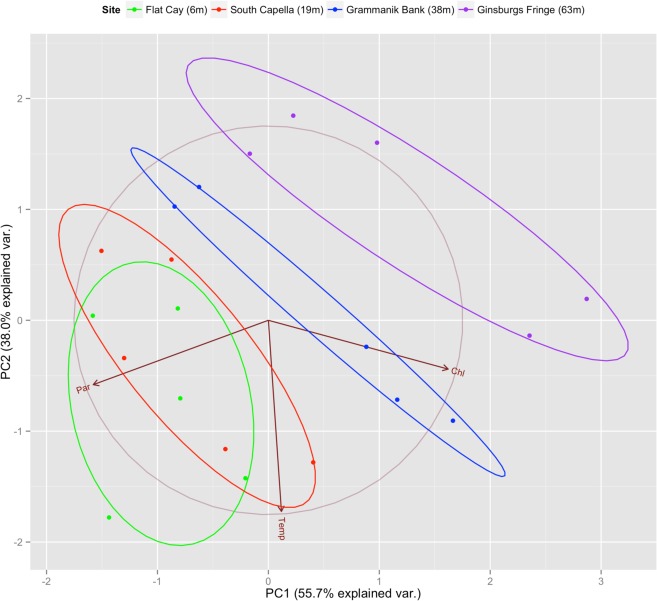
Measurements of PAR, chl-a fluorescence and temperature exhibited considerable variability across the sampling period (Fig 4). Principal component analysis of CTD data indicates a separation between mesophotic and shallow reefs (Fig 5). The first two principle components explain 93.7% of environmental variability at the sampling sites—with MCE sites exhibiting reduced PAR and increased chl-a fluorescence along PC1 and reduced PAR and temperature along PC2 (Fig 5; Table 4). PAR values exhibited statistically significant differences between sites while chl-a and temperature did not (Table 5). Tukey HSD post-hoc comparisons of PAR are found in Table 6 and highlight the light differences between mesophotic and shallow environments. While temperature and chl a were not statistically different between sites, each site exhibited high variability in these variables, with peaks at different periods over the sampling year.
Fig 4. Environmental Characterization of Sampling Sites.
PAR, chl-a fluorescence and temperature at sampling locations. Data points indicate the mean value taken within one meter of the sampling depth indicated in the legend.
Fig 5. Principle Component Analysis of CTD Data.
The first two principle components are indicated here along with arrows showing the influence of PAR, temperature and chl-a. Ellipsoids represent the default 68% confidence interval for each site.
Table 4. Principle Components Analysis Results.
| Principle Components | PC1 | PC2 | PC3 |
| PAR | -0.697 | -0.310 | 0.647 |
| Chlorophyll-a | 0.715 | -0.235 | 0.658 |
| Temperature | 0.052 | -0.921 | -0.386 |
| Importance of Components | PC1 | PC2 | PC3 |
| Standard deviation | 1.293 | 1.068 | 0.433 |
| Proportion of variance | 0.557 | 0.380 | 0.063 |
| Cumulative proportion | 0.557 | 0.937 | 1.000 |
Results of principle component analysis comparing PAR, chl-a and temperature across all sampling sites and dates.
Table 5. ANOVA Results.
| Df | Sum Sq | Mean Sq | F-value | p-value | ||
|---|---|---|---|---|---|---|
| Temperature | Site | 3 | 6.702 | 2.234 | 2.028 | 0.151 |
| Residuals | 16 | 17.626 | 1.102 | |||
| Chlorophyll-a | Site | 3 | 0.250 | 0.083 | 1.664 | 0.215 |
| Residuals | 16 | 0.802 | 0.050 | |||
| PAR | Site | 3 | 636521 | 212174 | 15.58 | 5.26x10-5 |
| Residuals | 16 | 217941 | 13621 |
ANOVA results comparing environmental factors across sites and sampling dates.
