Abstract
An explicit dosimetry model has been developed to calculate the apparent reacted 1O2 concentration ([1O2]rx) in an in-vivo model. In the model, a macroscopic quantity, g, is introduced to account for oxygen perfusion to the medium during PDT. In this study, the SOED model is extended for PDT treatment in phantom conditions where vasculature is not present; the oxygen perfusion is achieved through the air-phantom interface instead. The solution of the SOED model is obtained by solving the coupled photochemical rate equations incorporating oxygen perfusion through the air-liquid interface. Experiments were performed for two photosensitizers (PS), Rose Bengal (RB) and Photofrin (PH), in solution, using SOED and SOLD measurements to determine both the instantaneous [1O2] as well as cumulative [1O2]rx concentrations, where [1O2]rx = (1/τΔ) · ∫[1O2]dt. The PS concentrations varied between 10 and 100 mM for RB and ~200 mM for Photofrin. The resulting magnitudes of [1O2] were compared between SOED and SOLD.
Keywords: photodynamic therapy, PDT, singlet oxygen, SOLD, SOED, explicit PDT dosimetry
1. INTRODUCTION
Improving dosimetry for photodynamic therapy (PDT) is an ongoing goal for use in the treatment of cancer and other localized diseases. PDT is a multi-faceted, dynamic process that involves the interactions of light, photosensitizer, and ground state oxygen (3O2), that create reactive singlet oxygen (1O2) in a type II process or other reactive oxygen species (such as O2−•) in a type I process [1]. A macroscopic singlet oxygen explicit dosimetry (SOED) model has been previously developed and studied for various sensitizers [2–12]. The use of SOED can be advantageous due to the difficulty of measuring the singlet oxygen luminescence signal in vivo due to its short lifetime of 30–180 ns [13, 14]. Furthermore, PDT dose alone is not sufficient as a dosimetric quantity, particularly in hypoxic environments that are created with high fluence rate treatments. SOED was compared to a direct dosimetry method, singlet oxygen luminescence dosimetry (SOLD) in photosensitizer solution.
2. MATERIALS AND METHODS
2.1 SOED Model in Phantoms
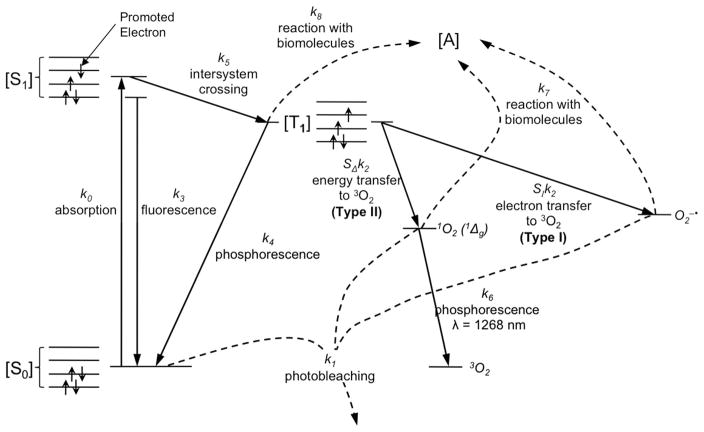
Singlet oxygen produced during illumination was calculated using an explicit dosimetry model. Based on both type I and type II processes modeled in figure 1, a set of coupled differential equations have been established for the photochemical reactions [11, 12, 15–17]:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Figure 1.
Jablonski diagram for PDT. In type I reactions, the triplet photosensitizer will transfer an electron to 3O2 react with molecular targets to produce radical species, or alternatively interact directly with the acceptor, [A], without oxygen mediation. In type II reaction, the energy is transferred from the triplet photosensitizer to ground state molecular oxygen (3O2), creating reactive singlet oxygen (1O2).
