Abstract
Long-term use of anti-diabetic agents has become commonplace as rates of obesity, metabolic syndrome and diabetes continue to escalate. Metformin, a commonly used anti-diabetic drug, has been shown to have many beneficial effects outside of its therapeutic regulation of glucose metabolism and insulin sensitivity. Studies on metformin’s effects on the central nervous system are limited and predominantly consist of in vitro studies and a few in vivo studies with short-term treatment in relatively young animals; some provide support for metformin as a neuroprotective agent while others show evidence that metformin may be deleterious to neuronal survival. In this study, we examined the effect of long-term metformin treatment on brain neurotrophins and cognition in aged male C57Bl/6 mice. Mice were fed control (C), high-fat (HF) or a high-fat diet supplemented with metformin (HFM) for 6 months. Metformin decreased body fat composition and attenuated declines in motor function induced by a HF diet. Performance in the Morris water maze test of hippocampal based memory function, showed that metformin prevented impairment of spatial reference memory associated with the HF diet. Quantitative RT-PCR on brain homogenates revealed decreased transcription of BDNF, NGF and NTF3; however protein levels were not altered. Metformin treatment also decreased expression of the antioxidant pathway regulator, Nrf2. The decrease in transcription of neurotrophic factors and Nrf2 with chronic metformin intake, cautions of the possibility that extended metformin use may alter brain biochemistry in a manner that creates a vulnerable brain environment and warrants further investigation.
Keywords: metformin, BDNF, water maze, Nrf2, NGF, neurotrophin 3
1. INTRODUCTION
The incidence of type II diabetes (T2DM) continues to emerge in epidemic proportions throughout the developed world. This disease is ranked fourth in leading causes of global death by disease [1]. The NIH reports that about 8.3% of the United States is affected by diabetes; and for those aged over 65 years, this rate increases to 26.9% [2, 3]. Given that obesity and physical inactivity are the primary factors that lead to the development of T2DM, the vast majority of cases could be controlled or reversed through lifestyle changes, such as increased exercise and a modification of dietary habits. Still, most type-2 diabetics require treatment with prescribed medication for prolonged periods of time [3], often several years. Consequently, chronic use of anti-diabetic agents is increasingly prevalent within general populations.
Several effective anti-diabetic agents have been developed and are currently in use. These agents provide benefits such as increased insulin sensitivity through up-regulation of glucose transporters, a suppressed rate of intestinal glucose absorption by inhibition of α-glucosidases, and increased insulin secretion from beta cells [4]. These treatments are important for regulating blood glucose and insulin levels, thereby preventing the consequential damage to peripheral organs and nerves.
Aside from the injurious effects on peripheral systems, T2DM also has deleterious effects on brain function. Several studies have shown impaired cognition and memory performance in patients with T2DM [5–7]. Others have shown an increased risk for the development of dementia and specifically, Alzheimer’s disease (AD) [8–10]. Additionally, studies suggest that insulin resistance in the brain is a major contributing factor to the symptoms of AD, ultimately affecting synaptic plasticity. Conversely, effective glycemic control is associated with cognitive improvement [11]. With this correlation between cognition andT2DM, it would be important to be aware of any potential risks and or benefits of commonly used diabetes treatments on brain health. Interestingly, one postmortem study, which examined the association between diabetes treatments and AD neuropathology, found that only in combination with insulin treatment are anti-diabetic drugs associated with decreased AD neuropathology [12]. Therefore, there is growing interest in evaluating the impact of anti-diabetic agents on brain chemistry and function.
Metformin is a highly prescribed anti-hyperglycemic drug. It is often a first line oral treatment for T2DM, and one of only two oral anti-diabetics listed in the 16th World Health Organization Model List of Essential Medicines and is currently being investigated, in clinical trials, as a potential treatment for AD. Metformin is known to elicit many of its effects through 5′ adenosine monophosphate-activated protein kinase (AMPK) activation which in turn decreases gluconeogenesis in the liver and improves insulin binding to receptors in liver and muscle thus increasing insulin sensitivity and glucose uptake [13]. AMPK activation by metformin has been shown to be secondary to its inhibition of mitochondrial respiratory chain complex 1[14]. Metformin has also been shown to decrease intestinal absorption of glucose [15, 16]. Studies on metformin’s effects in the central nervous system are limited and predominantly consist of in vitro studies, some of which show support for metformin as a neuroprotective agent [17], increasing hippocampal neurogenesis and enhancing cognitive function [18] however, other studies have shown evidence that metformin may increase risk of developing Alzheimer’s disease [19] and be deleterious to neuronal survival acting through its AMPK activating mechanism [20]. Most of these studies utilize a paradigm of short-term metformin treatment.
In this study, we used a mouse model to assess effects of a high-fat (HF) diet and long-term treatment with metformin on brain neurotrophins and cognitive function.
2. MATERIALS AND METHODS
2.1 Animals and Diets
Forty eight, twelve month-old male C57Bl/6 mice were obtained from the breeding colony at the National Institute on Aging (NIA). Mice were randomly assigned to one of three dietary groups: standard/control (C), high fat (HF), and high fat plus metformin (HFM) (n=16/group). Diets included the AIN-93G standard diet (C), AIN-93G modified high fat diet to provide 60% of calories from fat by the addition of hydrogenated coconut oil (HF), and the AIN-93G modified high fat diet with the addition of 1.0% metformin by weight (HFM). Mice were maintained on the diets for 6 months. During the first month all animals were fed ad libitum (AL). Food intake and body weight were measured on a biweekly basis. To ensure that effects seen in the metformin treated group did not result from differences in caloric intake, after the initial four weeks, HF-fed mice were fed daily an amount of HF diet that was equivalent to the average daily caloric intake of mice fed on the HFM diet. After 4 months on respective diets, mice were tested for learning and memory performance, motor coordination, body composition and glucose tolerance according to procedures described below.
