Abstract
Supramolecular host-guest interactions of trityl-nitroxide (TN) biradicals CT02-VT, CT02-AT and CT02-GT with methyl-β-cyclodextrin (M-β-CD), hydroxypropyl-β-cyclodextrin (H-β-CD) and γ-cyclodextrin (γ-CD) were investigated by EPR spectroscopy. In the presence of cyclodextrins (i.e., γ-CD, M-β-CD and H-β-CD), host-guest complexes of CT02-VT are formed where the nitroxide and linker parts possibly interact with the cyclodextrins’ cavities. Complexation with cyclodextrins leads to suppression of the intramolecular through-space spin-spin exchange coupling in CT02-VT, thus allowing determination of the through-bond spin-spin exchange coupling which was calculated to be 1.6 G using EPR simulations. Different types of cyclodextrins have variable binding affinity with CT02-VT with γ-CD (95 M−1) > M-β-CD (70 M−1) > H-β-CD (32 M−1). In addition, the effect of the linkers in TN biradicals on the host-guest interactions was also investigated. Among three TN biradicals studied, CT02-VT has the highest association constant with one designated cyclodextrin derivative. On the other hand, the complexes of CT02-GT (~ 22 G) and CT02-AT (7.7–9.0 G) with cyclodextrins have much higher through-bond spin-spin exchange couplings than that of CT02-VT (1.6 G) due to the shorter linkers than that of CT02-VT. Furthermore, the stability of TN biradicals towards ascorbate was significantly enhanced after the complexation with CDs, with an almost 2-time attenuation of the second-order rate constants for all the biradicals. Therefore, the supramolecular host-guest interactions with cyclodextrins will be an alternative method to modulate the magnitude of the spin-spin interactions and redox sensitivity of TN biradicals and the resulting complexes are promising as highly efficient DNP polarizing agents as well as EPR redox probes.
Introduction
Exchange-coupled biradicals have attracted considerable attention in the fields of chemistry, biology and related sciences. These biradicals have found applications as polarizing agents in high-field dynamic nuclear polarization (DNP),1–4 building blocks in molecular magnetic materials,5–8 polymerization initiators,9–13 spin labels for structural investigation of biomolecules using interspin distance determination,14–18 and molecular probes.19–21 An essential parameter in biradicals is represented by the spin-spin interaction (J) which have a dramatic effect on their physiochemical properties and potential applications.22, 23 This interaction can be through-bond and/or through-space, and its value varies by many orders of magnitude, depending on the nature of biradicals and the environments (e.g., temperature, solvent, etc.).24–27 In order to expand the applications of biradicals, it is desirable to tune precisely their spin-spin interactions. For instance, the spin-spin interaction in biradicals needs to be precisely controlled in order to obtain the maximal DNP enhancement: it should remain relatively small compared to nuclear Larmor frequency for the frequency matching but strong enough to optimize the polarization transfer.28–30
Besides the above mentioned method for controlling the spin-spin interaction via changing the linkers between two radical moieties, supramolecular interaction with cage-like host molecules such as cyclodextrins (CDs), cucurbit[n]uril, octaacid and resorcinarene has also been an effective tool to modulate the spin-spin interaction of nitroxide biradicals and oligoradicals.31–40 Typically, upon the complexation with hosts, the through-space spin-spin interaction in the biradicals or oligoradicals can be greatly decreased or completely suppressed due to the steric hindrance from the bulky hosts. Moreover, mechanically interlocked molecules such as rotaxanes, containing paramagentic species are also capable of controlling the spin-spin interaction between two paramagnetic centers by application of an appropriate stimulus such as redox reactions, photoactivation or variations in pH.35–37, 39, 41 These supramelocular interactions are very helpful for investigations of the molecular dynamics of host-guest complexation as well as the structure and stability of the resulting inclusion complexes.42–49 The resulting complexes also show great potential in molecular machine, magnetic material and DNP-enhanced magnetic resonance spectroscopy and imaging.50 Although extensive studies have been performed on the supramolecular interactions of homogeneous biradicals with hosts, there are very few related studies on heterogeneous biradicals.
Trityl-nitroxide (TN) biradicals which were developed by us are highly water soluble and have great potential as functional electron paramagnetic resonance (EPR) probes for simultaneous measurement of oxygneation and redox status as well as thiol concentration.51–53 In addition, these biradicals are so far the most promising high-field DNP polarizing agents.54 However, the optimal spin-spin interactions of TN biradicals are opposite for their uses as EPR probes and DNP polarizing agents. Recently, we have found that structural factors play a crucial role in controlling exchange coupling interactions in TN biradicals and the magnitude of J can be precisely tuned by varying the linker separating the two radical moieties and changing the temperature.55 Herein, as our continuous effort to fine-tune the exchange coupling interactions in TN biradicals, we describe the first example of the supramolecular interaction of the heterogeneous TN biradicals (Chart 1) with CDs. While TN biradicals CT02-GT, CT02-AT and CT02-VT were chosen as guest molecules, methyl-β-cyclodextrin (M-β-CD), hydroxypropyl-β-cyclodextrin (H-β-CD) and γ-cyclodextrin (γ-CD) were picked as host molecules. Effect of the linkers in the TN biradicals as well as type of CDs on the binding constants as well as the magnitude of the spin-spin interactions in the resulting complexes were explored by EPR and theoretical calculation. Additionally, effect of complexation on the redox sensitivity of TN biradicals was further studied.