Table 6. Tukey’s HSD post-hoc Analysis of PAR data.
| diff | lwr | upr | p-value | |
|---|---|---|---|---|
| GNS-FLC | -472.85 | -684.03 | -261.66 | <0.0001 |
| GRK-FLC | -379.28 | -590.46 | -168.10 | 0.0005 |
| SCP-FLC | -233.61 | -444.80 | -22.429 | 0.0276 |
| GRK-GNS | 93.565 | -117.62 | 304.75 | 0.5953 |
| SCP-GNS | 239.23 | 28.049 | 450.42 | 0.0238 |
| SCP-GRK | 145.67 | -65.516 | 356.85 | 0.2385 |
Tukey’s HSD results for PAR data. GNS: Ginsburgs Fringe, GRK: Grammanik Bank, SCP: South Capella, FLC: Flat Cay. Significance is indicated by asterisks.
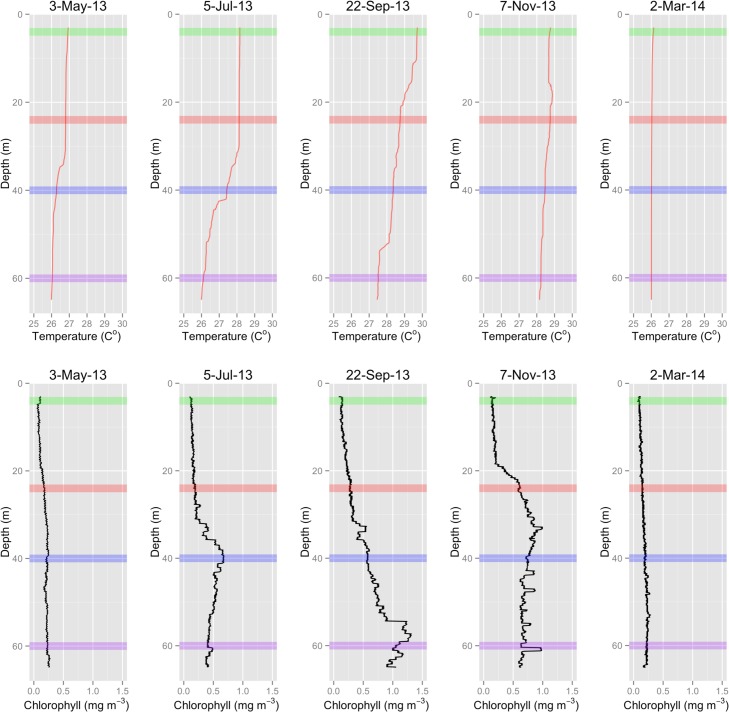
Seasonal changes in water column stratification can be identified in vertical profiles taken at the Ginsburgs Fringe site (Fig 6). During the early parts of both 2013 and 2014, the water column was well mixed to 60m depth, indicated by a consistent thermal regime and low variability in chl-a fluorescence. A weak thermocline was evident at 35m in May 2013. As the summer of 2013 progressed, temperatures increased across all depths, but more abruptly shallower than 30m. During July and September thermoclines were present, resulting in a temperature range of 2°C across the sampling depth range. The November 2013 cast showed a return to the well mixed regime measured in both spring samples; however, there were increased temperatures deeper than 30m compared to earlier in the year, most notably at depths exceeding 55m.
Fig 6. Seasonal Water Column Characterization.
Water column temperature and chlorophyll-a fluorescence measurements concurrent with each sampling event. Horizontal colored bars correspond to sampling depths.