With a focus on only the dynamic process of PDT in the time scale of a few seconds to hours, the time derivatives on the right hand sides of the equations for the excited singlet state photosensitizer, the triplet state photosensitizer, singlet oxygen, and superoxide anion (Eqs. (2), (3), (5), (6)) can be set to zero because these processes are known to be very fast (~μs or less). These can then be simplified to [11, 17]
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
where σII = k12τΔ, σI = k11 τS, τΔ = 1/(k6+k72[A]), τS = 1/k71[A], τf = 1/(k3+k5), , Φt=k5/(k3+k5), and β = k4+k8[A]/k2. It was assumed that σII([S0]+δ) ≪ 1 and σI ([S0]+δ) ≪ 1, which is true for this case. Here, ΦΔ = SΔΦt is the singlet oxygen quantum yield in the solvent used (methanol for Photofrin phantoms and water for Rose Bengal phantoms), ε is the extinction coefficient at 523 nm, and h is Planck’s constant. The parameters used for the calculation in each phantom are summarized in Table 1. This model has been used in in vivo systems previously where k7[A] ≫ k6 [2–12]. In phantoms, the substitute for biological substrate ([A]) to interact with the reactive singlet oxygen generated in the photodynamic process is sodium azide (NaN3), a well known singlet oxygen quencher. In the experiments performed without NaN3, [A] = 0.
Table 1.
Summary of photophysical and photochemical parameters for Photofrin (PH) and Rose Bengal (RB) in vitro
| Parameter | Definition | Photofrin | Rose Bengal |
|---|---|---|---|
| ε (cm−1 μM−1) | Photosensitizer extinction coefficient | 0.0089 @523nm | 0.059 @523nm |
| k0, ka @100mW/cm2 (s−1) | Photon absorption rate of photosensitizer as a function of photosensitizer concentration (in mM), k0 = εϕ/hν, for ϕ = 100 mW/cm2. | 3.89 [1] | 25.69 [1] |
| k12, kos (μM−1s−1) | Bimolecular rate for 1O2 reactions with ground-state photosensitizer | 2.1×10−1 [2] | 6.6×10−2 [2] |
| k2, kot (μM−1s−1) | Bimolecular rate of triplet photosensitizer quenching by 3O2 | 1.278×103 [3] | 1.2×103 (1.2 – 1.6)×103 [20, 21] |
| k3, kf (s−1) | Fluorescence rate of first excited singlet state photosensitizer to ground state photosensitizer | 2.9×107 [22] | 2.12×108 [4] |
| k4, kp (s−1) | Phosphorescence rate of monomolecular decay of the photosensitizer triplet state | 1.52×105 [5] | 1.43×104 [5] |
| k5, kisc (s−1) | Intersystem crossing (ISC) rate from first excited photosensitizer to triplet state photosensitizer | 4.94×107 [6] | 6.36×108 [6] |
| k6, kd (s−1) | Spontaneous phosphorescence rate of 1O2 to 3O2 | 1.1×105 [7] | 2.6×105 [7] |
| k72, koa (μM−1s−1) | Bimolecular rate of reaction of type II 1O2 with biological substrate [A] | 2.35×102 [7] | 2.58×101 [7] |
| β (μM) | Oxygen quenching threshold concentration | 11.9 [23] | 11.9 [9] |
| δ (μM) | Low concentration correction | 33 (33 – 150) [24] | 33 [8] |
| ξII (cm2mW−1s−1) | Specific oxygen consumption rate | 24.9×10−3 @ 523 nm | 195.2×10−3 @ 523 nm |
| σII (μM−1) | Specific photobleaching ratio where σII = k12τΔ | 1.0×10−6 [9] | 2.5×10−7 [9] |
| g (mM/s) | Macroscopic maximum oxygen supply rate | 0 [10] | 0 [10] |