Chart 1.
Molecular structure of TN biradicals studied in this paper
Experimental section
Materials
TN biradicals CT02-VT, CT02-AT and CT02-GT were synthesized according to our previously described method.55 γ-CD, M-β-CD and H-β-CD were purchased from Sigma-Aldrich. All other chemicals and solvents were of the highest grade commercially available.
EPR measurements
All EPR spectra were recorded at room temperature using a Bruker X-band EPR spectrometer. The following acquisition parameters were used: microwave power, 0.2–10 mW; modulation frequency, 30–100 kHz; time constant, 40.96 ms; conversion time, 40.96 ms; modulation amplitude, 0.03–1 G.
EPR spectra of TN biradicals (50 µM) in PBS (20 mM, pH 7.4) were recorded in the presence of various concentrations (0–100 mM) of γ-CD, M-β-CD and H-β-CD. Samples were loaded into EPR capillary tubes. The EPR signals of the biradicals alone were measured from spectra recorded without cyclodextrins, whereas the spectra of associated biradicals were determined in the presence of a high concentration of cyclodextrins (100 mM).
Kinetic study on the reduction of free and associated TN biradicals by ascorbic acid
Various concentrations of ascorbic acid was added to the solution of TN biradicals (50 µM) in the presence or absence of M-β-CD (50 mM) in PBS (50 mM, pH 7.4). Incremental EPR spectra were recorded immediately after mixing. The concentration of the trityl monoradicals at each time point was obtained by comparing their double integrated signal intensities relative to that of the reduced form of each TN biradical which was obtained by reaction of the corresponding TN biradical with ascorbic acid (1 mM) over two hours. Since ascorbic acid used (300–1000 µM) was in greater excess than the biradical (50 µM), the reaction kinetics of the biradical with ascorbic acid is a pseudo first-order reaction. Linear regression of kinetic data yields the biomolecular rate constants for reduction of TN biradicals by ascorbic acid in the presence or absence of M-β-CD (Table 2).
Table 2.
Second-order rate constants of TN biradicals with ascorbate in the absence (k) and presence (k’) of M-β-CD (50 mM).
| Rate constants | CT02-GT | CT02-AT | CT02-VT |
|---|---|---|---|
| k (M−1 s−1) | 10.33 ± 0.03 | 7.83 ± 0.16 | 6.84 ± 0.09 |
| k' (M−1 s−1) | 4.10 ± 0.09 | 3.29 ± 0.11 | 3.56 ± 0.07 |
EPR simulation
Computer simulations of the EPR spectra of free and associated biradicals were carried out using the EPR simulation program (ROKI\EPR).56, 57 The fitting routine used to determine the J values was similar to the method described in our previous studies.51–53, 55 The following parameters were optimized: g1 and g2; the hyperfine splitting constant of the nitrogen atom, αN; the relaxation (or linewidth variation) parameters α, β and γ which can be used to indicate the tumbling rates of free or associated biradicals; and J and its standard deviation ΔJ. The ratio of free and associated biradicals in the presence of cyclodextrins was used to calculate association constants (K). The relative concentrations of free and associated biradicals were determined by computer simulation as a function of CD concentrations and the association constant was determined from each spectra and the average values were given in the Table 1. Reliable K values were obtained when the concentrations of free and associated biradicals had comparable values. The best K values are just computed from the data in the intermediate CD concentration. Typically, the standard deviations of K values were 10–15% (Table 1). Since EPR spectra were recorded without field calibration, g factors obtained by EPR simulation are fictive. However, the difference of g factors of free and associated biradicals can be quantitatively determined to be 0.0001 or 0.0002 with the higher values for the associated biradicals.
Table 1.
The hyperfine splitting value (αN), magnitude of spin-spin interaction (J), standard deviation of J (ΔJ) and three relaxation parameters (α, β and γ) of free and complexed TN biradicals, and binding constants (K) of TN biradicals with cyclodextrins.