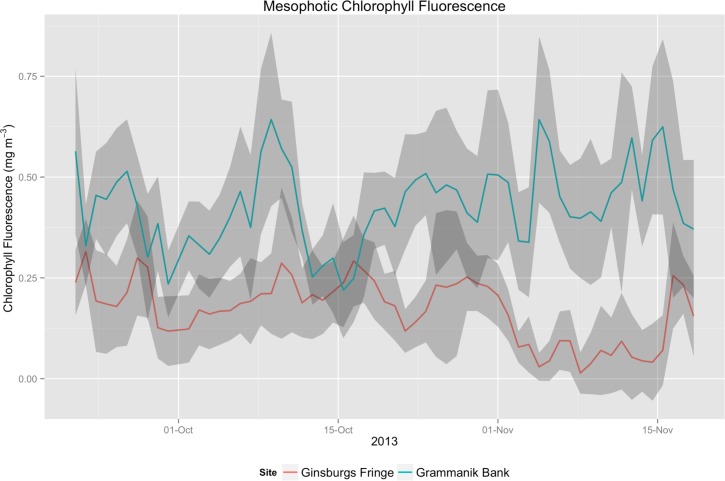
Vertical profiles of chlorophyll-a fluorescence values exhibited similar trends to temperature. The spring casts showed low chl-a fluorescence that were consistent across depths. During July and September, chl-a levels increased with maxima occurring at major thermocline depths. The November cast exhibited relatively consistent and high chl-a levels across the entire depth range below 20m. The chl-a fluorescence maximum in July occurred at the Grammanik Bank sampling site, and the September maximum encompassed Ginsburgs Fringe. Benthic recording of chl-a at these two sites in October and November 2013, between vertical profile sampling, showed that the Grammanik Bank had significantly higher and more variable chl-a values than Ginsburgs Fringe (Fig 7; MeanGrammanik = 0.430 ± 0.180 S.D., MeanGinsburgs Fringe = 0.171 ± 0.126 S.D; p<0.001 paired t-test). In many cases chlorophyll-a levels at Ginsburgs Fringe were near lower detection limits of the sensors, indicating very low abundance of phytoplankton.
Fig 7. Mesophotic Chlorophyll Fluorescence.
Mean daily Chlorophyl-a fluorescence at Grammanik Bank and Ginsburgs Fringe from 9/21/13 to 11/19/13. Shaded regions represent daily standard deviation.
Discussion
Perhaps the most striking pattern revealed here was increasing seasonal variability of energy content in coral tissues with depth. The shallowest sites for both species exhibited far less change in energetic content through time than deep sites. Furthermore, timing differences between O. faveolata and A. lamarcki energy variation across sampling periods, but within the same sites, suggests species-specific factors may be shaping energetic responses of corals inhabiting MCEs. Given the single-year timeframe of this study, we suggest that the main mechanism affecting the measured seasonal energetic drops in mesophotic colonies sampled is likely to be reproduction. It is important to note, however, that depth adaptations in the corals measured likely have a profound impact on the overall energetic measurements made. The refugia potential of mesophotic coral reefs will ultimately rely on the interplay between site-specific environmental characteristics and species-specific symbiont selection and reproductive activities.
Reproduction
One of the most influential energetic activity corals undertake is that of sexual reproduction. The production of gametes and larvae requires considerable energy investment on the part of the coral. Richmond showed that colonies of Pocillopora damicornis, a brooding species in the region he investigated, invest between 2 and 20% of their total energetic content into larvae production during each month of reproduction [40]. In addition, he suggested that P. damicornis were investing 1–10 times the calories into reproduction as they were into growth. Assuming that the energy demands of reproduction rival those of tissue growth and maintenance, the influence of reproduction on the overall energy content of corals is likely to be important.
The reproductive strategies of O. faveolata and A. lamarcki are different and may be very influential in the resilience of both species to future stress events. While reproduction was not measured directly in our study corals, we have inferred reproduction based on annual timing from the literature. Orbicella spp. are broadcast spawning species that release egg and sperm bundles that break up and fertilize in the water column [41]. Reproduction in O. faveolata is expected in either August or September, just prior to the third sampling period in 2013, in both shallow [42] and mesophotic environments [43]. Thus, the drop in energy content exhibited by the 38m colonies in September 2013 coincided with likely gamete release and this may explain the pattern of annual variation.
