Abstract
The CDC’s National Report on Biochemical Indicators of Diet and Nutrition in the US Population (Nutrition Report) is a serial publication that provides ongoing assessment of the population’s nutritional status. The Nutrition Report presents data on blood and urine biomarker concentrations (selected water- and fat-soluble vitamins and nutrients, trace elements, dietary bioactive compounds) from a representative sample of the population participating in the NHANES. The Second Nutrition Report (released in 2012) contains reference information (means and percentiles) for 58 biomarkers measured during all or part of 2003–2006, stratified by age, sex, and race-ethnicity. Where available, we presented cutpoint-based prevalence data during 2003–2006, and data on changes in biomarker concentrations or prevalence since 1999. Blood vitamin concentrations were generally higher in older (≥60 y) compared to younger (20–39 y) adults and lower in Mexican Americans and non-Hispanic blacks compared to non-Hispanic whites. Nearly 80% of Americans (≥6 y) were not at risk for deficiencies in any of the 7 vitamins studied (A, B-6, B-12, C, D, E and folate). Deficiency rates varied by age, sex, and race-ethnicity. About 90% of women (12–49 y) were not at risk for iron deficiency, but only 68% were not at risk for deficiencies in iron and all 7 vitamins. Young women (20–39 y) had median urine iodine concentrations bordering on insufficiency. First-time data are presented on plasma concentrations of 24 saturated, mono- and polyunsaturated fatty acids. Tabulation and graphical presentation of NHANES data in the Second Nutrition Report benefits those organizations involved in developing and evaluating nutrition policy.
INTRODUCTION
The NHANES provides the most comprehensive assessment of the health and nutritional status of the US population, and it is the only population representative survey that collects biological specimens and produces reference information on nutritional biomarkers (1–3). Policy makers and researchers rely on the NHANES results to provide information on and evaluate public health programs and policies, develop national reference intervals, and generate research hypotheses. Until recently, no publication existed that presented descriptive data on the collection of nutritional biomarkers measured as part of the continuous NHANES (1999 and forward) in a straightforward and consistent format to allow for comparisons across demographic subgroups and biomarkers.
The CDC’s National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population (Nutrition Report) fills this important gap. The Nutrition Report was established in 2008 and consists of a series of publications that use data from the continuous NHANES to provide an ongoing assessment of the US population’s nutritional status by describing blood and urine concentrations of dietary and nutrition-related biomarkers by age, sex, and race-ethnicity. The CDC Division of Laboratory Sciences at the National Center for Environmental Health (NCEH/DLS)4 conducted the laboratory and data analyses for 58 biochemical indicators presented in the Second Nutrition Report––the most current report of this series––which was released in April 2012 and covers data from all or part of the NHANES from 2003 through 2006 (4). Where available, data are also presented on the prevalence of low or high biomarker concentrations during 2003–2006 and on changes in biomarker concentrations or prevalence estimates over time since 1999. The First Nutrition Report was published in July 2008 and contained information on 27 biochemical indicators from all or part of the NHANES 4-y period from 1999 through 2002 (5). Both reports and an accompanying executive summary and factsheets for the second report are accessible online.
Measuring biomarkers and assessing nutrient intake from foods and dietary supplements are the 2 main tools used to assess the nutritional status of a population (6). The Nutrition Report covers the first of these 2 approaches. The overarching goal of this paper is to review the main features and findings of the Second Nutrition Report. Our specific objectives are: (a) to discuss the public health uses and the value of the report in the context of nutritional status assessment; (b) describe selected findings by summarizing data across various report chapters; and (c) highlight new data presented in the report.
SUBJECTS AND METHODS
The NHANES collects cross-sectional data on the health and nutritional status of the civilian non-institutionalized US population (3). Since 1999, the CDC National Center for Health Statistics (NCHS) has conducted the NHANES as a continuous survey with data released in 2-y cycles. The survey obtains a stratified, multistage, probability sample designed to represent the US population on the basis of self-reported age, sex, and race-ethnicity. NCHS personnel first interview survey participants in their homes where they collect information on demographic characteristics, dietary supplement use, and some health-related issues. Participants undergo a physical examination, blood draw and urine collection about 1–2 wk after the household interview in a Mobile Examination Center. A complete 24-h dietary recall is performed at that time, with a second recall performed by telephone 3–10 d later for a subset or all participants, depending on the survey cycle. All respondents gave informed consent, and the NHANES protocol was reviewed and approved by the NCHS Research Ethics Review Board. Interview and examination response rates for each survey period are publically available (7).
Biomarker laboratory methods
The NCEH/DLS laboratory analyzed biological specimens for all 58 nutritional biomarkers for which data were presented in the Second Nutrition Report. Detailed laboratory method information is provided elsewhere (8, 9).
Statistical analyses
A detailed description of all statistical analyses can be found in the Introduction chapter of the Second Nutrition Report (4). Statistical analyses were performed using SAS (version 9, SAS Institute Inc., Cary, NC) and SUDAAN (version 9, RTI, Research Triangle Park, NC) software. Because the NHANES sample design is a complex, multistage probability sample, we used sample weights to account for differential nonresponse or noncoverage and to adjust for oversampling of some groups. Statistics provided in the report for all biomarkers included unadjusted geometric means and selected percentiles with 95% confidence intervals. The data were grouped by age, sex, and race-ethnicity. With the exception of vitamin C and body iron, all biomarkers covered in the report were right-skewed; thus, a geometric mean provided a better estimate of central tendency. Arithmetic means were presented for vitamin C and body iron as the distributions of these biomarkers were reasonably symmetric. For calculation of geometric means, concentrations less than the limit of detection (LOD) were assigned an imputed value equal to the LOD divided by the square root of 2. If the percentage of results <LOD was greater than 40%, geometric means were not calculated. Standard error estimates were calculated by use of the Taylor series (linearization) method within SUDAAN. Percentile estimates were calculated by use of linear interpolation. Confidence intervals for percentiles were calculated by the Woodruff method (10). We used the unweighted sample size and an average design effect of 1.4 (as in the First Nutrition Report [5]) as the criteria to report percentiles of sufficient precision (11). Recent sample design guidance from NCHS for the continuous NHANES suggested a similar design effect of 1.5 (12). In order for percentiles to be considered reliable, at least 112 persons had to be represented to allow estimation of the 10th and 90th percentiles, 224 persons for the 5th and 95th percentiles, and 448 persons for the 2.5th and 97.5th percentiles. We noted percentiles for which these requirements were not met. Where possible, we also described biomarker concentrations across survey cycles during all or part of the 8-y period from 1999 through 2006.