| Biradicals | αN/G | J/G | ΔJ/G | α/G | β/G | γ/G | K /M−1 |
|---|---|---|---|---|---|---|---|
| CT02-GT | 17.2 | 60.0 | 7.2 | 1.11 | 0.08 | −0.01 | - |
| CT02-AT | 17.3 | 75.9 | 12.3 | 1.43 | 0.01 | 0.14 | - |
| CT02-VT | 16.8 | 21.5 | 9.5 | 1.43 | 0.55 | −0.63 | - |
| CT02-AT/γ-CD | 13.5 | 8.8 | 1.2 | 2.2 | 0.12 | −0.24 | 35 ± 5 |
| CT02-AT/H-β-CD | 13.8 | 7.7 | 3.8 | 2.5 | 0.53 | −0.13 | - |
| CT02-AT/M-β-CD | 13.5 | 7.1 | 1.0 | 2.1 | −0.1 | −0.26 | 15 ± 5 |
| CT02-GT/M-β-CD | 15.6 | 22.2 | 9.0 | 3.6 | −0.57 | −0.55 | 64 ± 8 |
| CT02-VT/γ-CD | 15.7 | 1.6 | 4.5 | 2.2 | −0.1 | −0.23 | 95 ± 10 |
| CT02-VT/H-β-CD | 16.0 | 1.6 | 3.4 | 1.9 | 0.18 | −0.1 | 32 ± 5 |
| CT02-VT/M-β-CD | 15.9 | 1.6 | 3.4 | 1.9 | 0.18 | −0.1 | 70 ± 10 |
Results and discussion
Much evidence showed that β-CD, γ-CD and their derivatives can form inclusion complexes with the TEMPO nitroxide moiety owing to their relatively large minimal internal diameters (β-CD, 5.8 Å and γ-CD, 7.4 Å ) but α-CD with a minimal internal diameter of only 4.4 Å does not.37, 39, 58 Therefore, we chose γ-CD and two β-CD derivatives (i.e., M-β-CD and H-β-CD) as hosts to investigate their complexations with TN biradicals.
As shown in Fig. 1, in the absence of CDs, the TN biradical CT02-VT has a strongly asymmetric EPR triplet signal consisting of a broad and almost invisible low-field peak, strong center-field peak and moderate high-field peak.55 Addition of γ-CD (10 mM) to the aqueous solution of CT02-VT (50 µM) resulted in the host-guest interaction between γ-CD and CT02-VT and induced the appearance of a new EPR component with three lines separating by 15.7 G (Fig. 1, #) plus a single line in the center field (Fig. 1, *) possibly due to the very weak spin-spin coupling of two radical moieties in the supramolecular complex. Further increase of the concentration of γ-CD led to a higher ratio of the new component and the signal from the EPR component with the relatively strong spin-spin coupling was almost completely changed to the signal of the weakly coupled component at the concentration of 100 mM for γ-CD (Fig. 1). Therefore, the supramolecular host-guest interaction with γ-CD effectively decreased the spin-spin coupling (J) of the bound CT02-VT which was further determined by EPR simulation to be 1.6 ± 4.5 G (Table 1). Comparatively, the J value of free CT02-VT under the experimental condition is 21.5 ± 9.5 G (Table 1). As described in our previous study, the ΔJ value is indicative of the flexibility of the linker between two radical moieties.55 The higher ΔJ value of the free CT02-VT (9.5 G) compared to its corresponding complex with γ-CD (4.5 G) is most likely because the complexation slows down the rotation of the bonds in the linker and decreases its flexibility accordingly. Given that a low concentration (50 µM) of CT02-VT was used, this very weak spin-spin interaction in the complex is intramolecular and most likely through-bond due to the blocking of the spatial proximity between the trityl and nitroxide moieties by the cyclodextrin macrocycle. In contrast to the nitroxide-nitroxide biradicals which did not show any new EPR peaks upon complexation with hosts such as cyclodextrin and cucurbit[n]uril,31–34 the appearance of these new peaks makes the complexation of TN biradicals with γ-cyclodextrin much easier to be monitored by EPR. Considering that the trityl part is very bulky, the complexation of CT02-VT with γ-CD most likely occurs through the nitroxide and linker parts. The smaller AN (15.7 G, Table 1) in the complex compared to the value observed for CT02-VT alone (AN = 16.8 G, Table 1) in aqueous solutions indicated that the nitroxide part in the complex localized into a relatively less polar environment than in aqueous media. The much weaker high-field peak due to the nitroxide part as compared to the other two low-field peaks in the complex is most likely due to the slow molecular tumbling resulting from the formation of the large complex of CT02-VT with γ-CD. The slow rotational motion of CT02-VT in the presence of γ-CD can be also verified by the significant increase of the relaxation parameter α (2.2 G versus 1.43 G, Table 1) of the biradical upon this supramolecular interaction.
Figure 1.
Effect of concentration of γ-CD on the EPR spectra of CT02-VT. Gray and black lines denote experimental and simulated spectra, respectively.