There are two factors that explain the opposing energetic trends between shallow and mesophotic O. faveolata during the reproductive period. First, greater solar irradiance allows for higher net productivity in corals at shallow depths [29]. The energy expended in reproductive activities of shallow colonies is likely fully replaced over a short time period by photosynthesis, evinced by the stability of shallow colony energy content throughout the reproductive seasons of both species. Second, it has been shown that gametogenesis in O. faveolata is delayed in mesophotic colonies relative to shallow colonies, but once initiated is more rapid in mesophotic corals [43]. The authors also showed across our same sampling region on the southeastern Puerto Rican Shelf that mesophotic colonies were hyper-fecund, producing greater numbers of gametes than shallow colonies. It is likely, therefore, that mesophotic O. faveolata experience a compressed period of strong reproductive activity, incurring the same or greater energy costs as shallow colonies over a much shorter period of time. The September drop in energetic content for mesophotic colonies is likely a result of intense gamete production followed by spawning. The lack of energetic drop in shallow colonies is possibly a result of prolonged and less intense gametogenesis that may be mostly or fully supported by photosynthesis.
At different times throughout the sampling period, O. faveolata colonies at 25m trended with both shallow and mesophotic colonies. While not statistically significant, the downward trend in energy status of 25m colonies in July, followed by an increase into September is likely due to environmental factors, and not an early spawning event. Spawning of O. faveolata across its depth range in the Caribbean is timed between August and October [35, 43] and has not been observed earlier, suggesting that early spawning is an unlikely explanation for energy content decreases prior to August. As the well-mixed water column of spring gives way to a stratified regime in summer, it is possible that colonies at South Capella were receiving increased heterotrophic food sources through tidal boring, and then increased sunlight during periods while above the thermocline. Colonies living at 25m appear to experience conditions reminiscent, but not identical, to both shallow and mesophotic reefs at different times of year. More work is necessary to understand the factors affecting colonies residing within the transition zone between shallow and mesophotic reefs.
In contrast to O. faveolata, A. lamarcki is a brooding species that undergoes internal fertilization and releases fully competent larvae during planulation [44]. The timing of reproduction in A. lamarcki is unknown, but it has been suggested that planulation may occur during the spring alongside other deep-living Caribbean agariciids [45–46]. The energetic minimum exhibited by mesophotic colonies in July 2013 supports the assertion that A. lamarcki are reproducing in the first half of the year. The disparity between shallow and mesophotic energetics during reproduction is likely attributable again to differences in photosynthetic net productivity. Shallow colonies experiencing higher light levels may be capable of supporting reproduction without marked losses of energy while mesophotic colonies are not. In addition, as may be the case with O. faveolata, mesophotic A. lamarcki may be placing greater energetic investment into reproduction for unknown reasons.
The timing of reproduction influences the extent to which energy content is affected in both species. Mesophotic O. faveolata and A. lamarcki both experienced similar energetic drops during their reproductive periods; however, since O. faveolata spawns in the fall, colonies may be at greater risk of disturbance in future stress events. Thermal stress across all sampling sites generally begins in the second half of September and continues through January (Fig 3). O. faveolata experience their energetic minimum in September, at the beginning of the thermal stress season. If algal symbionts are thermally stressed and this leads to a reduction in photosynthetic subsidies, such as occurs during bleaching events, then mesophotic corals may be more susceptible to mortality. Conversely, mesophotic A. lamarcki have considerably more time for energetic recovery following a July energetic minimum—with colonies exhibiting greater energetic content in September than O. faveolata.
Respiration, Photosynthesis, and Trophodynamics
Along with reproduction, variability in the environmental and physiological conditions across a depth gradient likely affects energy content of coral colonies. The effects of environmental variability and depth on coral growth are well reviewed in [47]. As light attenuates, corals adapt—exhibiting reduced calcification and denser skeletons [48–49]. Colonies display flattened, plating growth forms intended to better capture light and compensate for reduced photosynthesis to respiration ratios (P/R ratio) [29, 50]. The stability of energy content in shallow corals in this study suggests that colonies at those depths may be maintaining positive net productivity throughout the year. High light levels and low short-term thermal variability at the shallowest sites may assist in maintaining more constant energy levels.