| Φt | Triplet quantum yield | 0.91 [25] (0.63 – 0.93) [17, 25–27] | 0.75 in water [20, 28] |
| ΦΔ | Singlet oxygen quantum yield | 0.25 in MeOH [29, 30] (0.12–0.56) [26, 31] [32] [29,30] | 0.76 in vitro [33] |
| Φf | Fluorescence quantum yield , where A10 is spontaneous transition rate between S1 and S0. | 0.16 [20] | 0.08 [33] |
| τΔ (s) | Singlet oxygen lifetime , [A] = 0. | (9.4±0.2)×10−6 [11] | (3.8±0.3)×10−6 [11] |
| τf (s) | Fluorescence decay time | (5.5±1.2)×10−9 [34] | 1.18×10−10 [35] |
| τt (s) | Triplet state lifetime | (0.43±0.03)×10−6 [11] | (2.1±0.2)×10−6 [11] |
Calculated based on value of ε and ϕ = 100 mW/cm2: k0 = εϕ/(hν))
Photofrin: k0 = (0.0089 μM−1cm−1) / (6.022×1014 cm2μM−1) × (100 mW/cm2) / (3.80 ×10−16 mW s) = 3.89 s−1
Rose Bengal: k0 = (0.059 μM−1cm−1) / (6.022×1014 cm2μM−1) × (100 mW/cm2) / (3.80 ×10−16 mW s) = 25.69 s−1
Calculated based on value of σ that was fit to data (see Fig. 3 and 4) and measured τΔ: k1 =σ/τΔ
Photofrin: k1 = (2×10−6 μM−1) / (9.4×10−6 s) = (2.1 ×10−1 μM−1s−1)
Rose Bengal: k1 = (2.5×10−7 μM−1) / (3.8×10−6 s) = (6.6 ×10−2 μM−1s−1)
Calculated based on measured value of τt:
Photofrin: k2 = ((0.43×10−6 s−1) − (1.52×105 s−1))/(170 μM) = 1.278×103 μM−1s−1
Calculated based on the value of Φt and τf: k3 = (1 − Φt)/τf
Rose Bengal: k3 = (1 − 0.75) / (1.18×10−10 s) = 2.12×109 s−1
Calcualted based on assumed value of β: k4 = β×k2
Photofrin: k4 = (11.9 μM) × (1.278×103 μM−1s−1) = 1.52×105 s−1
Rose Bengal: k4 = (11.9 μM) × (1.278×103 μM−1s−1) = 1.43×104 s−1
Calculated based on value of k3 and Φt: k5 = Φt k3/(1 − Φt)
Photofrin: k5 = (0.63) × 2.9×107 s−1 / (1 − 0.63) = 4.94×107 s−1
Rose Bengal: k5 = (0.75) × (2.12×109 s−1) / (1 − 0.75) = 6.36×109 s−1
Calculated based on fit to data in figure 5.
Assumed to be the same as that of Photofrin
Due to absence of blood flow and reoxygenation in phantoms
Measured values from SOLD experiment when [A] = 0, i.e., without NaN3.
For the type II photosensitizers (PH and RB) used in this study, η = 0 since there is no direct triplet interaction. [3O2](t) and [S0](t) can be solved by the coupled differential Eqs. (12) and (13). Assuming that [3O2]0 ≫ β and there is minimal photobleaching of the photosensitizer, i.e., σI ≈ σII ≈ 0, thus [S0] = [S0]0 from Eq. (12), then Eq. (13) can be solved as
| (15) |
where γ = ξII (σII ([S0]0 + δ) + k7[A]τΔ) + ξI = ξIIσII ([S0] + δ) + ξI (when [A] = 0), is the PDT oxygen consumption rate per PDT dose rate and is 2.1×10−6 μM/s/(μMmW/cm2) for Photofrin and 4.1×10−6 μM/s/(μMmW/cm2) for RB for [S0] = 50 μM. The expression of the photosensitizer([S0]) can be solved by combining Eqs. (10), (11) and (12), regarless of the value of σ, to be
| (16) |
Thus the solution:
| (17) |
where
| (18) |
and
| (19) |
Oxygen measurements were compared with the modeled values of oxygen using both the full coupled differential equations (Eqs. (12)–(13)) as well as the simplified verion stated above (Eqs. (15) and (17)). In all our model, we have made the assumption that type I interaction is negligible, i.e., σI = 0 and ξI = 0.