For biomarkers with accepted cutoff values for low and/or high concentrations (e.g., folate, vitamins A, B-6, B-12, C, D, E, ferritin)—suggesting risk of deficiency or excess of certain micronutrients—we presented prevalence estimates by age, sex, or race-ethnicity for all or part of NHANES 1999–2006. We used the relative standard error (RSE) as a criterion for prevalence estimates of sufficient precision. Prevalence estimates were noted if 30% ≤ RSE <40%. Estimates were not provided if they were associated with an RSE ≥40%. All estimates presented in this paper were statistically reliable based on these criteria.
For biomarkers measured in urine, we presented separate tables for the concentration of each indicator expressed as per volume of urine (uncorrected table) and per gram of urine creatinine (creatinine-corrected table).
Where long-term trending information beyond the continuous NHANES was available and of public health interest, we presented figures in the highlights section of each chapter showing changes in biomarker concentrations from NHANES III (1988–1994) to 1999–2002 and 2003–2006 using age-adjusted geometric mean concentrations that have been generated in SUDAAN by use of age-standardizing proportions from the 2000 US Census population (using direct standardization). Statistically significant (P <0.05) differences between age-adjusted geometric means (time trend analysis) or between means or percentages comparing population groups were assessed through pairwise comparisons.
RESULTS
Features and public health uses of the Second Nutrition Report
The Second Nutrition Report contained more than twice the number of biomarkers compared to the first report (58 vs. 27). This was largely due to the addition of 24 plasma fatty acid concentrations. New features of the Second Nutrition Report included information on prevalence of nutrient deficiencies or excess, trends over time for the period 1999–2006, and the presentation of central 95% reference intervals (2.5th to 97.5th percentile), often used to describe normal concentrations in a population (Supplemental Table 1). Because concentrations outside the interval are generally considered to be unusual, 95% reference intervals can be used to study the association of biomarker concentrations with health outcomes in the absence of biologically derived clinical cutoff values.
The primary objective of the Nutrition Report is to inform public health scientists and policy makers about the biomarkers of diet and nutrition in the general US population and in selected subpopulations. These data help physicians, scientists and public health officials assess inadequate or excess intake and inform analyses on the relation between biochemical indicators and health outcomes. Other objectives and public health uses of the information include: (a) establishing and improving on existing population reference levels that can be used to determine whether an individual or a group has an unusually high or low concentration of a diet-and-nutrition biochemical indicator; (b) determining whether the nutrition status of special population groups, such as minorities, children, women of childbearing age, or the elderly, is different from that of other groups, or whether such nutrition status needs improvement; (c) tracking trends over time in the population’s biomarker concentrations; (d) assessing the effectiveness of public health efforts to improve the diet and nutrition status of the US population; (e) testing the appropriateness of cutoff values; and (f) guiding research to perform more in-depth analyses of the NHANES data and to generate hypotheses for future nutrition and human health studies.
Selected findings of the Second Nutrition Report
The biochemical indicators of diet and nutrition assessed in the Second Nutrition Report, why they were measured, what the recommended concentrations or cutoff values are, and selected report findings, are presented in Table 1. Compared to young adults (20–39 y), children had significantly higher blood concentrations for most vitamins: B-6 (measured as serum pyridoxal-5′-phosphate [PLP], the biologically active coenzyme form and best single indicator of status), B-12 (measured as serum total cobalamin), C (measured as serum ascorbic acid, an indicator of tissue stores), D (measured as serum 25-hydroxyvitamin D [25OHD], the circulating form and indicator of status) and folate (measured as serum and RBC folate, short- and long-term indicators of status) (Fig. 1, panel A). Children had significantly lower concentrations of serum vitamins A and E. Older persons (≥60 y) also had mostly significantly higher blood vitamin concentrations compared to younger adults, except for PLP and 25OHD concentrations (Fig. 1, panel B). Most vitamin concentrations were significantly lower in Mexican Americans (MA; Fig. 1, panel C) and non-Hispanic blacks (NHB; Fig.1, panel D) compared to non-Hispanic whites (NHW), except for serum vitamin B-12 where these two race-ethnic groups had significantly higher serum concentrations and for vitamin C where they had comparable serum concentrations to NHW. Compared to males, females had significantly higher concentrations of vitamins C and E, and folate (serum and RBC), comparable concentrations of vitamin B-12 and 25OHD, and significantly lower concentrations of vitamin A and PLP (Fig. 1, panel E).
Table 1.
Summary information on biochemical indicators of diet and nutrition assessed in the Second Nutrition Report1.
Nutrient or dietary bioactive compound
|
Why measured? Health impact |
Recommended biochemical concentrations2 (reference) | What did we find? | NHANES years covered |
|---|---|---|---|---|
Folate
|
Deficiency causes macrocytic anemia; low folate status increases risk of NTD and may modulate risk of chronic diseases (CVD cancer, cognitive function) | Clinical deficiency3: Serum folate <2 μg/L (21) RBC folate <95 μg/L (21) Functional deficiency: tHcy >13 μmol/L (33) |
Prevalence of folate deficiency was <1% in the era of post-fortification; NHB had lowest and NHW had highest folate status | 1999–2006 |
Vitamin B-6
|
Deficiency causes dermatitis, glossitis, confusion, anemia; low concentrations may modulate risk of chronic diseases (CVD, cancer, cognitive function) | Deficiency: PLP <20 nmol/L (14) |
Prevalence of vitamin B-6 deficiency was 11%, but differences noted by demographic subgroup; differences also found in PLP and 4PA concentrations by demographic subgroup | First-time assessment in 2005–2006 |