Conversely, mesophotic colonies of both species are likely to receive less light and experience greater short-term thermal variability. O. faveolata and A. lamarcki have been shown to modulate symbiont communities at mesophotic depths—favoring more productive but less thermally tolerant clades when light and temperature are reduced [31, 51–52]. Adapting zooxanthellate communities at depth is likely an effort to increase photosynthetic production in reduced light. While both subject species exhibit differing symbiont communities with depth, it is possible that they possess unequal heterotrophic capabilities. While A. lamarcki has been shown to successfully feed at mesophotic depths [32], the heterotrophic plasticity of O. faveolata is currently unclear—although Lesser and others have suggested that it may be less suited to heterotrophy than other coral species [53].
Whether O. faveolata is feeding heterotrophically or not, the seasonal drop in energy content of mesophotic colonies indicates that the combination of more productive symbionts with increased planktonic food sources in the water column cannot sufficiently support reproductive activities in deep living colonies without a drop in energetic content. Similarly, the precipitous seasonal energetic drop in A. lamarcki, followed by rapid energy recovery likely suggests that the combination of shifting symbiont communities and increased heterotrophic feeding does not allow mesophotic colonies to maintain consistent energy throughout the year. Ultimately, the timing of the energetic drop in both species—likely linked to reproductive activities—defines the energetic state of these species as they enter the season most associated with thermal stress events.
Another possible explanation for the low energetic variability exhibited by shallow colonies versus mid and deep colonies has to do with historic seasonal dynamics. Specifically, the history of bleaching and coral mortality at 6m is considerably different than at the other three sites. Thermal stress events affected shallow water corals in 2005 and 2010 [34], but the effect of thermal stress declined with depth [39]. Also, while there was mesophotic bleaching in 2012, it was not severe and did not result in loss of coral cover [39]. As such, it is possible that differential mortality has occurred between sites. It may be that the only surviving shallow colonies are those that had the most efficient energy maintenance regimes going into previous stress events, and therefore they showed constant energy content over the sampled year.
MCEs as Refugia
The seasonal energetic content of O. faveolata and A. lamarcki suggest differing refuge potential for each species in MCEs habitats. O. faveolata appears to be better adapted for shallow water living, and mesophotic colonies—though prevalent—may be at risk of future disturbance. Corals incur large energy costs during reproduction [40] and deep-living colonies appear to require a considerably longer recovery period than shallow colonies. In O. faveolata reproduction, energy content minima, and the annual thermal maximum period all coincide. Thus, the corals have the lowest energy stores during the period when there could be high temperature caused stress. If during this period there is photosynthetic stress and bleaching that limits energy production, energy stores may be insufficient to maintain colony vitality. In contrast, spring brooding and subsequent energetic minimum exhibited by A. lamarcki colonies may give them time to recover energy content prior to the annual thermal maximum. As such, A. lamarcki colonies living at or beyond 40m have a life history that allows them greater energy stores during the most stressful time of year.
The conclusion that MCEs may represent a better refuge for A. lamarcki than O. faveolata needs to be balanced by investigations into the ability of mesophotic larvae and newly recruited corals to survive stress events. Studies have shown that increased temperatures at the time of larval release and settlement can impact survivorship in coral larvae and recruits [54–56]. While the timing of A. lamarcki reproduction provides abundant energy stores for extant colonies entering the thermal maximum, larvae and newly recruited colonies are likely to experience stress shortly after settling. Alternatively, O. faveolata larvae may have the opportunity to settle shortly after the thermal maximum, providing new colonies a greater period of time to grow and store energy. The individual ways that coral species time life history events in relation to stressful conditions may contribute to the species-specific responses of corals to climate change—and likely affect the refugia potential of MCEs.
Supporting Information
(PDF)
Acknowledgments
We thank R. Brewer, K. Brown, I. Byrne, R. Ennis, L. Henderson, D. Holstein, J. Jossart, S. Kadison, M. Kammann, J. Kisabeth, R. Nemeth, S. Prosterman, A. Sabine, R. Sjoken, A. Tagini and V. Wright for field and laboratory assistance as well as data management. No funding agency participated in the design or interpretation of the research. This is contribution #128 from the Center for Marine and Environmental Studies, University of the Virgin Islands.