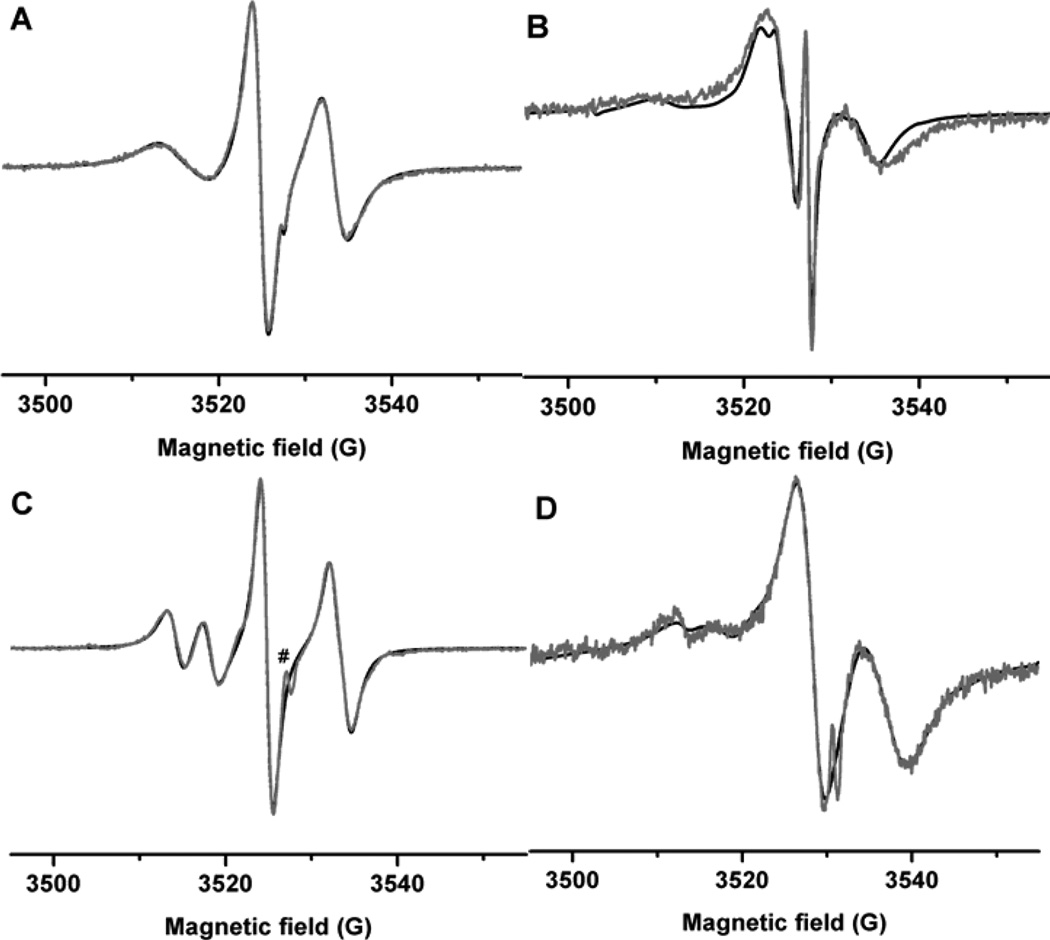
Besides γ-CD, β-CD derivatives (M-β-CD and H-β-CD) with smaller minimal internal diameters also showed the supramolecular host-guest interactions with CT02-VT. As shown in Fig. 2, in the presence of high concentration of either host (100 mM), the spin-spin exchange coupling of CT02-VT was almost completely inhibited as evidenced by the observation of only a very weakly coupled biradical signal. In order to further confirm whether the host-guest interaction with CDs inhibited the spin-spin exchange coupling of CT02-VT, 1-adamantane carboxylic acid (ACA), a guest competitor, was added to the solution containing CT02-VT and M-β-CD. Upon the addition of ACA, the relatively strong biradical signal was recovered with almost complete disappearance of the weakly coupled biradical signal (Fig. S1 in the supplementary information), confirming the formation of the host-guest complex between CT02-VT and M-β-CD. EPR simulations demonstrated that the complexes of CT02-VT with both M-β-CD and HOP-β-CD have the identical spin-spin dipolar interactions (1.6 G, Table 1) with the complex of γ-CD (1.6 G, Table 1) but exhibit slightly faster rotational motion compared to the latter as shown by their smaller relaxation parameters. Moreover, similar AN values for these three complexes (Table 1) indicate that the polarity around the nitroxide part in each complex are similar. However, the binding capacity of these CDs towards CT02-VT is different. The binding constants (K) which can be used to quantitatively describe their binding capacity were reliably obtained by EPR simulation when the concentration of free and associated biradicals had comparable values (See details in the Experimental section). Results show that γ-CD has the strongest binding capacity towards CT02-VT with a K value of 95 ± 10 M−1 (Table 1) as compared to M-β-CD (70 ± 10 M−1) and H-β-CD (32 ± 5 M−1) and thus the complex of γ-CD with CT02-VT has the highest stability among three complexes. Both the internal diameters and steric hindrance of the substituents of CDs may account for the difference in their binding capacity towards CT02-VT. The obtained binding constants of CDs (32–95 M−1) for CT02-VT were lower than the previously reported values (102–104 M−1) for nitroxide radicals42, 59, 60 possibly because of the linkage of the bulky trityl moiety in CT02-VT.
Figure 2.
Experimental (gray) and simulated (black) EPR spectra of CT02-VT in the presence of M-β-CD (100 mM) and H-β-CD (100 mM).