Vitamin B-12
|
Deficiency causes macrocytic anemia and may cause neurologic abnormalities; low concentrations may increase risk of NTD and may modulate risk of chronic diseases (CVD, cancer, cognitive function) | Deficiency: B-12 <200 ng/L (18) ;Functional deficiency: MMA >271 nmol/L (34) |
Prevalence of vitamin B-12 deficiency was higher in older persons (4%) than in the general population (2%); NHW had lowest vitamin B-12 status | 1999–2006 |
Vitamin C
|
Deficiency causes scurvy; has potential (in combination with vitamin E, zinc, β-carotene supplements) to slow progression of age-related macular degeneration | Clinical deficiency: Vitamin C <11.4 μmol/L (17) Low vitamin C concentrations: 11.4–23 μmol/L (17) |
Prevalence of vitamin C deficiency was 6%, but it varied by demographic subgroup | 2003–2006 |
Vitamin A
|
Deficiency may cause childhood blindness, night blindness, corneal thinning, and conjunctival metaplasia; low concentrations may impair immune function, growth and development | Severe deficiency: Vitamin A <0.35 μmol/L (19) Deficiency: Vitamin A <0.70 μmol/L (19) |
Prevalence of vitamin A deficiency was <1%; highest concentrations found in older persons | 1999–2002; 2005–2006 |
Vitamin E
|
Deficiency causes peripheral neuropathy | Deficiency: α-Tocopherol <11.6 µmol/L (20) |
Prevalence of vitamin E deficiency was <1% | 1999–2002; 2005–2006 |
Carotenoids
|
Good biological indicators of fruit and vegetable intake | No defined serum concentrations | Generally, highest concentrations in older persons | 2001–2002; 2005–2006 |
Vitamin D
|
Deficiency causes inadequate mineralization or demineralization of skeleton (rickets in children; osteomalacia in adults); low concentrations may affect muscle strength, risk for cancer or type 2 diabetes | At risk for deficiency: 25OHD <30 nmol/L (16) At risk for inadequacy: 25OHD 30–<50 nmol/L (16) Sufficient: 25OHD 50–75 nmol/L (16) Reason for concern: 25OHD >125 nmol/L (16) |
NHB had the lowest and NHW had the highest concentrations; likelihood of being vitamin D deficient was significantly influenced by race-ethnicity | 2001–2006 |
Fatty acids
|
Association between SFA and increased CVD risk; association between long-chain n3 PUFA and decreased CVD risk; potential association between long-chain n3 PUFA and improved visual and cognitive development in infant | No defined plasma concentrations | Race-ethnic differences found in concentrations of heart-healthy n3 PUFA EPA and DHA; ;no consistent race-ethnic patterns found within fatty acid groups; generally similar concentrations in men and women; generally lower concentrations in younger adults |
First-time assessment in 2003–2004 (fasted adults only) |
Iron
|
Deficiency has negative effects on cognitive development among infants and adolescents and eventually causes anemia | Depleted iron stores: Ferritin <15 μg/L (≥5 y) and <12 μg/L (<5 y) (22) Functional iron deficiency: Body iron <0 mg/kg (15) |
Prevalence of iron deficiency based on body iron was 10% for women of childbearing age and 7% for children, but it varied by race-ethnic group | 2003–2006 (children and women of childbearing age only) |
Iodine
|
Deficiency causes hypothyroidism, goiter, cretinism, growth and developmental abnormalities, and mental retardation | Population median urine iodine (μg/L) (13): Insufficient intake: <100 Adequate intake:100–199 Above requirements: 200–299 Excessive intake: ≥300 |
Iodine intake of US population was adequate; young women had lowest iodine intake, slightly above insufficient concentrations; urine iodine concentrations relatively stable over two decades (1988–2006) | 2001–2006 |
Phytoestrogens (isoflavones & lignans)
|
Association with reduced risk of hormone-dependent cancers, reduced severity of menopause-related symptoms, CVD, modulation of osteoporosis | No defined urine concentrations | No consistent patterns with regard to age, sex, or race-ethnicity; relatively similar concentrations from 1999–2006 | 1999–2006 |
Acrylamide
|
Neurotoxic and suspected human carcinogen; one exposure route is through food (starchy foods that are cooked at high-temperature and low-moisture conditions) | No defined hemoglobin adduct concentrations | No consistent patterns with regard to age, sex, or race-ethnicity for blood adduct concentrations; interesting patterns emerged for glycidamide-to-acrylamide adduct ratio (higher concentrations in children compared to other age groups; lower concentrations in NHB compared to other race-ethnic groups) | First-time assessment in 2003–2004 |
25OHD, 25-hydroxyvitamin D; 4PA, 4-pyridoxic acid; B-12, total cobalamin; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PLP (nmol/L), pyridoxal-5′-phosphate; tHcy, total homocysteine; MMA, methylmalonic acid; NHB, non-Hispanic black; NHW, non-Hispanic white; NTD, neural tube defect.
SI conversion factors are as follows: folate, ×2.266 (nmol/L); vitamin B-12, ×0.738 (pmol/L); ferritin, ×2.247 (pmol/L); iodine, ×7.88 (nmol/L).
Cutoff values used for serum (2 μg/L) and RBC folate (95 μg/L) are lower than the traditional microbiologic assay derived values (3 and 140 μg/L) to account for assay differences between the BioRad assay used in NHANES 2003–2006 and the microbiologic assay (21).
Figure 1.
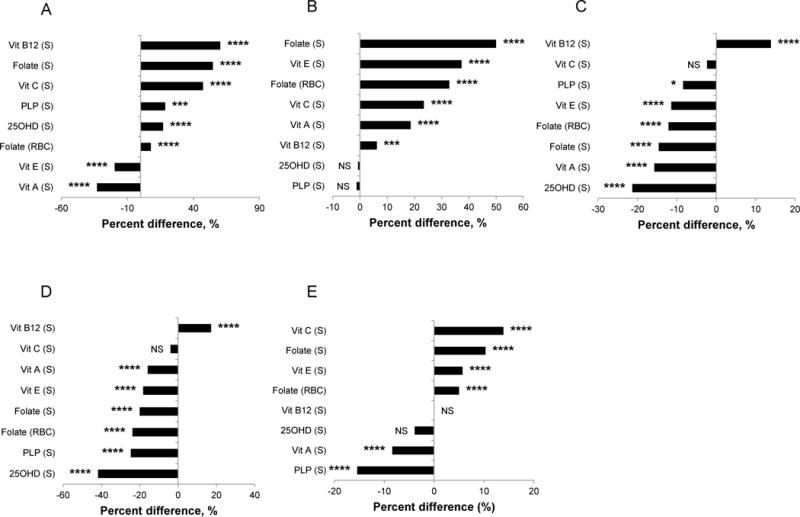
Relative differences in geometric mean concentrations (arithmetic mean for serum vitamin C) comparing two demographic subgroups in the US population aged ≥6 y, NHANES 2003–2006. Panel A: children (6–11 y) vs. young adults (20–39 y); panel B: older (≥60 y) vs. young (20–39 y) adults; panel C: MA vs. NHW; panel D: NHB vs. NHW; panel E: females vs. males. Vitamin B-6, A, and E data are only available for NHANES 2005–2006. The relative difference was obtained by calculating the difference between the geometric (arithmetic) mean concentration of the comparison group (mentioned first) and the reference group (mentioned second) divided by the geometric (arithmetic) mean concentration of the reference group, and expressed in percent. The level of significant difference is indicated as follows: * <0.05; ** <0.01; *** <0.001; **** <0.0001; NS, not significant, P <0.05. Refer to Supplemental Table 2 for sample sizes (n). 25OHD, 25-hydroxyvitamin D; MA, Mexican Americans; NHB, non-Hispanic blacks; NHW, non-Hispanic whites; PLP, pyridoxal-5′-phosphate; vit, vitamin.