Data Availability
The data have been served publicly using the Open Science Framework. They can be accessed at https://osf.io/tc7kz/ and with: DOI 10.17605/OSF.IO/TC7KZ | ARK c7605/osf.io/tc7kz.
Funding Statement
Funding was provided by the Black Coral Penalty Fund (US Justice Departments), the Virgin Islands Experimental Program to Stimulate Competitive Research (National Science Foundation #0814417) and the Lana Vento Charitable Trust. No funding agency participated in the design or interpretation of the research.
References
- 1.Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471 [Google Scholar]
- 2.Munday PL, Jones GP, Pratchett MS, William AJ. Climate change and the future for coral reef fishes. Fish Fish. 2008;9:261–285 [Google Scholar]
- 3.Glynn PW. In tandem reef coral and cryptic metazoan declines and extinctions. Bull Mar Sci. 2011;87:767–794 [Google Scholar]
- 4.Knowlton N. The future of coral reefs. P Natl A Sci. 2001;98:5419–5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoegh-Guldberg O, Ortiz JC, Dove S. The future of coral reefs. Science. 2011;334:1494–1495 [DOI] [PubMed] [Google Scholar]
- 6.Crook ED, Cohen AL, Rebolledo-Vieyra M, Hernandez L, Paytan A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. P Natl A Sci. 2013;110:11044–11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O. Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs. 2010;29:309–327 [Google Scholar]
- 8.Slattery M, Lesser MP, Brazeau D, Stokes MD, Leichter JJ. Connectivity and stability of mesophotic coral reefs. J Exp Mar Biol Ecol. 2011;408:32–41 [Google Scholar]
- 9.Thomas CJ, Bridge TCL, Figueiredo J, Deleersnijder E, Hanert E. Connectivity between submerged and near-sea-surface coral reefs: can submerged reef populations act as refuges? Divers Distrib. 2015;21:1254–1266 [Google Scholar]
- 10.Hinderstein LM, Marr JCA, Martinez FA, Dowgiallo MJ, Puglise KA, Pyle RL, et al. Theme section on “Mesophotic Coral Ecosystems: Characterization, Ecology, and Management”. Coral Reefs. 2010;29:247–251 [Google Scholar]
- 11.Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, et al. Community ecology of mesophotic coral reef ecosystems. Coral Reefs. 2010;29:255–275 [Google Scholar]
- 12.Lesser MP, Slattery M, Leichter JJ. Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol. 2009;375:1–8 [Google Scholar]
- 13.Leichter JJ, Genovese SJ. Intermittent upwelling and subsidized growth of the scleractinian coral Madracis mirabilis on the deep fore-reef of Discovery Bay, Jamaica. Mar Ecol Prog Ser. 2006;316:95–103 [Google Scholar]
- 14.Glynn PW. Coral reef bleaching: facts, hypotheses and implications. Glob Change Biol. 1996;2:495–509 [Google Scholar]
- 15.Riegl B, Piller WE. Possible refugia for reefs in times of environmental stress. Int J Earth Sci. 2003;92:520–531 [Google Scholar]
- 16.Gleason DF, Edmunds PJ, Gates RD. Ultraviolet radiation effects on the behavior and recruitment of larvae from the reef coral Porites astreoides. Mar Biol. 2006;148:503–512 [Google Scholar]
- 17.van Oppen MJH, Bongaerts P, Underwood JN, Peplow LM, Cooper TF. The role of deep reefs in shallow reef recovery: an assessment of vertical connectivity in a brooding coral from west and east Australia. Mol Ecol. 2011. 10.1111/j.1365-294X.2011.05050.x [DOI] [PubMed] [Google Scholar]