Fluorescence spectra as well as absoroption spectra were used to determine the experimentally measured values of [S0] and absorption properties to compare with expected calculated values.
2.2 SOLD Instrumentation
Singlet oxygen luminescence dosimetry was performed using a compact, fiber optic probe-based singlet oxygen luminescence detection system [18]. The near-infrared luminescence probe was coupled to a compact InGaAs/InP single photon avalanche diode (SPAD) detector. Samples were irradiated with a 523 nm wavelength pulsed-laser source coupled into the delivery fiber via a collimation package. Patterned time gating was used to limit the unwanted dark counts and eliminate the strong photosensitizer luminescence background. The luminescence signal of singlet oxygen at 1270 nm was confirmed through spectral filtering and lifetime fitting for Rose Bengal and Photofrin.
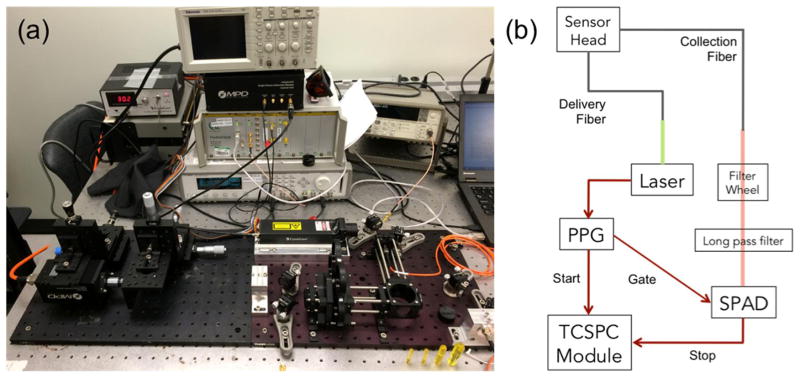
Figure 2 shows a photo and schematic of the experimental setup. A pulsed 523 nm wavelength laser with 10 ns pulses at a repetition rate of 18.2 kHz was coupled into the delivery fiber with a collimation package. The laser outputs an electrical signal that is sent to a programmable Pulse Pattern Generator (PPG). Each pulse generates outputs on two separate channels, each with pulse shape designed to match the intended input The first output is a single pulse sent to the ‘start’ channel of the time-correlated single-photon counter (TCSPC), while the second is a pattern of pulses sent to the SPAD control module. The SPAD is turned on for a pre-assigned time, only when the control module receives a pulse from the PPG.
Figure 2.
SOLD instrumentation setup (a) on an optical bench and (b) schematic diagram of the experimental arrangement. The 523 nm laser source is coupled into the delivery fiber with a collimation package. The laser outputs a synchronous signal that is sent to a programmable Pulse Pattern Generator (PPG). Light from the collection fiber is coupled out through another collimation package, directed through a filter wheel (FW) for the bandpass filter selection, and then a long pass filter. The fiber core is imaged onto the face of the SPAD detector.
2.3 Comparison Study
Liquid phantoms were created using the appropriate solvent (methanol (MeOH) for Photofrin and water (H2O) for Rose Bengal) in cuvettes. Rose Bengal is a commonly used, well-characterized model compound of a single molecular species that was used for verification of the singlet oxygen signal. Photofrin is a clinically-active photosensitizer that is FDA approved and currently in use for many clinical trials. Phantoms were made with various concentrations of each sensitizer in cuvettes.
Ground state oxygen measurements were performed with an Oxford Optronix OxyLite system (Oxford Optronix Ltd., Oxford, United Kingdom). Illumination light was briefly turned off during these measurements, and multiple values were recorded for a single phantom. Oxygen partial pressure was measured in mmHg and converted to μM by using a factor of α = 1.3 [9, 19].
3. RESULTS
Singlet oxygen explicit dosimetry modeling was validated in two methods: ground state oxygen (3O2) modeling and singlet oxygen (1O2) modeling. 3O2 was modeled for a phantom system with no external oxygen perfusion. This is due to the illumination of the phantom happneing at a depth of at least 1 cm below the water-air surface. Diffusion of oxygen in standard conditions could not supply oxygen to the illumination area with the set-up.