The iodine intake of the US population was adequate on the basis of median urine iodine concentrations recommended by the WHO (13) (Fig. 2). However, young women (20–39 y) had significantly lower iodine intake compared to other female age groups (except for women 40–59 y), just slightly above the “insufficient intake” category. Furthermore, children (6–11 y) had significantly higher iodine intake compared to other age groups (except for men ≥60 y), placing children, particularly boys, into the “above requirements” category.
Figure 2.
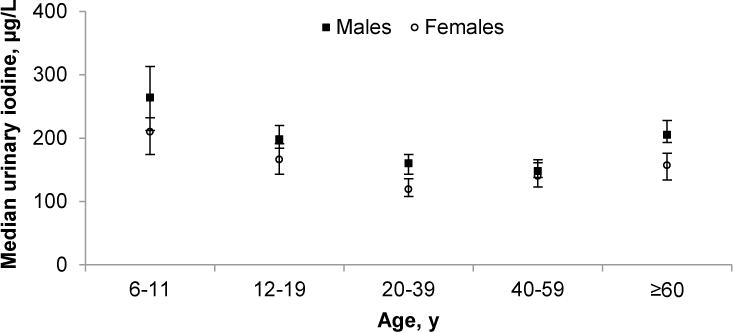
Median concentrations of urine iodine in the US population aged ≥6 y by age group and sex, NHANES 2001–2006. Median urine iodine concentrations (μg/L) <100, 100–199, 200–299, and ≥300 represent insufficient, adequate, above requirements, and excessive intake, respectively. Error bars represent 95% CI. Sample sizes (n) were as follows: males: 307 [6–11 y], 693 [12–19 y], 512 [20–39 y], 454 [40–59 y], 511 [≥60 y]; females: 359 [6–11 y], 750 [12–19 y], 622 [20–39 y], 465 [40–59 y], 502 [≥60 y]. The SI conversion factor (nmol/L) for urine iodine is ×7.88.
Some of the nutritional biomarkers that were measured as part of the continuous NHANES were also previously measured in NHANES III (1988–1994), allowing us to provide long-term trending information on changes in biomarker concentrations over time. Age-adjusted geometric mean concentrations of serum and RBC folate markedly and significantly increased (~50% and more) after the introduction of fortification of cereal-grain products with folic acid in 1998 (Fig. 3, panels A and B). We observed modest but significant decreases (~10%) in 25OHD concentrations from NHANES III to 2001–2002 (Fig. 2, panel C) and modest but significant increases (~15%) in urine iodine concentrations during the same time period (Fig. 2, panel D). Concentrations of serum vitamins A, B-12 and E remained generally unchanged with small fluctuations (<10%; in some cases significant) over almost two decades (Supplemental Fig. 1, panels A–C). Serum ferritin concentrations decreased modestly but significantly (~10%) in women of childbearing age from NHANES III to 2001–2002 (Supplemental Fig. 1, panel D).
Figure 3.
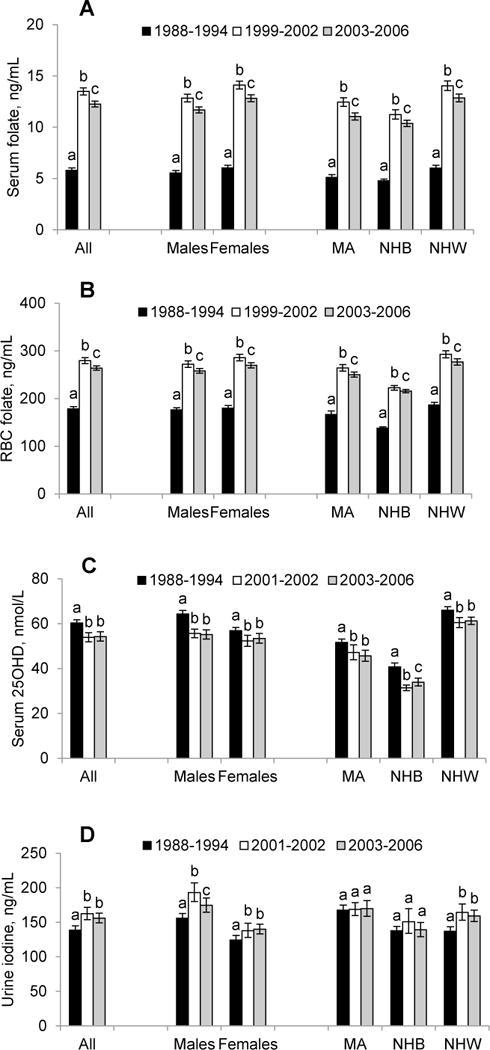
Time trends of age-adjusted geometric mean concentrations of serum folate (panel A, persons ≥4 y) and RBC folate (panel B, persons ≥4 y), serum 25-hydroxyvitamin D (panel C, persons ≥12 y), and urine iodine (panel D, persons ≥6 y) by sex and race-ethnicity in the US population, NHANES 1988–2006. Error bars represent 95% CI. Within a demographic group, bars not sharing a common letter differ (P <0.05). Age adjustment was done using direct standardization. Refer to Supplemental Table 3 for sample sizes (n). MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white. SI conversion factors are as follows: folate, ×2.266 (nmol/L); iodine, ×7.88 (nmol/L).
The prevalence of low concentrations for any single selected individual nutrient in the general US population (children and women for iron) was ~10% or less in NHANES 2003–2006 (Fig. 4). The four nutrients with the highest prevalence of persons at risk for deficiency (6–10.5%) were vitamin B-6 [PLP <20 nmol/L (14); ≥1 y], iron [serum body iron <0 mg/kg (15); children 1–5 y and women 12–49 y], vitamin D [25OHD <30 nmol/L (16); ≥1 y], and vitamin C [serum ascorbic acid <11.4 μmol/L (17); ≥6 y]. The four nutrients with the lowest prevalence (≤2%) were vitamins B-12 [serum total cobalamin <200 ng/L (148 pmol/L) (18); ≥1 y], A [serum retinol <70 μmol/L (19); ≥6 y], and E [serum alpha-tocopherol <11.6 μmol/L (20); ≥6 y], and folate [RBC folate <95 μg/L (215 nmol/L) (21); ≥1 y].
Figure 4.