2.2 Housing
Animal housing conditions consisted of a 12-hour light/dark cycle with temperatures between 22–24°C (according to animal protocols and NIH guidelines). Mice were single housed in cages which contained a voluntary free-spinning running wheel. Running wheels were connected to a computerized automated monitoring system which kept record of the number of wheel rotations for each individual cage (Columbus Instruments, Columbus, OH).
2.3 Rotarod Motor Performance
Motor coordination performance was tested using an automated, motorized rotarod treadmill for mice (Med Associates Inc., St. Albans, VT). The rotating drum (3 cm in diameter) is divided into test zones, by round divider plates, allowing for 5 mice to be tested at one time. Mice were habituated to the rotarod one day prior to testing by first being placed on the non-rotating drum for 10seconds(s) immediately followed by a 120s period with the drum rotating at a constant 4.0 rpm. During testing, mice were placed on the rotating drum which was set to gradually accelerate from 4 to 40 rpm over a 300s interval. Mice were forced to move at increasing speeds to avoid the 16.5 cm fall to the platform. Each mouse received 3 trials, with a 30-min inter-trial interval. Latencies before falling were measured and averaged across the 3 trials as the dependent variable.
2.4 Morris Water Maze
The Morris water maze test was used to measure hippocampal based spatial memory and learning function. The water maze apparatus consisted of a white circular plastic tank (100 cm diameter and 70 cm high) which was filled with water (24±1 C) made opaque by the addition of white DryTemp® paint powder (Palmer Paint Products Inc., Troy, MI, USA). Visual cues of objects varying in geometric shapes and shades were affixed to a clear, plastic cylinder surrounding the interior wall of the pool and extending approximately 30 cm above the pool surface. A clear, circular (10 cm diameter) escape platform was submerged a few millimeters below the water surface. Mice were trained 4 trials/day, for 5 consecutive days. Each acquisition trial was started by placing the mouse in the water facing the wall of the tank. The location of entry of the mouse was changed for every trial such that mice entered the maze from each of 4 different start positions each day representing four quadrants of the maze. The order of start position randomly set each day. During each trial, mice were given 60s to locate the submerged platform. If a mouse did not locate the platform, it was gently led to the platform. After either finding or being led to the platform, mice were left on the platform for 30s in order to get familiarized with its location with respect to the visual cues. A camera mounted on the ceiling in the center of the pool was used to track the swim route of the mouse. Data were collected using a computerized animal tracking system (HVS Image with Water 2020 Software, Buckingham, UK) which recorded path length, swim speed, time spent in each quadrant of the pool and time taken to reach platform (latency). Animals were tested in squads of 8 mice, and all treatment groups were represented within each testing squad. Inter-trial interval for each mouse was approximately 20 minutes. Time (%) spent in each quadrant was recorded and averaged for each group. On day 6 mice performed a probe trial where the escape platform was removed, and the amount of time spent searching in each quadrant of the pool was recorded for 60 seconds to assess recall of the platform location. Day 7 included a visual test, which tests the mice ability to locate the escape platform when it is marked with a clearly visible flag. Time and path taken to reach the platform was recorded. Trials were consistently performed from 2pm to 4pm.
2.5 Body Composition
Measurements of total lean tissue, fat and fluid mass of live mice were acquired using the Minispec LF90 nuclear magnetic resonance spectroscopy (NMR) analyzer (Bruker Optics, Inc.). Mice were guided into a plastic animal restrainer which was then placed into the Minispec probe. Data are automatically acquired within 90 seconds. The mice are then immediately returned to their home cage.
2.6 Glucose Tolerance Test (GTT)
For oral glucose tolerance tests (OGTTs), mice that had been fasted overnight (16 hrs) received an oral dose of 2 g/kg bodyweight of D-glucose in a 20% solution by oral gavage. Immediately before, and 15, 30, and 60 min after glucose administration, glucose was measured in retro-orbital blood using an Ascensia Elite glucose meter (Bayer, Mishawaka, IN).
2.6 Sacrifice
At the end of the 6-month period, mice were anesthetized with isoflurane. Brains and other tissues were excised, flash frozen in liquid nitrogen, and stored at −80°C until use.
2.7 Western Blot
Sagittally cut hemi-brains brains were homogenized in ice-cold RIPA buffer (PBS, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS) with freshly added protease inhibitors (Protease Inhibitor Cocktail P8340 and, Phosphatase Inhibitor Cocktail 1&2, Sigma-Aldrich, St. Louis, MO; Phenylmethylsulfonylfluoride (PMSF), Fluka-Biochemica, Switzerland), incubated on ice for 30 min and centrifuged at 14,000 g for 10 min at 4°C. After centrifugation, the supernatant was collected and total protein concentrations were measured using the Bradford method. Tissue lysate was added to electrophoresis sample buffer and separated by SDS/PAGE under reducing conditions on a 12% separation gel. Proteins were transferred to nitrocellulose membranes using the iBlot dry-blotting system (Invitrogen Corp., Carlsbad, CA). Unspecific binding was blocked by incubation in 5% milk blocking buffer (PBS, 5% nonfat milk and 0.1% Tween 20). Membrane bound proteins were immunoblotted with antibodies to BDNF, NGF, NT3, Nrf2 (Santa Cruz Biotech, Santa Cruz, CA), p-AMPK, AMPKα (Cell Signaling, Beverly, MA), p-Nrf2 (BIOSS Antibodies, Woburn, MA) and β-actin (Abcam Inc., Cambridge, MA). Signals were developed using ECL reagent (Amersham Pharmacia Biotech, Buckinghamshire, England). Densities of the bands were evaluated using Image J software.