In order to check if the linkers in the TN biradicals affect their complexations with CDs, the interactions of the biradicals CT02-AT and CT02-GT with M-β-CD were also investigated by EPR. The linkers of CT02-AT and CT02-GT have two and three less C-C bonds than that of CT02-VT, respectively (see Chart 1). Both biradicals can also form the complexes with M-β-CD. As shown in Fig. 3, CT02-AT had an asymmetric EPR triplet in the absence of M-β-CD which was changed to a much broader signal consisting of a sharp trityl peak and a relatively broad signal upon complexation with M-β-CD, characteristic of a relatively decoupled biradical with a calculated J value of 9.0 ± 6.0 G. In contrast, the complexation of CT02-GT with M-β-CD did not result in a significant change of its EPR spectral feature in which a well-resolved asymmetric quartet was changed into a broad quartet with a calculated J value of 22.2 ± 9.0 G. EPR simulation shows that three relaxation parameters are enhanced for the associated forms of both CT02-GT and CT02-AT due to the slower rotational motions (Table 1). Much stronger spin-spin coupling for the complex of CT02-GT with M-β-CD relative to those of CT02-AT (J = 9 G) and CT02-VT (J = 1.6 G) with M-β-CD is most likely due to the shorter linker in CT02-GT which provides an extensive through-bond interaction. Therefore, for the host molecule M-β-CD, the through-bond spin-spin interaction of the supramolecular complex has an opposite relationship with the length of the linker between two radical moieties. Interestingly, the association constants of TN biradicals with CDs don’t not have direct correlation with the linker length. CT02-AT with a moderate linker has the smallest association constants (15 M−1, Table 1) with M-β-CD among the three TN biradicals (70 M−1 for CT02-VT and 64 M−1 for CT02-GT).
Figure 3.
Experimental (gray) and simulated (black) EPR spectra of free CT02-AT (A) and its complex with M-β-CD (B) and free CT02-GT (C) and its complex with M-β-CD (D).
It is well known that the hyperfine splitting (αN) of the nitrogen in nitroxide radicals is sensitive to the microenvironment around the nitroxide part in nitroxide monoradicals or biradicals and the difference (ΔB) of αN between the free and bound monoradicals or biradicals can be used as a good indicator for their localization in the host molecules. For the same TN biradical, the type of CDs has no significant effect on their αN’s in the complexes (Table 1). For instance, the αN values for the complexes of CT02-VT with CDs are in a narrow range from 15.7–16.0 G. Similar results were observed for the CT02-AT complexes with CDs (13.5–13.8 G). However, there is a big difference in the ΔB values for different TN biradicals with the order of CT02-AT (3.65 G) > CT02-GT (1.56 G) > CT02-VT (0.92 G). This result implies that the nitroxide part of CT02-AT locates into the most hydrophobic region whereas the position of the nitroxide in CT02-VT is close to the aqueous solution. Considering that CDs have a strong binding with the hydrophobic linker and the TEMPO group can thread through the cavities of γ-CD and β-CD derivatives, the pseudorotaxane between TN biradicals and CD derivatives may be formed in which the linker and/or nitroxide part are surrounded by the macrocycle.58 Therefore, CDs may bind to the linker of CT02-VT due to its long linker, thus exposing its TEMPO group to the aqueous solution. In contrast, the nitroxide part is the main binding site of CT02-AT with CDs. As for CT02-GT, the binding site with CDs is the region between the linker and nitroxide part.
Since the nitroxide parts in TN biradicals can reside in the cavity of CDs, this interaction is expected to increase the stability of the corresponding nitroxide parts and slow down their reduction rates by reductants such as ascorbate. We have recently showed that TN biradicals are unique probes for simultaneous measurement of redox status and oxygenation and the use of the more stable pyrrolidinyl nitroxide instead of the piperidinyl nitroxide for the synthesis of TN biradicals can significantly decrease their reduction rates by biological reductants, thus enhancing their sensitivity to redox status.51, 52 As observed from our previous studies,51, 52 upon addition of ascorbate to the solution of TN biradicals, a partially overlapped triplet EPR signal from the trityl-hydroxylamine monoradical was observed which was further changed into the well resolved signal under anaerobic conditions with a hyperfine splitting constant of 0.22 G due to the amide-N (I = 1) of the linker (See Figure S2 in the supplementary information). The sensitivity of the EPR spectra of the resulting trityl monoradicals to oxygen enables these TN biradicals to measure oxygen concentrations in biological systems by EPR.51, 52 Figure 4A shows the formation kinetics of the trityl monoradical in the reaction of CT02-GT with various concentrations of ascorbate in the presence or absence of M-β-CD. While high concentrations of ascorbate have a positive effect on the production of the trityl monoradical, addition of M-β-CD significantly slows down this process. According to the data shown in Fig. 4A, values of k[Asc] were obtained which can be further used to calculate the second-order rate constants of CT02-GT with ascorbate (Fig. 4B). As shown in Table 2, the supramolecular interaction with M-β-CD significantly slows down the reduction of CT02-GT by ascorbate with the calculated second-order rate constants of 10.33 ± 0.03 M−1 s−1 and 4.10 ± 0.09 M−1 s−1 in the absence and presence of M-β-CD, respectively. Similar results were observed from the other two TN biradicals (See Fig. S3 and Fig. S4 in the supplementary information). In general, the second-order rate constants of TN biradicals with ascorbate in the absence of M-β-CD is 1.9–2.5 times higher than those in the presence of M-β-CD under our experimental conditions (Table 2). Therefore, the supramolecular interaction of TN biradicals with CDs can be an alternative method to increase the stability of TN biradicals and thus enhance their sensitivity to redox status.