Singlet oxygen luminescence counts were compared to singlet oxygen concentrations calculated with equation (10). The parameters used for each sensitizer are summarized in table 1. The values are for in vitro conditions using their respective solvents.
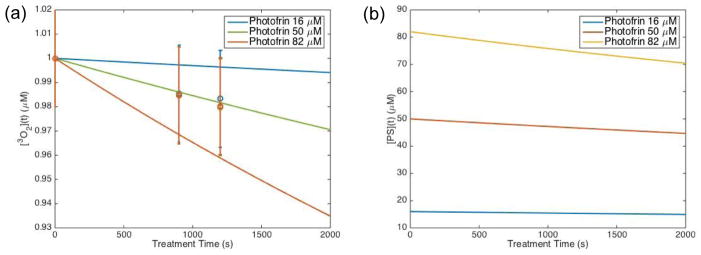
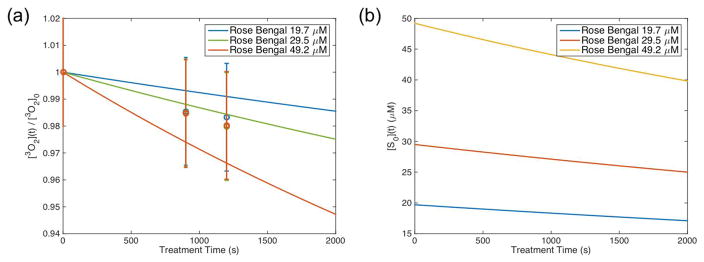
Figures 3(a) and 4(a) show a comparison between the measured oxygenation versus the explicit model-calculated values of oxygen for Photofrin and Rose Bengal, respectively. Data was plotted relative to the measured initial oxygen concentration, which is around 170 μM (2% uncertainties). The measured values had large standard deviations; however, the model was able to look at the reduction of oxygen in the phatom with the treatment conditions outlined. Further studies can be done with more measurements at the initial drop off as well as with different light dose and sensitizer concentrations to validate the model in more detail.
Figure 3.
Explicit model calculation of (a) relative ground state oxygen ([3O2]) and gound state sensitizer ([S0]) plotted for Photofrin using the parameters summarized in Table 1. Open circles represent oxygen measurements performed in phantoms after illumination with 523nm light for 15 minutes and then another 5 minutes. Ground state oxygen is plotted relative to the initial oxygen concentration ([3O2]0) that was measured at ~ 170 μM.
Figure 4.
Explicit model calculation of (a) relative ground state oxygen ([3O2]) and gound state sensitizer ([S0]) plotted for Rose Bengal using the parameters summarized in Table 1. Open circles represent oxygen measurements performed in phantoms after illumination with 523nm light for 15 minutes and then another 5 minutes. Ground state oxygen is plotted relative to the initial oxygen concentration ([3O2]0) that was measured at ~ 170 μM.
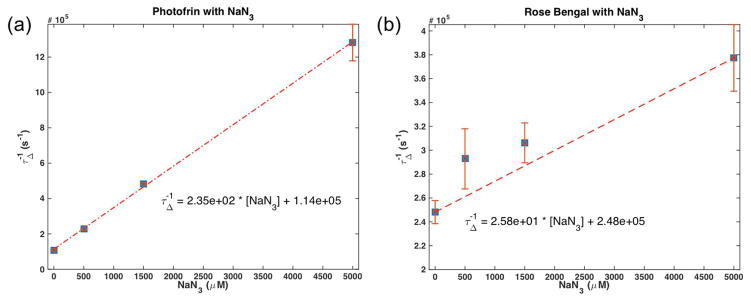
Using a NaN3, a singlet oxygen quencher, singlet oxygen lifetimes could be used to determine parameters k6 and k7, the spontaneous phosphorescence rate of 1O2 to 3O2 and the bimolecular rate of reaction for 1O2 with a substrate ([A]), respectively. By plotting the inverse of singlet oxygen lifetime against the concentration of singlet oxygen quencher, the following equation can be fit with a line
| (14) |
The values obtained are summarized in table 1 and are consistent with values obtained for k6 without any quencher. Figure 5 shows the plot of τΔ−1 versus [A].