2.8 Transcription Analysis
Total RNA from hemisected brain tissue were extracted by using Qiagen RNeasy Kit (Qiagen) and quantified by a spectrophotometer (Thermoscientific NanoDrop 1000). Only samples that met the criteria of quality (260/280 nm > 1.8) were included in the experiments. Reverse transcriptase (RT) was performed by using 2 μg of total RNA with Applied Biosystem’s High capacity cDNA reverse Transcription Kit (Applied Biosystems). Real-time PCR for NGF, NTF3, BDNF, Nrf2, AMPK and GAPDH utilized Taq Man Gene Expression Assay kits (Applied Biosystems, Foster City, CA). All real-time PCR was performed using the ABI Prism 7700 sequence detection system (Applied Biosystems). Each PCR contained between 2– 5.0 ng of reverse-transcribed RNA (depending on the gene). cDNA samples were processed at the same time and in triplicate for each gene. The samples were also tested without RT to verify that there was no contamination with genomic DNA. The thermal cycle profile consisted of 10 min at 95°C, followed by 40 cycles at 95° C for 15 sec and 60° C for 60 sec. A standard curve was created using a calibrator DNA sample, and gene expression was calculated based on threshold cycles that were converted to quantities by interpolation from the standard curve which was applied to each plate. Real-time data were analyzed using the Sequence Detector System 1.7 (Applied Biosystems). Data were normalized to the reference gene, GAPDH.
2.9 Statistical Analyses
Results are presented as mean ± standard error of the mean (SEM). Data were analyzed using Stat View 5.0 software (SAS Institute Inc., Cary, NC USA). A one-way ANOVA was used to determine significant differences between groups. When a significant effect was found, differences between means were determined by Bonferroni-Dunn post hoc test. P ≤ 0.05 was considered a statistically significant difference.
3. RESULTS
3.1 Metformin reduced body fat composition
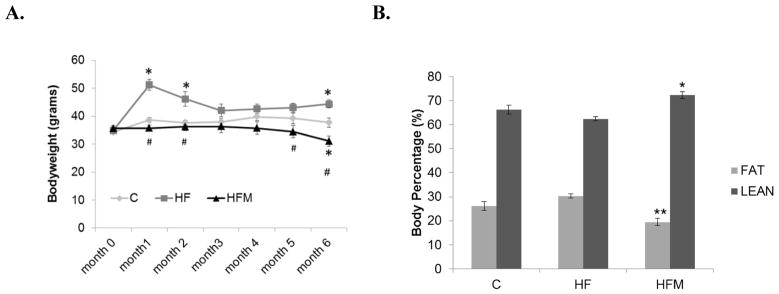
Mice were fed ad libitum during the first month on respective diets. One way ANOVA followed by Bonferroni-Dunn post-hoc analysis showed that body weight in the HF group differed significantly from the C and HFM groups at the end of 1st month when all groups were fed ad libitum (Figure 1A). After the 1st month, HF animals were pair-fed (by calories) to C animals, a procedure which led to a decrease of weight differences between the groups. During the third, fourth and fifth months, body weights did not differ significantly. During the last two months, weight disparity increased slightly between the groups leading to a significant difference between all group comparisons at month 6 (Fig. 1A). Bars indicate SEM (n= 9,8,8 for C, HF, and HFM groups respectively, for months 0–4. For months 5 and 6 n= 8,7,6 for C, HF and HFM groups respectively, p<0.05).
Figure 1. Effect of high fat diet and metformin on body weight and body composition.
(A) Bodyweight changes during the 6-month dietary treatment *= significantly different from C. # = significantly different from HF. p<0.05 (B) Body fat percentage taken at month 4 of dietary treatment. **=significantly different from HF and C, p=0.022(HFM vs C), p<0.001 (HFM vs. HF). * = significantly from HF, p< 0.001 Error bar = ± standard error of mean (n=9,8, and 7 for C HF and HFM groups respectively).
Body composition analysis, taken after four months of dietary treatments, showed a significantly lower body fat percentage in HFM mice relative to both C and HF mice (Fig. 1B) along with a corresponding increase in lean body mass percentage. Metformin treated mice had approximately 10% and 7% lower body fat content relative to HF and C groups, respectively.
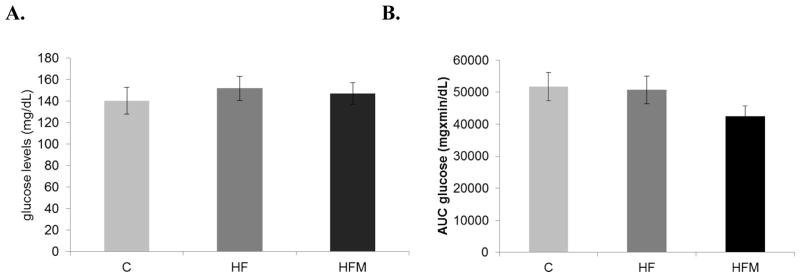
3.2 Metformin had no effect on fasting glucose levels but tended to increase glucose uptake
Mice were fasted for 16 hours overnight before undergoing an oral glucose tolerance test. Fasting blood glucose levels were not different between the groups (C = 154±12.4, HF = 173.6±9.1, HFM = 145.1±7.7). Analysis of area under the curve showed no difference in overall blood glucose levels between groups (Fig 2B.). However, a 20% decrease in blood glucose levels was observed in the HFM-fed mice compared with SD and HF-fed mice, at the final, 2 hr. time-point (p=0.025), indicating an increased rate of glucose uptake with metformin treatment. C and pair-fed HF groups did not differ from each other in glucose tolerance.
Figure 2. Effect of high fat diet and metformin on fasting glucose levels and glucose tolerance.
(A)Fasting blood glucose levels were measured as part of the oral glucose tolerance test, after 5 months of dietary treatment. (B) Blood glucose levels at various time points after an oral dose of 2g/kg(body weight) of glucose by gavage. * Significantly different from corresponding time point of C and HF groups, p=0.025. Error bar = ± standard error of mean. (C) Analysis of area under the curve showed no significant differences between groups p=0.225 (n= 6, 7 and 7 for C, HF and HFM groups respectively).