Figure 4.
(A) Plot of the concentrations of trityl monoradicals as a function of time which were generated by the reaction of CT02-GT (50 µM) with 500 µM (square), 800 µM (circle) and 1000 µM (triangle) of ascorbic acid in the presence (unfilled) or absence (filled) of M-β-CD (50 mM) in phosphate buffer (50 mM, pH 7.4). (B) Plot of k[Asc] as a function of the concentrations of ascorbate (Asc). Values of k[Asc] were obtained according to the data shown in Fig. 4A. Linear regression of kinetic data to yield the second-order rate constants for reduction of CT02-GT by ascorbate in the presence (circle) or absence (square ) of M-β-CD. Data were shown in Table 2.
Conclusions
TN biradicals CT02-VT, CT02-GT and CT02-AT can form supramolecular host-guest complexes with M-β-CD, H-β-CD and γ-CD. Both the linker of the biradicals and the type of cyclodextrins affect this host-guest interaction. While CT02-VT has the highest association constant with one designated cyclodextrin derivative as compared to the other two biradicals, γ-CD, among three cyclodextrins, has the strongest tendency to form the complexes with the biradicals as evidenced by its high association constants for TN biradicals. The complexation with CDs suppresses the through-space spin-spin exchange coupling in TN biradicals and the resulting complexes has the through-bond spin-spin interaction with the J values of 1.6 G for CT02-VT, 7.7–9.0 G for CT02-AT and 22.2 G for CT02-GT. We have recently reported that the spin-spin exchange coupling of TN biradicals can be also controlled by changing structural factors.55 We also demonstrate in this work that this host-guest interaction significantly enhances the redox sensitivity of TN biradicals by increasing their stability towards biological reductants such as ascorbate. Therefore, the supramolecular host-guest interaction will be another effective method to enhance the sensitivity of TN biradicals to redox status and modulate their spin-spin exchange coupling which could further expand their applications especially in the field of DNP. Since cyclodextrins have a relatively weak binding with TN biradicals, new studies can be directed to investigate the supramolecular host-guest interaction of TN biradicals with more efficient hosts such as cucurbit[n]uril.32, 33, 35
Supplementary Material
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (81201126, 21572161 and 31500684), Science & Technology Projects of Tianjin (15JCZDJC32300 and 15JCYBJC23700), and NIH grant (EB016096).
References
- 1.Ni QZ, Daviso E, Can TV, Markhasin E, Jawla SK, Swager TM, Temkin RJ, Herzfeld J, Griffin RG. Acc. Chem. Res. 2013;46:1933–1941. doi: 10.1021/ar300348n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ysacco C, Karoui H, Casano G, Le Moigne F, Combes S, Rockenbauer A, Rosay M, Maas W, Ouari O, Tordo P. Appl. Magn. Reson. 2012;43:251–261. [Google Scholar]
- 3.Dane EL, Maly T, Debelouchina GT, Griffin RG, Swager TM. Org. Lett. 2009;11:1871–1874. doi: 10.1021/ol9001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz-Gomez JL, Marin-Montesinos I, Lloveras V, Pons M, Vidal-Gancedo J, Veciana J. Org. Lett. 2014;16:5402–5405. doi: 10.1021/ol502644x. [DOI] [PubMed] [Google Scholar]
- 5.Miller JS, Drillon M, editors. Magnetism: Molecules to Materials. Weinheim, Germany: Wiley-VCH; 2001–2003. [Google Scholar]
- 6.Iwamura H, Koga N. Acc. Chem. Res. 1993;26:346–351. [Google Scholar]
- 7.Lahti PM, editor. Magnetic Properties of Organic Materials. New York: Marcel Dekker; 1999. [Google Scholar]
- 8.Rajca A. Chem. Rev. 1994;94:871–893. [Google Scholar]
- 9.Yoshida E, Takeda K. Polym. J. 2001;33:590–596. [Google Scholar]
- 10.Bothe M, Schmidt-Naake G. Macromol. Chem. Phys. 2004;205:208–216. [Google Scholar]