Figure 5.
Inverse of singlet oxygen lifetime versus singlet oxygen quencher concentration for (a) Photofrin and (b) Rose Bengal. Values for parameters k6 and k7 are given by the fits to data according to equation (9).
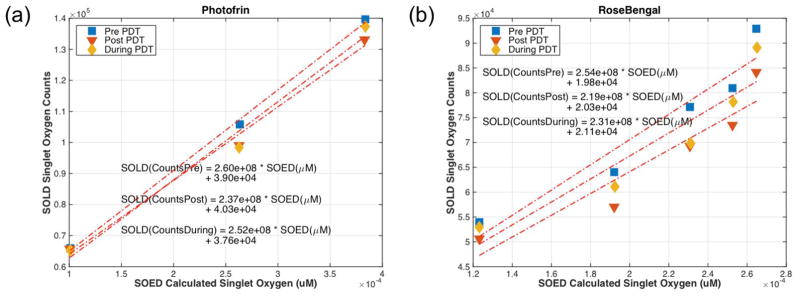
Figure 6 shows the comparison of SOLD singlet oxygen counts versus SOED model calculated singlet oxygen. With two different sensitizers in two different solvents, there were differences in photochemical parameters. Using the values summarized, the comparison shows that the SOLD system and SOED system are consistent even with two very different type II photosensitizers. The calculation of instantaneous singlet oxygen was done using Eq. 8. The slope between SOLD and SOED calculated singlet oxygen is the same regardless photosensitizers used, (2.5 ± 0.1) × 108 for Photofrin and (2.3 ± 0.2) × 108 for RB.
Figure 6.
SOLD system singlet oxygen counts plotted against SOED calculated singlet oxygen for (a) Photofrin and (b) Rose Bengal. The plots show that the two systems are comparible with two different sensitizers.
4. CONCLUSION
Singlet oxygen luminescence detection (SOLD) technology was compared with singlet oxygen explicit dosimetry (SOED) calculations for phantoms using Photofrin and Rose Bengal. Oxygen measurements were used to validate one aspect of SOED, while SOLD photon counts of singelt oxygen signal at 1270 nm were compared to SOED-calculated singlet oxygen to validate their correspondence using two different sensitizers and their solvents. SOED system validation is performed in vitro.
Acknowledgments
This work is supported by grants from the National Institute of Health (NIH) R01 CA154562 and P01 CA87971 (T.C.Z., M.M.K., R.P.) and the Princess Margaret Cancer Foundation (B.W., I.V.). R.H.H. acknowledges support from the European Research Council via Consolidator Grant IRIS. R.H.H. and G.S.B. acknowledge support from the UK Engineering and Physical Sciences Research Council grant EP/K015338/1, and the UK Quantum Technology Hub in Quantum Enhanced Imaging, QuantIC, EP/M01326X/1.