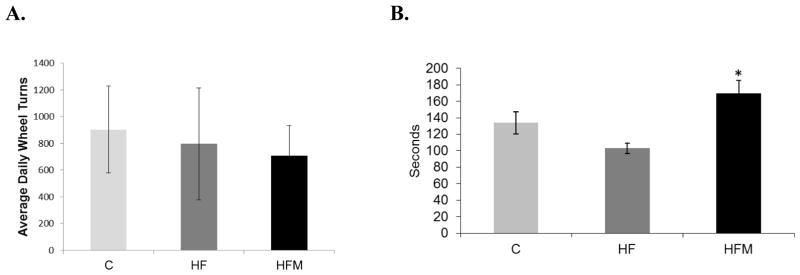
3.3 Metformin prevented impairment of motor coordination
Mean daily voluntary wheel running distances varied considerably within groups and showed no significant differences between groups (Fig 3A). Rotarod performance was significantly impacted by metformin treatment. The average of three, 5min trials, for each animal, was used to calculate each group’s average latency before falling. Metformin treated mice performed significantly better that HF-fed mice and were able to maintain balance for an average of 66 seconds longer than HF mice (p=0.003) (Fig. 3B). Neither the HF nor HFM group differed significantly from the control group.
Figure 3. Effect of high fat diet and metformin treatment on motor activity.
(A) Average daily voluntary wheel running activity. (B) Motor coordination via rodarod performance. * HFM significantly different from HF, p=0.003. Error bar = ± standard error of mean225 (n= 15, 13 and 11 for C, HF and HFM groups respectively).
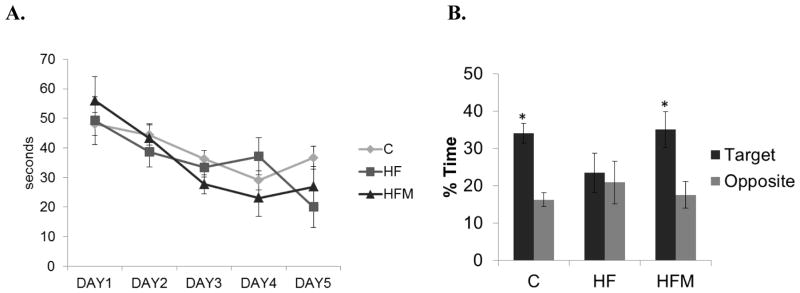
3.4 Metformin prevented decline in memory performance
Morris Water maze testing was used to determine the effect of metformin on spatial learning and memory after 4 months of dietary treatments. During the five days of acquisition testing, we noted no significant differences in performance among the three groups (Fig. 4A). All groups showed a decline in latency to the escape platform with each consecutive training day. Metformin treatment did not have any significant effect on escape latency with training. During the probe test which assesses spatial memory, both C and HFM mice spent approximately twice as much time in the target quadrant compared to time spent in the opposite quadrant (p=0.002 for C, p=0.04 for HFM ). HF mice did not differ in the time spent in opposite and target quadrants (Fig 4B).
Figure 4. Effect of high fat diet and metformin treatment on Morris water maze performance.
(A) Latencies to reach the hidden escape platform. (B) Duration of time spent in target and opposite quadrants of the maze. * = significantly different compared to time spent in the opposite quadrant. (p=0.002 for C, p=0.04 for HFM). C=control, HF= high-fat, HFM = High-fat plus metformin. Error bar = ± standard error of mean (n=7,4,5 in C, HF and HFM groups respectively).
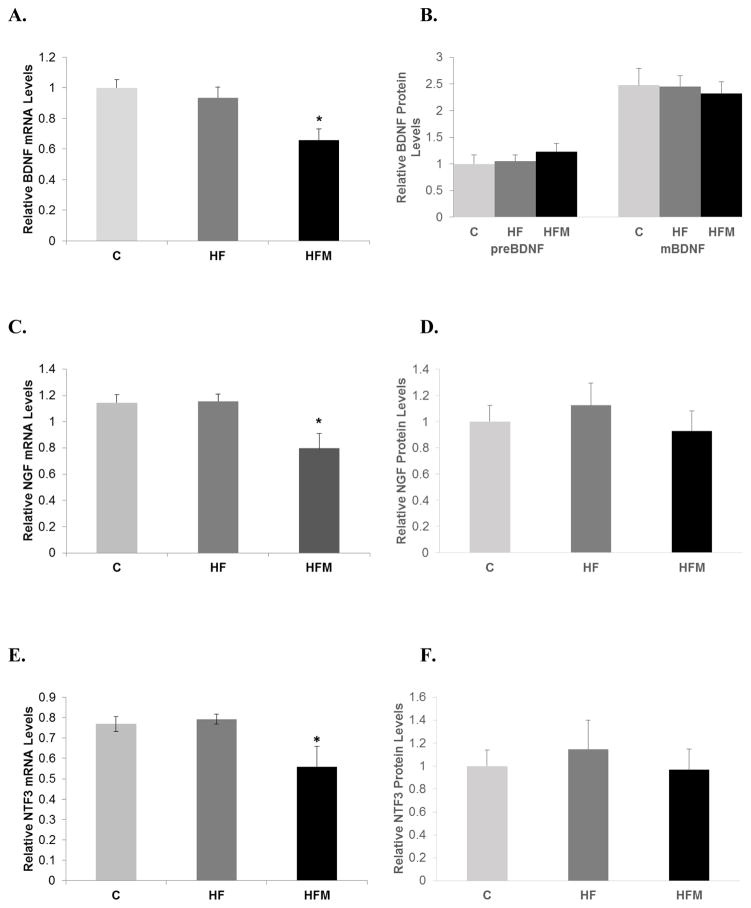
3.5 Metformin decreased BDNF, NGF and NTF3 mRNA levels but did not significantly alter protein levels
Messenger RNA levels of BDNF, NGF, and NT3 in cortical brain tissue were determined by RT-qPCR. Levels of mRNA for all three neurotrophic factors measured in mice in the HFM group were significantly decreased relative to both C and HF groups (ANOVA p values: p=0.006, p=0.009, and p=0.008 for BDNF, NGF and NTF3, respectively). Western blot analysis of protein levels of the three neurotrophins showed no differences between groups (pre-BDNF p=0.581, mature BDNF p=0.918, NGF p=0.688, NT3 p=0.835). See figure 5.