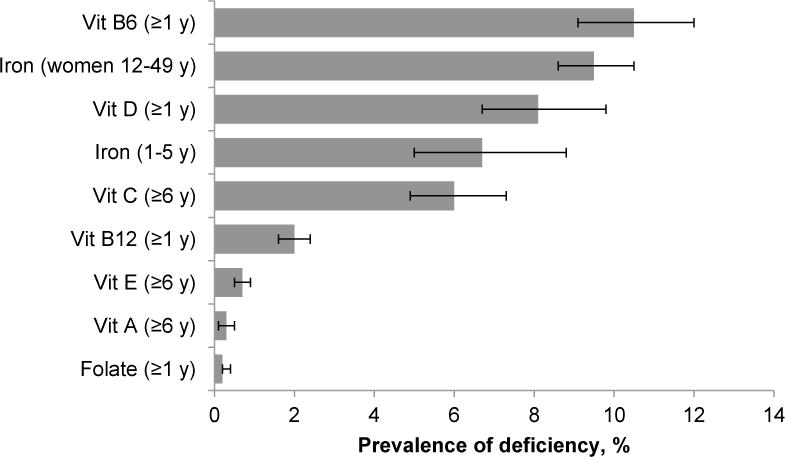
Prevalence estimates for risk of nutrient deficiencies in the US population, NHANES 2003–2006. Error bars represent 95% CI. Nutritional biomarkers were measured in different age (e.g., ≥1 y, ≥6 y) and population groups (e.g., women 12–49 y, children 1–5 y). Cutoff values (sample size) used to estimate prevalence are: vitamin B-6 (serum pyridoxal-5′-phosphate <20 nmol/L, [n = 8311]), iron (serum body iron <0 mg/kg, [n = 1369 children and 4476 women]), vitamin D (serum 25-hydroxyvitamin D <30 nmol/L, [n = 16604]), vitamin C (serum ascorbic acid <11.4 μmol/L, [n = 14579]), vitamin B-12 (serum total cobalamin <200 ng/L [148 pmol/L], [n = 16316]), vitamin A (serum retinol <0.70 μmol/L, [n = 7254]), vitamin E (serum α-tocopherol <11.6 µmol/L, [n = 7254]), and folate (RBC folate <95 μg/L [215 nmol/L], [n = 16670]). Vitamin B-6, A and E data are only available for NHANES 2005–2006.
For many nutritional biomarkers, the prevalence of low concentrations varied further by age, sex, or race-ethnicity. For example, compared to young adults (20–39 y; 9.9%) children and adolescents were significantly less likely to be at risk for vitamin B-6 deficiency (≤5%), while older adults were significantly more likely to be at risk (16%) (Fig. 5, panel A). NHB (31%) and MA (12%) were significantly more likely to be at risk for vitamin D deficiency compared to NHW (3%) (Fig. 5, panel B). MA children (11%) were significantly more likely to be at risk for iron deficiency compared to NHB (5%) and NHW children (6%) (Fig. 5, panel C). MA (13%) and NHB (16%) women of childbearing age were significantly more likely to be at risk for iron deficiency compared to NHW women (7%) (Fig. 5, panel D).
Figure 5.
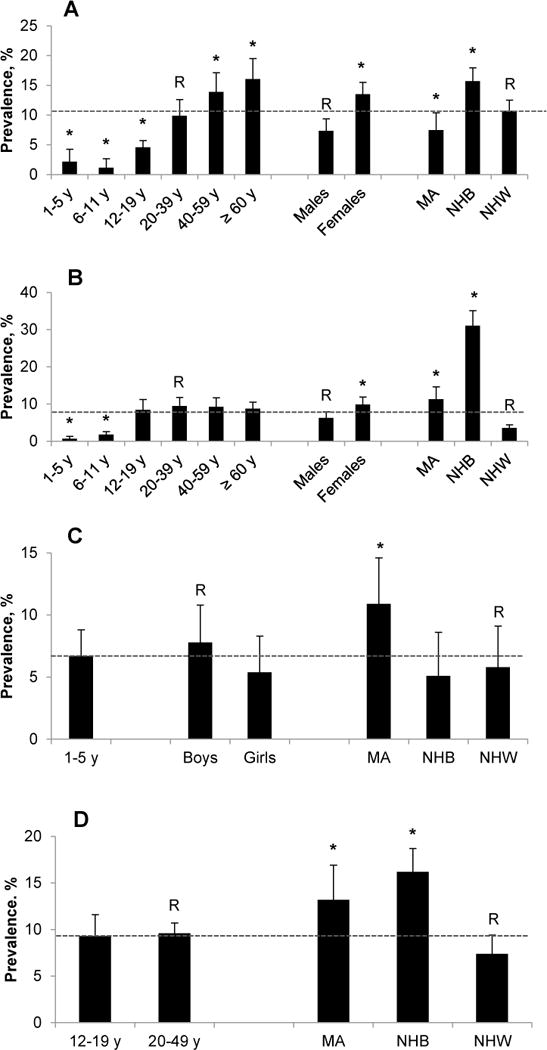
Prevalence estimates for risk of nutrient deficiencies by age, sex, and race-ethnicity in the US population, NHANES 2003–2006. Panel A: vitamin B-6 deficiency in persons ≥1 y (serum pyridoxal-5′-phosphate <20 nmol/L); panel B: vitamin D deficiency in persons ≥1 y (serum 25-hydroxyvitamin D <30 nmol/L); panel C: iron deficiency in children 1–5 y (serum body iron <0 mg/kg); panel D: iron deficiency in women 12–49 y (serum body iron <0 mg/kg). Error bars represent 95% CI. Within a demographic group, bars labeled with an asterisk indicate a significant difference (P <0.05) relative to the reference group R. Dotted horizontal lines represent the overall prevalence for the population captured in each panel. Vitamin B-6 data are only available for NHANES 2005–2006. Refer to Supplemental Table 4 for sample sizes (n). MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white.
Since the release of the Second Nutrition Report, we assessed the prevalence of multiple low biomarker concentrations in NHANES 2005–2006 to better estimate the burden of risk for deficiencies in the US population. Nearly 8 of every 10 Americans (78%) and almost three-quarters of women of childbearing age (12–49 y) were not at risk for deficiencies in any of the 7 vitamins (A, B-6, B-12, C, D, E and folate) studied in the Second Nutrition Report (Table 2). Sixteen percent of the population was at risk for deficiency in 1 vitamin and 6% had 2 or more vitamin concentrations low enough to be at risk for deficiencies. About 9 of every 10 women of childbearing age were not at risk for iron deficiency, but only two-thirds of women were not at risk for deficiencies in iron and all 7 vitamins. Twenty-three percent of women of childbearing age had 1 and 9% had 2 or more vitamin concentrations suggesting risk for deficiencies.
Table 2.