- 11.Hill NL, Braslau R. Macromolecules. 2005;38:9066–9074. [Google Scholar]
- 12.Ruehl J, Hill NL, Walter ED, Milihauser G, Braslau R. Macromolecules. 2008;41:1972–1982. doi: 10.1021/ma702358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaim A, Pietrasik K, Stoklosa T. Eur. Polym. J. 2010;46:519–527. [Google Scholar]
- 14.Hubbell WL, Cafiso DS, Altenbach C. Nat. Struct. Biol. 2000;7:735–739. doi: 10.1038/78956. [DOI] [PubMed] [Google Scholar]
- 15.Hagelueken G, Ingledew WJ, Huang H, Petrovic-Stojanovska B, Whitfield C, ElMkami H, Schiemann O, Naismith JH. Angew. Chem.-Int. Edit. 2009;48:2904–2906. doi: 10.1002/anie.200805758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berliner LJ, Eaton GR, Eaton SS, editors. Distance Measurements in Biological Systems by EPR. New York: Kluwer; 2000. [Google Scholar]
- 17.Yang ZY, Liu YP, Borbat P, Zweier JL, Freed JH, Hubbell WL. J. Am. Chem. Soc. 2012;134:9950–9952. doi: 10.1021/ja303791p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shevelev GY, Krumkacheva OA, Lomzov AA, Kuzhelev AA, Rogozhnikova OY, Trukhin DV, Troitskaya TI, Tormyshev VM, Fedin MV, Pyshnyi DV, Bagryanskaya EG. J. Am. Chem. Soc. 2014;136:9874–9877. doi: 10.1021/ja505122n. [DOI] [PubMed] [Google Scholar]
- 19.Marx L, Rassat A. Angew. Chem.-Int. Edit. 2000;39:4494–4496. doi: 10.1002/1521-3773(20001215)39:24<4494::aid-anie4494>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Ishiguro K, Ozaki M, Sekine N, Sawaki Y. J. Am. Chem. Soc. 1997;119:3625–3626. [Google Scholar]
- 21.Roshchupkina GI, Bobko AA, Bratasz A, Reznikov VA, Kuppusamy P, Khramtsov VV. Free Radic. Biol. Med. 2008;45:312–320. doi: 10.1016/j.freeradbiomed.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajca A. Chem. Rev. 1994;94:871–893. [Google Scholar]
- 23.Abe M. Chem. Rev. 2013;113:7011–7088. doi: 10.1021/cr400056a. [DOI] [PubMed] [Google Scholar]
- 24.Forbes MDE, Dukes KE, Avdievich NI, Harbron EJ, DeSimone JM. J. Phys. Chem. A. 2006;110:1767–1774. doi: 10.1021/jp053183q. [DOI] [PubMed] [Google Scholar]
- 25.Rajca A, Mukherjee S, Pink M, Rajca S. J. Am. Chem. Soc. 2006;128:13497–13507. doi: 10.1021/ja063567+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiesewetter MK, Corzilius B, Smith AA, Griffin RG, Swager TM. J. Am. Chem. Soc. 2012;134:4537–4540. doi: 10.1021/ja212054e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajca A, Takahashi M, Pink M, Spagnol G, Rajca S. J. Am. Chem. Soc. 2007;129:10159–10170. doi: 10.1021/ja0712017. [DOI] [PubMed] [Google Scholar]
- 28.Hu KN. Solid State Nucl. Magn. Reson. 2011;40:31–41. doi: 10.1016/j.ssnmr.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu KN, Debelouchina GT, Smith AA, Griffin RG. J. Chem. Phys. 2011;134:125105. doi: 10.1063/1.3564920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Can TV, Ni QZ, Griffin RG. J. Magn. Reson. 2015;253:23–35. doi: 10.1016/j.jmr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ionita G, Meltzer V, Pincu E, Chechik V. Org. Biomol. Chem. 2007;5:1910–1914. doi: 10.1039/b704112h. [DOI] [PubMed] [Google Scholar]
- 32.Porel M, Ottaviani F, Jockusch S, Jayaraj N, Turro NJ, Ramamurthy V. Chem. Commun. 2010;46:7736–7738. doi: 10.1039/c0cc02587a. [DOI] [PubMed] [Google Scholar]
- 33.Yi S, Captain B, Ottaviani MF, Kaifer AE. Langmuir. 2011;27:5624–5632. doi: 10.1021/la2005198. [DOI] [PubMed] [Google Scholar]
- 34.Porel M, Ottaviani MF, Jockusch S, Turro NJ, Ramamurthy V. RSC Adv. 2013;3:427–431. [Google Scholar]
- 35.Mileo E, Casati C, Franchi P, Mezzina E, Lucarini M. Org. Biomol. Chem. 2011;9:2920–2924. doi: 10.1039/c0ob01160f. [DOI] [PubMed] [Google Scholar]
- 36.Romano F, Manoni R, Franchi P, Mezzina E, Lucarini M. Chem.-Eur. J. 2015;21:2775–2779. doi: 10.1002/chem.201406301. [DOI] [PubMed] [Google Scholar]