- 18.Yamashiro H, Oku H, Onaga K. Effect of bleaching on lipid content and composition of Okinawan corals. Fisheries Sci. 2005;71:448–453 [Google Scholar]
- 19.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189 [DOI] [PubMed] [Google Scholar]
- 20.Anthony KRN, Connolly SR, Hoegh-Guldberg O. Bleaching, energetics, and coral mortality risk: effects of temperature, light, and sediment regime. Limnol Oceanogr. 2007;52(2):716–726 [Google Scholar]
- 21.Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Functional Ecology. 2009;23:539–550 [Google Scholar]
- 22.Anthony KRN, Fabricius KE. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol. 2000;252:221–253 [DOI] [PubMed] [Google Scholar]
- 23.Borell EM, Yuliantri AR, Bischof K, Richter C. The effect of heterotrophy on photosynthesis and tissue composition of two scleractinian corals under elevated temperature. J Exp Mar Biol Ecol. 2008;364:116–123 [Google Scholar]
- 24.Palardy JE, Rodrigues LJ, Grottoli AG. The importance of zooplankton to the daily metabolic carbon requirements of healthy and bleached corals at two depths. J Exp Mar Biol Ecol 2008;367:180–188 [Google Scholar]
- 25.Palardy JE, Grottoli AG, Matthews KA. Effect of naturally changing zooplankton concentrations on feeding rates of two coral species in the Eastern Pacific. J Exp Mar Biol Ecol. 2006;331:99–107 [Google Scholar]
- 26.Alamaru A, Loya Y, Brokovich E, Yam R, Shemesh A. Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: Insights from stable isotope analysis of total organic material and lipids. Geochim Cosmochim Ac. 2009;73:5333–5342 [Google Scholar]
- 27.Ziegler M, Roder CM, Büchel C, Voolstra CR. Limits to physiological plasticity of the coral Pocillopora verrucosa from the central Red Sea. Coral Reefs. 2014;33:1115–1129 [Google Scholar]
- 28.Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. P Natl A Sci. 2008;105:10444–10449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesser MP, Slattery M, Stat M, Ojimi M, Gates RD, Grottoli A. Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology. 2010;91:990–1003 [DOI] [PubMed] [Google Scholar]
- 30.Thornhill DJ, Rotjan RD, Todd BD, Chilcoat GC, Iglesias-Prieto R, Kemp DW, et al. A connection between colony biomass and death in Caribbean reef-building corals. 2011. PLoS One 6(12): e29535 10.1371/journal.pone.0029535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bongaerts P, Frade PR, Ogier JJ, Hay KB, van Bleijswijk J, Englebert N, et al. Sharing the slope: depth partitioning of agariciid corals and associated Symbiodinium across shallow and mesophotic habitats (2–60 m) on a Caribbean reef. BMC Evol Biol. 2013;13:205–219 10.1186/1471-2148-13-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crandall JB, Teece MA, Estes BA, Manfrino C, Ciesla JH. Nutrient acquisition strategies in mesophotic hard corals using compound specific stable isotope analysis of sterols. J Exp Mar Biol Ecol. 2015;474:133–141 [Google Scholar]
- 33.Richmond RH. Energetic considerations in the dispersal of Pocillopora damicornis planulae. In: Gomez E (ed.) Proc. Fourth Int. Coral Reef Symp., Manila. 1981.