Percentage of the US population ≥6 y and of women of childbearing age at risk for nutritional deficiencies, NHANES 2005–2006.
| Unweighted sample size | Weighted percentage1 | Estimated total number of persons2 | |
|---|---|---|---|
| Population ≥6 y, 7 nutrients considered3 | |||
| 0 deficiency | 5188 | 78.0 (74.7, 81.0) | 208 500 000 |
| 1 deficiency | 1404 | 16.3 (14.3, 18.6) | 43 600 000 |
| ≥2 deficiencies | 449 | 5.7 (4.4, 7.3) | 15 200 000 |
| Women 12–49 y, iron considered4 | |||
| 0 deficiency | 2344 | 89.6 (88.4, 90.7) | 71 000000 |
| Women 12–49 y, 7 nutrients considered3 | |||
| 0 deficiency | 1506 | 73.6 (69.1, 77.6) | 58 300 000 |
| 1 deficiency | 574 | 20.1 (17.7, 22.7) | 15 900 000 |
| ≥2 deficiencies | 167 | 6.4 (4.3, 9.4) | 5 100 000 |
| Women 12–49 y, 8 nutrients considered5 | |||
| 0 deficiency | 1358 | 67.5 (63.5, 71.3) | 53 500 000 |
| 1 deficiency | 621 | 23.3 (20.9, 25.9) | 18 500 000 |
| ≥2 deficiencies | 266 | 9.2 (6.7, 12.5) | 7 300 000 |
Estimates provided are percent (%) with 95% CI in parenthesis
Current Population Survey 2005–2006 available at (7).
Biochemical indicators and cutoffs used for deficiency definition: folate (RBC folate <95 μg/L [215 nmol/L]); vitamin B-6 (serum pyridoxal-5′-phosphate <20 nmol/L); vitamin B-12 (serum total cobalamin <200 ng/L [148 pmol/L]); vitamin C (serum ascorbic acid <11.4 μmol/L); vitamin A (serum retinol <0.70 μmol/L); vitamin E (serum alpha-tocopherol <11.6 μmol/L); and vitamin D (serum 25-hydroxyvitamin D <30 nmol/L)
Iron deficiency (body iron <0 mg/kg)
Biochemical indicators and cutoffs used for deficiency definition: same 7 vitamin deficiency indicators and cutoffs as listed above plus 1 indicator for iron deficiency (body iron <0 mg/kg)
New data presented in the Second Nutrition Report
A panel of 24 saturated, mono-, and polyunsaturated fatty acids was measured for the first time in NHANES during the 2003–2004 survey period (plasma from fasted adults ≥20 y). In general we found that younger adults (20–39 y) had lower plasma fatty acid concentrations compared to older adults (≥60 y). Plasma fatty acid concentrations were generally similar in men and women. For most fatty acids we did not find race-ethnic patterns that were consistent within class (SFA, MUFA or PUFA). However, geometric means of the PUFA eicosapentaenoic acid (EPA), which is typically derived from seafood and supplements, were significantly higher in NHB and NHW compared to MA adults. In addition, plasma concentrations of the related long-chain PUFA docosahexaenoic acid (DHA) were significantly higher in NHB compared to MA and NHW adults.
A new marker of iron status, body iron, was used for the first time in this report to generate reference information for children and women of childbearing age. This marker is calculated from the ratio of soluble transferrin receptor to serum ferritin (15), is less affected by inflammation than the acute-phase protein ferritin, and can assess the full spectrum of iron status of populations. Body iron is in a positive balance (≥0 mg/kg) when there is residual storage iron or in negative balance (<0 mg/kg) when there is functional iron deficiency (15). We found a significantly lower prevalence (mean [95% CI]) of risk for iron deficiency in women of childbearing age (12–49 y) using body iron (<0 mg/kg; 9.5% [8.6–10.5%]) compared to using serum ferritin as an indicator (<15 μg/L (22); 13.6% [12.2–15.2%]), possibly because serum ferritin can be elevated in the presence of inflammation, thus interfering with its association with iron deficiency.
The Second Nutrition Report presents a first assessment of acrylamide exposure in the US population. Acrylamide occurs in a wide range of food products commonly consumed by a large portion of the population; potato chips, French fries and some baked goods contain notable concentrations. Exposure to acrylamide from food and other sources (e.g., smoking) is of concern, because acrylamide is “reasonably anticipated to be a human carcinogen” and a potentially endocrine disrupting chemical. Hemoglobin adducts of acrylamide and glycidamide, an epoxide of acrylamide formed in the body, reflect the internal dose of acrylamide during the preceding 2–4 mo. We found detectable concentrations of these hemoglobin adducts in nearly all blood samples measured as part of NHANES 2003–2004.
DISCUSSION
The CDC Second Nutrition Report provides the single most comprehensive biochemical assessment of the US population’s nutritional status to date. Other sources of nutritional biomarker reference data, such as textbooks and research publications, may be outdated, generally do not provide information by demographic subgroups, or use inconsistent data analysis approaches, resulting in data that cannot be readily compared. NCHS has historically released or commissioned a variety of products presenting NHANES results (23). NHANES Series Reports (mainly Series 11) and Life Sciences Research Office Reports from surveys prior to the continuous NHANES have been of particular value to the nutrition community; however such reports have not been available for the continuous NHANES.
The consistent data analysis approach of the Nutrition Report is one of its major strengths. By presenting reference data on population distributions in standardized tables and graphs, it is now possible to conduct comparative analyses across demographic subgroups and biomarkers, as well as across survey periods. This feature is of particular value when generating research hypotheses, and a necessity when evaluating the effectiveness of public health interventions to improve the nutritional status of the population. Another strength of the Nutrition Report is that it is based on data from 2 survey cycles (when available), which provides adequate sample size to compute statistically reliable estimates for the main race-ethnic categories as well as robust central 95% reference intervals for nutritional biomarkers by age, sex, or race-ethnicity. It also allows multi-level stratifications to describe population distributions by combinations of age, sex, and race-ethnicity.
The number of report findings is too large and topic areas too numerous to permit a comprehensive discussion in this work; however, a selection of noteworthy findings will be discussed, the first being the significant public health success of folic acid fortification in the United States. The sustained positive impact of adding folic acid to the US food supply since 1998 was highlighted in the Second Nutrition Report and has also been reported in a recent analysis of NHANES data up to 2010 (24). Other countries that introduced folic acid fortification also found the prevalence of folate deficiency to be <1% post-fortification, for example in the general Canadian population from the Canadian Health Measures Survey (25) and in a convenience sample of Chilean women of reproductive age (26).
The Second Nutrition Report finding that risk for vitamin D deficiency [defined by the Institute of Medicine cutoff value of <30 nmol/L (16)] was highest among black Americans compared to the other 2 race-ethnic groups (NHW and MA) has also been confirmed in a recent analysis of NHANES 2001–2006 data (27). Further research is needed to understand whether African Americans have lower requirements for vitamin D to maintain bone health, as clinical data show that they have greater bone density and fewer fractures than other race-ethnic groups (28), or possibly factors other than vitamin D affect these outcomes.