References
- 1.Dougherty TJ. Photodynamic Therapy. Photochem Photobiol. 1993;58(6):896–900. doi: 10.1111/j.1751-1097.1993.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim MM, Finlay JC, Zhu TC. Macroscopic singlet oxygen model incorporating photobleaching as an input parameter. Proc SPIE. 2015;9308:93080V-93081–93086. doi: 10.1117/12.2079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MM, Liu B, Miller J, Busch TM, Zhu TC. Parameter determination for BPD-mediated vascular PDT. Proc SPIE. 2014;8931:89311D-89311–89316. doi: 10.1117/12.2040305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MM, Penjweini R, Zhu TC. In vivo outcome study of BPD-mediated PDT using a macroscopic singlet oxygen model. Proc SPIE. 2015;9308:93080A-93081–93088. doi: 10.1117/12.2077803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X, Wang KK, Zhu TC. Singlet oxygen dosimetry modeling for photodynamic therapy. Proc SPIE. 2012;8210:82100T-82101–82107. doi: 10.1117/12.908317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Kim MM, Gallagher-Colombo SM, Busch TM, Zhu TC. Comparison of PDT parameters for RIF and H460 tumor models during HPPH-mediated PDT. Proc SPIE. 2014;8931:89311C-89311–89316. doi: 10.1117/12.2040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillan DD, Chen D, Kim MM, Liang X, Zhu TC. Parameter determination for singlet oxygen modeling of BPD-mediated PDT. Proc SPIE. 2013;8568:856810. doi: 10.1117/12.2003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penjweini R, Kim MM, Zhu TC. In vivo outcome study of HPPH mediated PDT using singlet oxygen explicit dosimetry (SOED) Proc SPIE. 2015;9308:93080N-93081–93086. doi: 10.1117/12.2076441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penjweini R, Liu B, Kim MM, Zhu TC. Explicit dosimetry for 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH) mediated photodynamic therapy: macroscopic singlet oxygen modeling. J Biomed Opt. 2015;20(12):128003-128001–128008. doi: 10.1117/1.JBO.20.12.128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HW, Putt ME, Emanuele MJ, Shin DB, Glatstein E, Yodh AG, Busch TM. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Can Res. 2004;64:7553–7561. doi: 10.1158/0008-5472.CAN-03-3632. [DOI] [PubMed] [Google Scholar]
- 11.Zhu TC, Finlay JC, Zhou X, Li J. Macroscopic modeling of the singlet oxygen production during PDT. Proc SPIE. 2007;6427:1–12. doi: 10.1117/12.701387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu TC, Kim MM, Liang X, Finlay JC, Busch TM. In-vivo singlet oxygen threshold doses for PDT. Photon Lasers Med. 2015;4(1):59–71. doi: 10.1515/plm-2014-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay JC, Mitra S, Patterson MS, Foster TH. Photobleaching kinetics of Photofrin in vivo and in multicell tumour spheroids indicate two simultaneous bleaching mechanisms. Phys Med Biol. 2004;49:4837–4860. doi: 10.1088/0031-9155/49/21/001. [DOI] [PubMed] [Google Scholar]
- 14.Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53(4):549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang KK, Finlay JC, Busch TM, Hahn SM, Zhu TC. Explicit dosimetry for photodynamic therapy: macroscopic singlet oxygen modeling. J Biophoton. 2010;3(5–6):304–318. doi: 10.1002/jbio.200900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang KK, Mitra S, Foster TH. A comprehensive mathematical model of microscopic dose deposition in photodynamic therapy. Med Phys. 2007;34(1):282–293. doi: 10.1118/1.2401041. [DOI] [PubMed] [Google Scholar]