Figure 5. Effect of high fat diet and metformin treatment on neurotrophic factor expression in brain.
(A) BDNF mRNA levels. (B) Protein levels of preBDNF and mature BDNF. (C) NGF mRNA levels (D) NGF protein levels, (E) NTF3 mRNA levels. (F) NTF3 Protein levels. * = significantly different from C and HF, p < 0.001. All mRNA levels were normalized to GAPDH and protein levels were normalized to β-actin protein or ponsceau S. Error bar = ± standard error of mean.
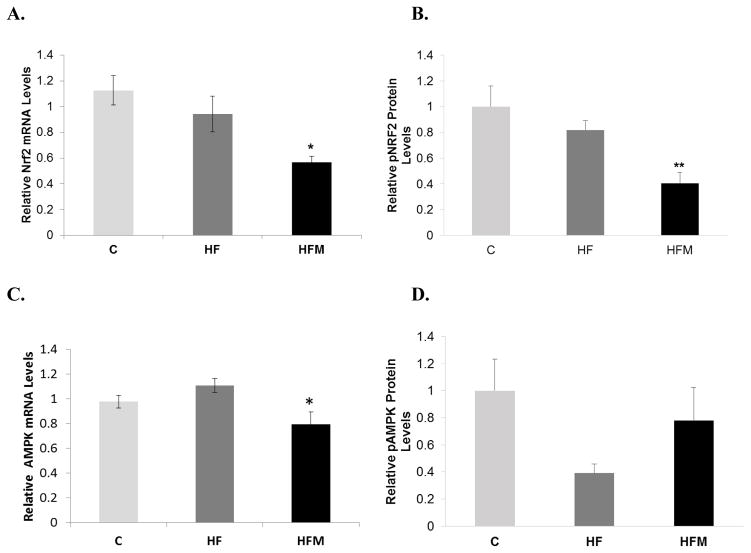
3.6 Metformin decreased transcription and activation of the antioxidant regulator Nrf2
Levels of phosphorylated Nrf2 protein were significantly decreased in the metformin treated group compared to both C and HF (HFM vs. C, p=0.007; HFM vs. HF, p=.049; HF vs. C, p=0.678). mRNA levels of Nrf2 showed a pattern the corresponded to protein levels with the HFM having decreased transcription relative to both C and HF groups. Results are shown in figures 6A and 6B.
Figure 6. Effect of metformin treatment on Nrf2 and AMPK expression.
(A) Nrf2 mRNA levels. (n=9,10,7 for C, HF and HFM respectively). (B) Protein levels of pNrf2 relative to total Nrf2 protein (n= 8,12,6 for C, HF and HFM respectively) ** = significantly different from C (p=0.007) and HF (p=0.049). (C) AMPK mRNA levels (n=14,13,7 for C, HF and HFM respectively); * = significantly different from C and HF (p=0.01). (D) Protein levels of pAMPK relative to total AMPK protein (n=9,9,10 for C, HF and HFM respectively). P=0.068. Error bar = ± standard error of mean.
3.7 Metformin showed a decrease in AMPK transcription and a trend toward increased p-AMPK protein
There was a reduction in transcription of AMPK in the HFM group compared to the HF group (p= 0.01, Fig 6C). There were no differences between HF versus C (p=0.36) or HFM versus C (p=0.21) (n= 14,13,7 for C, HF and HFM respectively). Protein levels of p-AMPK trended toward reduced levels in HF-fed mice relative to C. The HFM group showed a strong trend toward increased pAMPK levels compared to the HF group (p=0.068, Fig. 6D)(n = 9,9, 10 for C, HF and HFM respectively).
4. DISCUSSION
Metformin has been shown to have a myriad of beneficial effects from anti-cancer to improved hormonal balance, and increased fertility [21–23]. Cogent evidence that metformin, taken orally, rapidly crosses the blood brain barrier [20, 24–26] indicates that this molecule also has the potential to directly impact the central nervous system. One study in particular demonstrated that cerebrospinal fluid (CSF) levels of metformin were significantly and substantially higher than levels measured in plasma after the delivery of an oral dose (150mg/kg) of metformin in rats [27]). Several studies have now shed light on its effects on brain tissue and function including its neuroprotective effects [28–31]. In this study, we sought to uncover mechanisms impacting the behavioral and cognitive effects of long term metformin treatment.
In line with previous metformin studies, we show that metformin intake induced a reduction in body fat composition in mice on a high fat diet. A recent study on human diabetic patients supports this finding by showing an increase in lean to fat ratio in diabetic patients on metformin treatment [32]). Metformin’s ability to affect food intake and hypothalamic activity regulating feeding behavior has been documented [25, 33–36]. However, the difference in the body fat content of mice in this study was not due to decreased feeding behavior, as caloric intake was equalized among the groups. Instead, this effect is more likely due to an increase in fat oxidation, a proposed mechanism of action for metformin treatment [37–39]. In a recent in vitro study using primary hepatocytes and mouse embryonic fibroblasts [40], metformin increased β-oxidation and decreased lipid synthesis.
In the current study we also wanted to determine if intake of a high fat diet without development of obesity would result in decreased glucose tolerance that would be prevented by metformin treatment. HF mice in this study, showed no differences in fasting blood glucose levels or glucose tolerance compared to controls, indicating the necessity for increased caloric intake or obesity to induce glucose intolerance and perhaps also insulin resistance in these mice. Metformin treated mice; however, showed significantly enhanced glucose tolerance compared to both C and HF groups. Given that the increased glucose tolerance in HFM mice is coupled to an overall decrease in fat mass found in this group, this finding is supportive of a direct relationship between body fat composition and glucose tolerance, even within a non-obese bodyweight range. In corroboration, a positive correlation between body fat mass and carbohydrate tolerance has been reported in both obese and non-obese human subjects [41]. Additionally, in a related human study it was shown that lean muscle mass inversely predicts glucose tolerance in glucose intolerant subjects [42]. However, it is also well documented in human studies that body fat distribution is an important factor in glucose tolerance and insulin resistance [43]. In mice, insulin resistance and glucose tolerance are associated with visceral fat accumulation as opposed to subcutaneous fat [44]. Regional fat distribution was not evaluated in this study.