- 37.Casati C, Franchi P, Pievo R, Mezzina E, Lucarini M. J. Am. Chem. Soc. 2012;134:19108–19117. doi: 10.1021/ja3073484. [DOI] [PubMed] [Google Scholar]
- 38.Graziano C, Masiero S, Pieraccini S, Lucarini M, Spada GP. Org. Lett. 2008;10:1739–1742. doi: 10.1021/ol8003832. [DOI] [PubMed] [Google Scholar]
- 39.Mezzina E, Fani M, Ferroni F, Franchi P, Menna M, Lucarini M. J. Org. Chem. 2006;71:3773–3777. doi: 10.1021/jo0601720. [DOI] [PubMed] [Google Scholar]
- 40.Chen JYC, Jayaraj N, Jockusch S, Ottaviani MF, Ramamurthy V, Turro NJ. J. Am. Chem. Soc. 2008;130:7206–7207. doi: 10.1021/ja801667w. [DOI] [PubMed] [Google Scholar]
- 41.Coskun A, Spruell JM, Barin G, Dichtel WR, Flood AH, Botros YY, Stoddart JF. Chem. Soc. Rev. 2012;41:4827–4859. doi: 10.1039/c2cs35053j. [DOI] [PubMed] [Google Scholar]
- 42.Kotake Y, Janzen EG. J. Am. Chem. Soc. 1989;111:2066–2070. [Google Scholar]
- 43.Lucarini M, Luppi B, Pedulli GF, Roberts BP. Chem.-Eur. J. 1999;5:2048–2054. [Google Scholar]
- 44.Karoui H, Rockenbauer A, Pietri S, Tordo P. Chem. Commun. 2002:3030–3031. doi: 10.1039/b209787g. [DOI] [PubMed] [Google Scholar]
- 45.Franchi P, Fani M, Mezzina E, Lucarini M. Org. Lett. 2008;10:1901–1904. doi: 10.1021/ol800405b. [DOI] [PubMed] [Google Scholar]
- 46.Polovyanenko DN, Marque SRA, Lambert S, Jicsinszky L, Plyusnin VF, Bagryanskaya EG. J. Phys. Chem. B. 2008;112:13157–13162. doi: 10.1021/jp8050164. [DOI] [PubMed] [Google Scholar]
- 47.Rossi S, Bonini M, Lo Nostro P, Baglioni P. Langmuir. 2007;23:10959–10967. doi: 10.1021/la7011638. [DOI] [PubMed] [Google Scholar]
- 48.Bardelang D, Casano G, Poulhes F, Karoui H, Filippini J, Rockenbauer A, Rosas R, Monnier V, Siri D, Gaudel-Siri A, Ouari O, Tordo P. J. Am. Chem. Soc. 2014;136:17570–17577. doi: 10.1021/ja509586k. [DOI] [PubMed] [Google Scholar]
- 49.Bardelang D, Banaszak K, Karoui H, Rockenbauer A, Waite M, Udachin K, Ripmeester JA, Ratcliffe CI, Ouari O, Tordo P. J. Am. Chem. Soc. 2009;131:5402–5404. doi: 10.1021/ja900306m. [DOI] [PubMed] [Google Scholar]
- 50.Mao JF, Akhmetzyanov D, Ouari O, Denysenkov V, Corzilius B, Plackmeyer J, Tordo P, Prisner TF, Glaubitz C. J. Am. Chem. Soc. 2013;135:19275–19281. doi: 10.1021/ja409840y. [DOI] [PubMed] [Google Scholar]
- 51.Liu YP, Villamena FA, Rockenbauer A, Zweier JL. Chem. Commun. 2010;46:628–630. doi: 10.1039/b919279d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu YP, Villamena FA, Song YG, Sun J, Rockenbauer A, Zweier JL. J. Org. Chem. 2010;75:7796–7802. doi: 10.1021/jo1016844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu YP, Song YG, Rockenbauer A, Sun J, Hemann C, Villamena FA, Zweier JL. J. Org. Chem. 2011;76:3853–3860. doi: 10.1021/jo200265u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathies G, Caporin MA, Michaelis VK, Liu Y, Hu KN, Mance D, Zweier JL, Rosay M, Baldus M, Griffin RG. Angew. Chem. Int. Ed. 2015;54:11770–11774. doi: 10.1002/anie.201504292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu YP, Villamena FA, Rockenbauer A, Song YG, Zweier JL. J. Am. Chem. Soc. 2013;135:2350–2356. doi: 10.1021/ja311571v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rockenbauer A, Korecz L. Appl. Magn. Reson. 1996;10:29–43. [Google Scholar]
- 57.Rockenbauer A, Szabo-Planka T, Arkosi Z, Korecz L. J. Am. Chem. Soc. 2001;123:7646–7654. doi: 10.1021/ja0102888. [DOI] [PubMed] [Google Scholar]
- 58.Wenz G, Han BH, Muller A. Chem. Rev. 2006;106:782–817. doi: 10.1021/cr970027+. [DOI] [PubMed] [Google Scholar]
- 59.Okazaki M, Kuwata K. J. Phys. Chem. 1984;88:3163–3165. [Google Scholar]
- 60.Ionita G, Caragheorgheopol A, Caldararu H, Jones L, Chechik V. Org. Biomol. Chem. 2009;7:598–602. doi: 10.1039/b817290k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.