- 17.Zhu TC, Liu B, Kim MM, McMillan DD, Liang X, Finlay JC, Busch TM. Comparison of singlet oxygen threshold doses for PDT. Proc SPIE. 2014;8931:89310I-89311–89310. doi: 10.1117/12.2039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gemmell NR, McCarthy A, Kim MM, Veilleux I, Zhu TC, Buller GS, Wilson BC, Hadfield RH. A Compact Fiber Optic Probe-Based Singlet Oxygen Luminescence Detection System. 2016 doi: 10.1002/jbio.201600078. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu TC, Liu B, Penjweini R. Study of tissue oxygen supply rate in a macroscopic photodynamic therapy singlet oxygen model. J Biomed Opt. 2015;20(3):038001-038001–038013. doi: 10.1117/1.JBO.20.3.038001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmond RW, Gamlin JN. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem Photobiol. 1999;70(4):391–475. [PubMed] [Google Scholar]
- 21.Miller JS. Rose bengal-sensitized photooxidation of 2-chlorophenol in water using solar simulated light. Water Res. 2005;39:412–422. doi: 10.1016/j.watres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Sterenborg HJCM, van Gemert MJC. Photodynamic therapy with pulsed light sources: a theoretical analysis. Phys Med Biol. 1996;41:835–849. doi: 10.1088/0031-9155/41/5/002. [DOI] [PubMed] [Google Scholar]
- 23.Georgakoudi I, Nichols MG, Foster TH. The Mechanism of Photofrin Photobleaching and Its Consequences for Photodynamic Dosimetry. Photochem Photobiol. 1997;65(1):135–144. doi: 10.1111/j.1751-1097.1997.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 24.Dysart JS, Singh G, Patterson MS. Calculation of singlet oxygen dose from photosensitizer fluorescence and photobleaching during mTHPC photodynamic therapy of MLL cells. Photochem Photobiol. 2005;81:196–205. doi: 10.1562/2004-07-23-RA-244. [DOI] [PubMed] [Google Scholar]
- 25.Smith GJ. The effects of aggregation on the fluorescence and triplet state yield of hematoporphyrin. Photochem Photobiol. 1985;41(2):123–126. [Google Scholar]
- 26.Mitra S, Foster TH. Photophysical Parameters, Photosensitizer Retention and Tissue Optical Properties Completely Account for the Higher Photodynamic Efficacy of meso-Tetra-Hydroxyphenyl-Chlorin vs Photofrin. Photochem Photobiol. 2005;81:849–859. doi: 10.1562/2005-02-22-RA-447. [DOI] [PubMed] [Google Scholar]
- 27.Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen Consumption and Diffusion Effects in Photodynamic Therapy. Radiat Res. 1991;126:296–303. doi: 10.2307/3577919. [DOI] [PubMed] [Google Scholar]
- 28.Gandin E, Lion Y, Van de Vorst A. Quantum yield of singlet oxygen production by xanthene derivatives. Photochem Photobiol. 1983;37(3):271–278. [Google Scholar]
- 29.Patterson MS, Madsen SJ, Wilson BC. Experimental Tests of the Feasibility of Singlet Oxygen Luminescence Monitoring in vivo During Photodynamic Therapy. J Photochem Photobiol B. 1990;5:69–84. doi: 10.1016/1011-1344(90)85006-i. [DOI] [PubMed] [Google Scholar]
- 30.Keir WF, Land EJ, MacLennan AH, McGarvey DJ, Truscott TG. Pulsed radiation studies of photodynamic sensitizers: the nature of DHE. Photochem Photobiol. 1987;46(5):587–589. doi: 10.1111/j.1751-1097.1987.tb04818.x. [DOI] [PubMed] [Google Scholar]
- 31.Lovell JF, Liu TW, Chen J, Zheng G. Activatable Photosensitizers for imaging and therapy. Chem Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 32.Tanielian C, Schweitzer C, Mechin R, Wolff C. Quantum yield of singlet oxygen production by monomeric and aggregated forms of hematoporphyrin derivative. Free Radic Biol Med. 2000;30(2):208–212. doi: 10.1016/s0891-5849(00)00460-3. [DOI] [PubMed] [Google Scholar]
- 33.Neckers DC. Rose Bengal. J Photochem Photobiol A. 1989;47:1–29. doi: 10.1111/j.1751-1097.1988.tb08842.x. [DOI] [PubMed] [Google Scholar]
- 34.Russell JA, Diamond KR, Collins TJ, Tiedje HF, Hayward JE, Farrell TJ, Patterson MS, Fang Q. Characterization of Fluorescence Lifetime of Photofrin and Delta-Aminolevulinic Acid Induced Protoporphyrin IX in Living Cells Using Single-and Two-Photon Excitation. IEEE J Sel Top Quant Electron. 2008;14(1):158–166. [Google Scholar]
- 35.Cramer LE, Spears KG. Hydrogen Bond Strengths from Solvent-Dependent Lifetimes of Rose Bengal Dye. J Am Chem Soc. 1978;100(1):221–227. [Google Scholar]