A third important finding of the Second Nutrition Report was that young women (20–39 y) had the lowest iodine status compared to most other age groups. This is of concern because consuming an adequate amount of iodine during pregnancy is critical for fetal neurologic development. Perrine et al. identified dairy products as an important contributor to iodine status in women of reproductive age in the United States, and the authors concluded that some subgroups of women may be at risk for iodine deficiency, particularly women who do not consume dairy products (29).
Nutrient deficiencies are well documented, and have characteristic signs and symptoms; however, cutoff values that define nutrient inadequacies are not without controversy and generally define increased risk. For example, there is a lack of scientific consensus regarding cutoff values to determine low vitamin B-12 status and Bailey et al. have shown that depending on the cutoff value used for either serum B-12 or methylmalonic acid, widely different prevalence estimates are obtained (30). Furthermore, not all generally accepted cutoff values necessarily represent clinical deficiency. The cutoff value of 20 nmol/L for low serum PLP concentrations was selected by the Institute of Medicine as the basis for the Estimated Average Requirement and it may overestimate the vitamin B-6 requirement for health maintenance of more than half the group (11). A plasma PLP concentration of 20 nmol/L is not accompanied by observable health risks but it allows a moderate safety margin to protect against the development of signs or symptoms of deficiency. Epidemiologic studies often determine nutritional biomarker concentrations that are “suboptimal” and are associated with an increased risk of adverse health effects. Determining the concentrations of nutritional biomarkers that may indicate risk for disease and the concentrations that are of negligible health concern requires research studies that allow making causal inferences and are separate from NHANES or the Nutrition Report. Nonetheless, the Nutrition Report provides the population data to estimate the magnitude of a health problem once threshold levels have been determined.
Using generally accepted cutoff values for nutrient deficiencies in the Second Nutrition Report, we found that a large portion (~80%) of the US population was not at risk for deficiencies in 7 vitamins. Yet, 6% of the population was at risk for deficiencies in 2 or more vitamins. Having such information for a population is not only invaluable, but also globally unique. Not many countries conduct nationally representative nutrition surveys, and even if they do, the number of nutrients covered and nutritional biomarkers assessed is much smaller than in the NHANES.
The Second Nutrition Report presented first-time reference data for many nutritional biomarkers. Data on vitamin B-6 and C provided a more complete assessment of vitamin status. The first measurements of fatty acids in the US population provided a baseline to track fatty acid concentrations over time, which will evaluate our nation’s progress toward more heart-healthy diets. The inclusion of a new marker of iron deficiency improved the diagnosis of iron status in the two vulnerable groups, children and women of childbearing age.
The Nutrition Report has limitations. First, it does not cover information beyond biochemical indicators, such as dietary intake, supplement usage, hematologic measurements, and anthropometric body measurements, which are generally used together with the biomarker information to comprehensively assess nutritional status. The USDA’s What We Eat in America is the dietary intake interview component of NHANES and provides data on food and nutrient intakes of Americans (31). Second, adequate biomarker concentrations of specific nutrients may not necessarily indicate that people are eating healthy and balanced diets because nutritional biomarkers reflect cumulative intakes from foods, some fortified with micronutrients, and from dietary supplements, as well as physiological effects such as bioavailability. Third, the Nutrition Report does not make recommendations about diet or lifestyle. The Dietary Guidelines for Americans (32) provide recommendations about what to eat, and professional associations offer guidelines on nutrition related risk factors that influence health outcomes. Other limitations of the Nutrition Report and of NHANES are that certain population groups (pregnant and lactating women, infants <1 y of age) are under-represented or missing completely, resulting in a lack of normative data in these important groups, not all nutrients are covered, and not all race-ethnic groups are adequately oversampled to provide basic statistical information such as means and percentiles. An important limitation of the Nutrition Report is that it describes the biochemical characteristics of the population and of subgroups, but provides only limited interpretation of relative differences in nutritional status by age, sex, and race-ethnicity.
As an extension of the Second Nutrition Report, we have conducted descriptive modeling analyses examining the association of 10 preselected sociodemographic (age, sex, race-ethnicity, education, and income) and lifestyle variables (dietary supplement use, smoking, alcohol consumption, BMI, and physical activity) with 29 biomarkers of nutritional status in order to assess whether demographic differentials in nutritional status were confounded by the above mentioned variables. The findings from these analyses, presented in the collection of papers in this journal supplement, shed light on important intermediaries between the association of diet and health. Biomarkers are mediators of this important association and factors influencing biomarkers are often understudied. Each paper addresses a different class of diet and nutrition biomarkers covered in the Second Nutrition Report: water-soluble vitamins, fat-soluble vitamins and nutrients, trace elements, phytoestrogens (isoflavones and lignans), and acrylamide hemoglobin adducts. Two additional papers address topics complementary to these analyses: 1 paper discusses the statistical approach used for these analyses and how this approach differs from other approaches used to model observational data; the other paper addresses the association of nutritional biomarkers with selected preanalytical and physiological variables, such as fasting, time of specimen collection, inflammation, renal function, and pregnancy, to add another dimension to the interpretation of biomarker concentrations. As with the Nutrition Report, we conducted these analyses in a systematic way to allow a comparison of findings across biomarkers and therefore chose to forfeit insight into the association between variables unique to each individual biomarker. The collective information from these analyses will not only help investigators better interpret currently available data on biomarker concentrations, but it will also provide a foundation to investigators planning future nutrition studies or developing predictive models to study the association of nutritional status with health outcomes.
Supplementary Material
Acknowledgments
The authors acknowledge contributions from the following laboratory members: William Brown, Kathleen Caldwell, Madhu Chaudhary-Webb, Huiping Chen, Kara Dobbins, Dana Henahan, Leigha Ingham, Jeff Jarrett, Donna LaVoie, Tunde Meyers, Shahzad Momin, Jenny Pao, Daniel Parker, Elizabeth Pendergrast, Carissa Powers, Deanna Scott, Antoinette Smith, Hubert Vesper, Mary Xu, and Mindy Zhang. C.M.P, R.L.S, and M.R.S. designed the overall research project; C.M.P, R.L.S, M.R.S., B.M.H.H. conducted most of the research; M.R.S. analyzed most of the data; and C.M.P. wrote the initial draft, which was modified after feedback from all coauthors, and had primary responsibility for content. All authors read and approved the final manuscript.
Footnotes
No specific sources of financial support. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry or the Department of Health and Human Services.