Mice in the current study were housed in cages containing exercise wheel systems. Weekly wheel running activity measurements showed no significant differences in running activity between the groups. Thus, differences in locomotor activity likely did not affect body composition. A rotarod test of muscle endurance and motor coordination, however, revealed enhanced performance in metformin treated mice indicative of improved skeletal muscle function and metabolic properties. Other studies are in support of this finding [30, 40], showing similarly improved motor performance with metformin treatment. The significantly increased performance on the rotarod was likely to be at least partially due to enhanced metabolic properties of skeletal muscle, perhaps subsequent to improved mitochondrial function. Studies on the effects of metformin on skeletal muscle mitochondrial function are conflicting, with some reporting increased mitochondrial function [45, 46], and others reporting either no change or impairment in mitochondrial function [47, 48]. More focused and detailed studies on molecular mechanisms of metformin on skeletal muscle are needed.
The enhanced cognitive performance of mice receiving metformin treatment relative to the HF group was only evident in the probe trial of the water maze testing which measures reference spatial memory. Metformin had no effect on spatial learning. Other studies have shown improved cognitive performance with metformin using similar testing paradigms [18, 49, 50]. Interestingly, a sub group analysis of participants from the Primary Research in Memory Clinics study showed that among patients with diabetes, worse cognitive performance was associated with metformin use [51]. One rodent study also reported that metformin in drinking water (2mg/ml) had a detrimental effect on cognitive performance in male mice while enhancing performance in female mice [52], thus suggesting that gender is an important factor when considering the actions of this drug. Our findings in male mice showed that metformin treatment prevented the detrimental cognitive effects of the high fat diet on performance during probe testing. This result suggests that metformin may have a specific effect on memory retention compared to HF mice which showed memory impairment. It is possible that the effects of metformin on memory are multidimensional, perhaps having negligible effect unless there is some pre-existing dysfunction which is then attenuated or normalized by the drug. Since a control diet group supplemented with metformin (CM) was not incorporated into this study, we can only speculate about whether metformin may have enhanced glucose tolerance in the CM group relative to the C group.
One of the major findings of this study is that 6 months of metformin treatment led to a decrease in neurotrophic mRNA levels without a corresponding change in protein levels. Neurotrophins are known to induce neuronal growth and synaptogenesis in animal models and are associated with increased cognitive function [53–55]. The decrease in mRNA levels of BDNF, NGF and NTF3 suggests a potential state of vulnerability for the brain and a decrease in capacity for neuroplasticity which would be necessary for enhanced cognitive effects. The lack of correspondence in protein levels however, explains why this also did not relate to a decrease in cognition as measured during water maze testing. In a related study, mice treated with metformin (500 mg/kg) attenuated the detrimental effects on memory impairment and locomotor activity, of MPTP-treated mice [30]. That study showed an increase in BDNF levels in metformin treated mice relative to MPTP treated mice, but mice in that study were a much younger cohort and were treated for only 21 days. Mice in our study remained on diets for 6 months and behavioral testing was done during the 4th month of treatment. It is possible that there is both an aging as well as a treatment duration effect accounting for the disparate findings of neurotrophin levels and memory performance in our studies. Alternatively, an age-related transition in metabolic function in our animals may have occurred sometime between the 4th month and 6th month of treatment. In line with this possibility, we found that during the last month of the study, despite equal caloric intake, HFM and C animals began to show a slight but steady decrease in body weight which may be indicative of an age-related transition in metabolic conditions. We may also speculate that had animals been sacrificed shortly after water maze testing, an increase in neurotrophin transcription and or protein levels may have been observed. The lack of correlation between protein and mRNA levels found in this study likely due to the various mechanisms that may uncouple transcription and translation under specific conditions [56]. Post transcriptional mechanisms including activities of regulatory proteins, sRNA, and rRNA; as well as protein degradation potentially account for the discrepancy between protein and mRNA levels of the neurotrophins measured. Future studies on post-transcriptional mechanisms are necessary to address the lack of correlation definitively.
Among its various reported benefits, metformin has also been shown to have anti-oxidative properties [57–59]. Phosphorylation of the antioxidant master regulator, Nrf2 induces its translocation into the nucleus, subsequent activation of the antioxidant response element (ARE), and transcription of anti-oxidant genes[60]. We therefore measured brain levels of mRNA and protein of the transcription factor, Nrf2. Our study showed that in mouse brain, long-term metformin treatment significantly decreased Nrf2 expression and activation. Increased Nrf2 levels have been associated with lifespan and health span extension [61], with decreased levels presumably having an opposite effect. In a related study, metformin was shown to induce reactive oxygen species (ROS) generation and mitochondrial dysfunction in neuroblastoma cells in a dose-dependent manner (Picone et al 2015). Although Nrf2 levels were not determined in that study, one could speculate that the increased ROS may have been linked to a reduction in Nrf2-dependent anti-oxidative enzyme activity. In a previous study using two doses of metformin, the higher of which was used in this study, higher metformin dosage was associated with a decreased lifespan [40]. Although, Nrf2 was not measured in the previous study, it is possible that the decreased lifespan may have correlated with decreased Nrf2 activation.