- 34.Smith TB, Kadison E, Ennis R, Gyory J, Brandt ME, Wright V, et al. The United States Virgin Islands Territorial Coral Reef Monitoring Program 2014 Annual Report. University of the Virgin Islands, United States Virgin Islands: 273pp. 2014. 10.13140/RG.2.1.3791.5688 [DOI] [Google Scholar]
- 35.Szmant AM, Gassman NJ. The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs. 1990;8:217–224 [Google Scholar]
- 36.Golley FB. Energy values of ecological materials. Ecology. 1961;42:581–584 [Google Scholar]
- 37.Paine RT. Endothermy in bomb calorimetry. Limnol Oceanogr. 1966;11(1):126–129 [Google Scholar]
- 38.NOAA. Tropical Ocean Coral Bleaching Indices National Oceanic and Atmospheric Administration, Silver Springs, Maryland: 2006. http://coralreefwatch.noaa.gov/satellite/methodology/methodology.php [Google Scholar]
- 39.Smith TB, Gyory J, Brandt ME, Miller WJ, Jossart J, Nemeth RS. Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob Change Biol. 10.1111/gcb.13175 [DOI] [PubMed] [Google Scholar]
- 40.Richmond RH. Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull Mar Sci. 1987;41:594–604 [Google Scholar]
- 41.Steiner SCC. Spawning in scleractinian corals from SW Puerto Rico (West Indies). Bull Mar Sci. 1995;56:899–902 [Google Scholar]
- 42.Szmant AM, Weil E, Miller MW, Colón DE. Hybridization within the species complex of the scleractinian coral Montastraea annularis. Mar Biol. 1997;129:561–572 [Google Scholar]
- 43.Holstein DM, Smith TB, Gyory J, Paris CB. Fertile fathoms: Deep reproductive refugia for threatened shallow corals. Scientific Reports. 2015;5:12407 10.1038/srep12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richmond RH, Hunter CL. Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar Ecol Prog Ser. 1990;60:185–203 [Google Scholar]
- 45.van Moorsel GWNM. Different reproductive strategies in two closely related stony corals (Agaricia, Scleractinia). In: Gomez E (ed.) Proc. Fourth Int. Coral Reef Symp., Manila. 1982
- 46.Steneck RS, Arnold SN, Mumby PJ. Experiment mimics fishing on parrotfish: insights on coral reef recovery and alternative attractors. Mar Ecol Prog Ser. 2014;506:115–127 [Google Scholar]
- 47.Pratchett MS, Anderson KD, Hoogenboom MO, Widman E, Baird AH, Pandolfi JM, et al. Spatial, temporal and taxonomic variation in coral growth—Implications for the structure and function of coral reef ecosystems. Ocean Mar Biol Ann Rev. 2015;53:215–295 [Google Scholar]
- 48.Baker PA, Weber JN. Coral growth rate: variation with depth. Phys Earth Plan Int. 1975;10:135–139 [Google Scholar]
- 49.Allemand D, Tambutté E, Zoccola D, Tambutté S. Coral calcification, cells to reefs In: Dubinsky Z, Stambler N (eds.) Coral Refs: An Ecosystem in Transition, Amsterdam: Springer; 119–150 [Google Scholar]
- 50.Todd PA. Morphological plasticity in scleractinian corals. Biol Rev. 2008;83:315–337 [DOI] [PubMed] [Google Scholar]
- 51.Cooper TF, Ulstrup KE, Dandan SS, Heyward AJ, Kühl M, Muirhead A, et al. Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proc Royal Soc B. 2011;278:1840–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bongaerts P, Carmichael M, Hay KB, Tonk L, Frade PR, Hoegh-Guldberg O. Prevalent endosymbiont zonation shapes the depth distributions of scleractinian coral species. R Soc Open Sci. 2015;2:140297 10.1098/rsos.140297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lesser MP, Mazel C, Phinney D, Yentsch CS. Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol Oceanogr. 2000;45:76–86 [Google Scholar]
- 54.Edmunds PJ, Gates R, Gleason D. The biology of larvae from the reef coral Porites astreoides, and theire response to temperature disturbance. Coral Reefs. 2001;139:981–989 [Google Scholar]
- 55.Putnam HM, Edmunds PJ, Fan TY. Effect of temperature on the settlement choice and photophysiology of larvae from the reef coral Stylophora pistillata. Biol Bull. 2008;215:135–142 [DOI] [PubMed] [Google Scholar]
- 56.Anlauf H, D’Croz L, O’Dea A. A corrosive concoction: The combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative. J Exp Mar Biol Ecol. 2011;1:13–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The data have been served publicly using the Open Science Framework. They can be accessed at https://osf.io/tc7kz/ and with: DOI 10.17605/OSF.IO/TC7KZ | ARK c7605/osf.io/tc7kz.