Author disclosures: C.M. Pfeiffer, M.R. Sternberg, R.L. Schleicher, B.M.H. Haynes, M.E. Rybak, J.L. Pirkle, no conflicts of interest.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: 25OHD, 25-hydroxyvitamin D; LOD, limit of detection; MA, Mexican Americans; NCEH/DLS, National Center for Environmental Health/Division of Laboratory Sciences; NCHS, National Center for Health Statistics; NHB, non-Hispanic blacks; NHW, non-Hispanic whites; PLP, pyridoxal-5′-phosphate; RSE, relative standard error.
References
- 1.Yetley E, Johnson C. Nutritional applications of the Health and Nutrition Examination Survey (HANES) Annu Rev Nutr. 1987;7:441–63. doi: 10.1146/annurev.nu.07.070187.002301. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Borrud LG, McDowell MA, Wang C-Y, Radimer K, Johnson CL. Nutrition assessment in the National Health and Nutrition Examination Survey 1999-2002. J Am Diet Assoc. 2007;107:822–9. doi: 10.1016/j.jada.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. [cited 2012 Aug 24]. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 4.U.S. Centers for Disease Control and Prevention. Second National Report on Biochemical Indicators of Diet and Nutrition in the US Population 2012. Atlanta, GA: National Center for Environmental Health; Apr, 2012. [cited 2013 Feb 4]. Available from: http://www.cdc.gov/nutritionreport. [Google Scholar]
- 5.U.S. Centers for Disease Control and Prevention. National Report on Biochemical Indicators of Diet and Nutrition in the US Population 1999–2002. Atlanta, GA: National Center for Environmental Health; Jul, 2008. [cited 2013 Feb 01]. Available from: http://www.cdc.gov/nutritionreport. [Google Scholar]
- 6.Pfeiffer CM, Schleicher RL, Johnson CL, Coates PM. Assessing vitamin status in large population surveys by measuring biomarkers and dietary intake – two case studies: folate and vitamin D. Food Nutr Res. 2012;56:5944. doi: 10.3402/fnr.v56i0.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Centers for Disease Control and Prevention. NHANES response rates and CPS totals. [cited 2013 Feb 4]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm.
- 8.U.S. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2003–2004 Lab Methods. [cited 2013 Feb 4]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/lab_methods_03_04.htm.
- 9.U.S. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2005–2006 Lab Methods. [cited 2013 Feb 4]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/lab_methods_05_06.htm.
- 10.Woodruff RS. Confidence intervals or medians and other position measures. J Am Stat Assoc. 1952;57:622–7. [Google Scholar]
- 11.U.S. Centers for Disease Control and Prevention. NHANES analytic guidelines, the Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) Hyattsville (MD): National Center for Health Statistics; Oct, 1996. [cited 2013 Feb 4]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf. [Google Scholar]
- 12.Curtin LR, Mohadjer L, Dohrmann S, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL. The National Health and Nutrition Examination Survey: Sample design, 1999–2006. National Center for Health Statistics. Vital Health Stat. 2(155):2012. [PubMed] [Google Scholar]
- 13.World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd. Geneva (Switzerland): World Health Organization; 2007. ((WHO/NHD/01.1)). [cited 2013 Feb 4]. Available from: http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf. [Google Scholar]
- 14.Institute of Medicine Food Nutrition Board. Dietary reference intakes: Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, D.C: National Academy Press; 1998. [PubMed] [Google Scholar]
- 15.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–64. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine Food and Nutrition Board. Dietary reference intakes for calcium and vitamin D. Washington, D.C: National Academies Press; 2011. [PubMed] [Google Scholar]
- 17.Gibson RS. Principles of nutritional assessment. 2nd. New York: Oxford University Press; 2005. [Google Scholar]
- 18.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr. 2011;94:348S–58S. doi: 10.3945/ajcn.111.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West KP., Jr . Vitamin A: deficiency and interventions. In: Caballero B, Allen L, Prentice A, editors. Encyclopedia of human nutrition. 2nd. Amsterdam: Elsevier Ltd; 2006. pp. 348–59. [Google Scholar]
- 20.Beers MH, editor. Merck Manual of Diagnosis and Therapy. 18th. Whitehouse Station, (NJ): Merck & Co Inc; 2006. Vitamin deficiency, dependency, and toxicity. [cited 2013 Feb 4]. Available from: http://www.merck.com/mmpe/sec01/ch004/ch004l.html. [Google Scholar]
- 21.Wright JD, Bialostosky K, Gunter EW, Carroll MD, Najjar MF, Bowman BA, Johnson CL. Blood folate and vitamin B12: United States, 1988–1994. National Center for Health Statistics, Vital Health Stat Series No. 1998;11(243) [PubMed] [Google Scholar]
- 22.WHO. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. ((WHO/NMH/NHD/MNM/11.2)). [cited 2013 Feb 4]. Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf. [Google Scholar]
- 23.U.S. Centers for Disease Control and Prevention. Survey results and products from the National Health and Nutrition Examination Survey. [cited 2013 Feb 4]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_products.htm.
- 24.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, Yetley EA, Rader JI, Sempos CT, Johnson CL. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr. 2012;142:886–93. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colapinto CK, O’Connor DL, Tremblay MS. Folate status of the population in the Canadian Health Measures Survey. Can Med Assoc J. 2011;183:E100–6. doi: 10.1503/cmaj.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertrampf E, Cortes F, Erickson JD, Cayazzo M, Freire W, Bailey LB, Howson C, Kauwell GPA, Pfeiffer C. Consumption of folic acid-fortified bread improves folate status in women of reproductive age in Chile. J Nutr. 2003;133:3166–9. doi: 10.1093/jn/133.10.3166. [DOI] [PubMed] [Google Scholar]
- 27.Ganji V, Zhang Xu, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142:498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 28.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88:545S–50S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrine CG, Herrick K, Serdula MK, Sullivan KM. Some subgroups of reproductive age women in the United States may be at risk for iodine deficiency. J Nutr. 2010;140:1489–94. doi: 10.3945/jn.109.120147. [DOI] [PubMed] [Google Scholar]
- 30.Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B12 nutritional status in the United States using plasma methylmalonic acid and serum vitamin B12. Am J Clin Nutr. 2011;94:552–61. doi: 10.3945/ajcn.111.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Department of Agriculture. What We Eat in America. [cited 2013 Feb 4]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=13793.
- 32.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th. Washington, DC: U.S. Government Printing Office; Dec, 2010. [cited 2013 Feb 4]. Available from: http://www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacques PF, Selhub J, Bostom AG, Wilson PF, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 34.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: Usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol. 1990;34:90–8. doi: 10.1002/ajh.2830340204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