Metformin dosage amount and timing is an important factor to consider in rodent studies. Dosages used in animal studies vary widely with reports of both beneficial and detrimental effects across the wide range of metformin doses. With respect to the duration of metformin treatment, the vast majority of studies used significantly shorter treatment times compared to the 6 month treatment implemented in this study. Our study combined long-term metformin use with a relatively high daily dose. In our related studies, a lifespan extending effect was found with a lower (0.1%) dose of metformin in male mice. The higher metformin dose was shown to be initially effective at producing beneficial metabolic and cognitive effects. However, prolonged used ultimately resulted in detrimental effects, possibly due to decreased levels of neurotrophic factors, which are critical for neuronal plasticity and survival. In a similar study, metformin failed to improve cognitive deficits in high-fat fed rats despite effectively reducing insulin resistance [62] The age at which metformin treatment was initiated also potentially plays a large role in the outcomes reported. Anisimov and colleagues [63] reported that ability of metformin to extend life-span decreased as metformin treatment was initiated later in life [63]. Mice in this study began metformin treated at an older age (12 months). The study by Anisimov et al. [63] showed that metformin’s life-extending effects were most potent when treatment began at 3 months and had a slight effect at 9 months with no effect at 15 months.
Our finding of decreased transcription of neurotrophic factors and decreased Nrf2 activation, seen with long-term metformin use, cautions against extended use of higher metformin doses. Further studies on age, dose and treatment duration effects of metformin use are needed to clearly uncover any age-related effects of prolonged metformin use that may lead to alterations in brain biochemistry in a manner that creates increased vulnerability of the central nervous system.
Metformin Paper highlights.
A mouse model was used to determine the effect of high fat diet and metformin treatment on cognition, brain neurotrophic factor expression and Nrf2 expression.
Transcripts of three major neurotrophic factors were decreased in brains of metformin treated mice.
Brain protein and mRNA levels of the antioxidant regulatory factor Nrf2 were decreased by metformin treatment
Long-term high dose metformin use may create disadvantageous biochemical conditions in the brain.
Acknowledgments
We are grateful to Dawn Nines, Dawn Phillips-Boyer and Justine Lucas for their exemplary animal care service. We thank Federico Butelman from Farmhispania S.A., a FDA-approved cGMP company, for providing the metformin used in this study. This research was supported by the Intramural Research Program of the National Institute on Aging (NIA), National Institutes of Health and also by NIA extramural Grant# 1R25AG047843-01.
Abbreviations
- HF
high fat
- HFM
high fat plus metformin
- BDNF
brain derived neurotrophic factor
- NGF
nerve growth factor
- NT3
neurotrophin 3
- Nrf2
nuclear factor erythroid 2-related factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Federation ID; Federation ID, editor. IDF Diabetes Atlas. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Services USDoHaH, editor. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 4.Moses RG. Repaglinide/metformin fixed-dose combination to improve glycemic control in patients with type 2 diabetes: an update. Diabetes Metab Syndr Obes. 2010;3:145–54. doi: 10.2147/dmsott.s6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooradian AD, Perryman K, Fitten J, Kavonian GD, Morley JE. Cortical function in elderly non-insulin dependent diabetic patients. Behavioral and electrophysiologic studies. Archives of internal medicine. 1988;148:2369–72. [PubMed] [Google Scholar]
- 6.Perlmuter LC, Hakami MK, Hodgson-Harrington C, Ginsberg J, Katz J, Singer DE, et al. Decreased cognitive function in aging non-insulin-dependent diabetic patients. The American journal of medicine. 1984;77:1043–8. doi: 10.1016/0002-9343(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 7.Hassenstab JJ, Sweat V, Bruehl H, Convit A. Metabolic syndrome is associated with learning and recall impairment in middle age. Dementia and geriatric cognitive disorders. 2010;29:356–62. doi: 10.1159/000296071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? British journal of clinical pharmacology. 2011;71:365–76. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strachan MW. R D Lawrence Lecture 2010. The brain as a target organ in Type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabetic medicine : a journal of the British Diabetic Association. 2011;28:141–7. doi: 10.1111/j.1464-5491.2010.03199.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: a population-based cohort study. Diabetologia. 2009;52:1031–9. doi: 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- 11.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes care. 2006;29:345–51. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- 12.Beeri MS, Schmeidler J, Silverman JM, Gandy S, Wysocki M, Hannigan CM, et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008;71:750–7. doi: 10.1212/01.wnl.0000324925.95210.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, et al. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54:3101–10. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey CJ. Metformin and intestinal glucose handling. Diabetes/metabolism reviews. 1995;11(Suppl 1):S23–32. doi: 10.1002/dmr.5610110505. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Iwata K, Murakami H. Inhibitory effect of metformin on intestinal glucose absorption in the perfused rat intestine. Biochemical pharmacology. 2000;59:887–90. doi: 10.1016/s0006-2952(99)00396-2. [DOI] [PubMed] [Google Scholar]
- 17.Hettich MM, Matthes F, Ryan DP, Griesche N, Schroder S, Dorn S, et al. The anti-diabetic drug metformin reduces BACE1 protein level by interfering with the MID1 complex. PloS one. 2014;9:e102420. doi: 10.1371/journal.pone.0102420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc. 2012;60:916–21. doi: 10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3907–12. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palomba S, Falbo A, La Sala GB. Metformin and gonadotropins for ovulation induction in patients with polycystic ovary syndrome: a systematic review with meta-analysis of randomized controlled trials. Reproductive biology and endocrinology : RB&E. 2014;12:3. doi: 10.1186/1477-7827-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nature reviews Endocrinology. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 23.Rosilio C, Ben-Sahra I, Bost F, Peyron JF. Metformin: a metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer letters. 2014;346:188–96. doi: 10.1016/j.canlet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Beckmann R. Absorption, distribution in the organism and elimination of metformin. Diabetologia. 1969;5:318–24. doi: 10.1007/BF00452906. [DOI] [PubMed] [Google Scholar]
- 25.Lv WS, Wen JP, Li L, Sun RX, Wang J, Xian YX, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain research. 2012;1444:11–9. doi: 10.1016/j.brainres.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica; the fate of foreign compounds in biological systems. 1994;24:49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 27.Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacological reports : PR. 2010;62:956–65. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 28.Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metabolic brain disease. 2014;29:47–58. doi: 10.1007/s11011-013-9475-2. [DOI] [PubMed] [Google Scholar]
- 29.Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain, behavior, and immunity. 2014;40:131–42. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014;277:747–54. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Zhu XC, Jiang T, Zhang QQ, Cao L, Tan MS, Wang HF, et al. Chronic Metformin Preconditioning Provides Neuroprotection via Suppression of NF-kappaB-Mediated Inflammatory Pathway in Rats with Permanent Cerebral Ischemia. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8866-7. [DOI] [PubMed] [Google Scholar]
- 32.Aghili R, Malek M, Valojerdi AE, Banazadeh Z, Najafi L, Khamseh ME. Body composition in adults with newly diagnosed type 2 diabetes: effects of metformin. Journal of diabetes and metabolic disorders. 2014;13:88. doi: 10.1186/s40200-014-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aubert G, Mansuy V, Voirol MJ, Pellerin L, Pralong FP. The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metabolism: clinical and experimental. 2011;60:327–34. doi: 10.1016/j.metabol.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Duan Y, Zhang R, Zhang M, Sun L, Dong S, Wang G, et al. Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus. Neural regeneration research. 2013;8:2379–88. doi: 10.3969/j.issn.1673-5374.2013.25.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Park EY, Oh MJ, Park SS, Shin KH, Choi SH, et al. Central administration of metformin into the third ventricle of C57BL/6 mice decreases meal size and number and activates hypothalamic S6 kinase. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305:R499–505. doi: 10.1152/ajpregu.00099.2013. [DOI] [PubMed] [Google Scholar]
- 36.Lee CK, Choi YJ, Park SY, Kim JY, Won KC, Kim YW. Intracerebroventricular injection of metformin induces anorexia in rats. Diabetes & metabolism journal. 2012;36:293–9. doi: 10.4093/dmj.2012.36.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. American journal of physiology Endocrinology and metabolism. 2006;291:E182–9. doi: 10.1152/ajpendo.00272.2005. [DOI] [PubMed] [Google Scholar]
- 38.Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Current opinion in endocrinology, diabetes, and obesity. 2014;21:323–9. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Liu F, Yuan Y, Wu J, Wang H, Zhang L, et al. Metformin suppresses lipid accumulation in skeletal muscle by promoting fatty acid oxidation. Clinical laboratory. 2014;60:887–96. doi: 10.7754/clin.lab.2013.130531. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nature communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratzmann KP, Knospe S, Heinke P, Schulz B. Relationship between body fat mass, carbohydrate tolerance and IRI response during glucose infusion in subjects with early diabetes. Acta diabetologica latina. 1979;16:67–75. doi: 10.1007/BF02590765. [DOI] [PubMed] [Google Scholar]
- 42.Kwankaew J, Saetung S, Chanprasertyothin S, Leelawattana R, Rattarasarn C. Lean mass inversely predicts plasma glucose levels after oral glucose load independent of insulin secretion or insulin sensitivity in glucose intolerance subjects. Endocrine journal. 2014;61:77–83. doi: 10.1507/endocrj.ej13-0241. [DOI] [PubMed] [Google Scholar]
- 43.Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, et al. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. The Journal of nutrition. 2001;131:354S–60S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- 44.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism: clinical and experimental. 1993;42:1405–9. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 45.Kane DA, Anderson EJ, Price JW, 3rd, Woodlief TL, Lin CT, Bikman BT, et al. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free radical biology & medicine. 2010;49:1082–7. doi: 10.1016/j.freeradbiomed.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kristensen JM, Larsen S, Helge JW, Dela F, Wojtaszewski JF. Two weeks of metformin treatment enhances mitochondrial respiration in skeletal muscle of AMPK kinase dead but not wild type mice. PloS one. 2013;8:e53533. doi: 10.1371/journal.pone.0053533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer & metabolism. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wessels B, Ciapaite J, van den Broek NM, Nicolay K, Prompers JJ. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PloS one. 2014;9:e100525. doi: 10.1371/journal.pone.0100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alzoubi KH, Khabour OF, Al-Azzam SI, Tashtoush MH, Mhaidat NM. Metformin Eased Cognitive Impairment Induced by Chronic L-methionine Administration: Potential Role of Oxidative Stress. Current neuropharmacology. 2014;12:186–92. doi: 10.2174/1570159X11666131120223201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao RR, Xu XC, Xu F, Zhang WL, Zhang WL, Liu LM, et al. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochemical and biophysical research communications. 2014;448:414–7. doi: 10.1016/j.bbrc.2014.04.130. [DOI] [PubMed] [Google Scholar]
- 51.Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes care. 2013;36:2981–7. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiTacchio KA, Heinemann SF, Dziewczapolski G. Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2015;44:43–8. doi: 10.3233/JAD-141332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, et al. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–72. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Bothwell M. NGF, BDNF, NT3, and NT4. Handbook of experimental pharmacology. 2014;220:3–15. doi: 10.1007/978-3-642-45106-5_1. [DOI] [PubMed] [Google Scholar]
- 55.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handbook of experimental pharmacology. 2014;220:223–50. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 56.Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–73. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 57.Srividhya S, Anuradha CV. Metformin improves liver antioxidant potential in rats fed a high-fructose diet. Asia Pac J Clin Nutr. 2002;11:319–22. doi: 10.1046/j.1440-6047.2002.00306.x. [DOI] [PubMed] [Google Scholar]
- 58.Esteghamati A, Eskandari D, Mirmiranpour H, Noshad S, Mousavizadeh M, Hedayati M, et al. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clin Nutr. 2013;32:179–85. doi: 10.1016/j.clnu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Formoso G, De Filippis EA, Michetti N, Di Fulvio P, Pandolfi A, Bucciarelli T, et al. Decreased in vivo oxidative stress and decreased platelet activation following metformin treatment in newly diagnosed type 2 diabetic subjects. Diabetes Metab Res Rev. 2008;24:231–7. doi: 10.1002/dmrr.794. [DOI] [PubMed] [Google Scholar]
- 60.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–74. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 61.Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3722–7. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia. 2012;55:3061–70. doi: 10.1007/s00125-012-2686-y. [DOI] [PubMed] [Google Scholar]
- 63.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–57. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]