ABSTRACT
Background: Folate intakes that do not meet or greatly exceed requirements may be associated with negative health outcomes. A better understanding of contributors that influence the input side will help establish dietary guidance that ensures health benefits without associated risks. Colonic microbiota produce large quantities of folate, and [13C5]5-formyltetrahydrofolate infused during colonoscopy is absorbed. However, it is unclear if significant quantities of folate are absorbed in an intact microbiome.
Objective: We determined whether and how much of a physiologic dose of [13C5]5-formyltetrahydrofolate delivered in a pH-sensitive enteric caplet to an intact colonic microbiome is absorbed.
Design: Healthy adults ingested a specially designed pH-sensitive acrylic copolymer–coated barium sulfate caplet that contained 855 nmol (400 ¼g) [13C5]5-formyltetrahydrofolate. After a washout period ≥4 wk, subjects received an intravenous injection of the same compound (214 nmol). Serially collected blood samples before and after each test dose were analyzed by using a microbiological assay and liquid chromatography–tandem mass spectrometry.
Results: Caplet disintegration in the colon was observed by fluoroscopic imaging for 6 subjects with a mean (±SD) complete disintegration time of 284 ± 155 min. The mean (±SEM) rate of appearance of [13C5]5-methyltetrahydrofolate in plasma was 0.33 ± 0.09 (caplet) and 5.8 ± 1.2 (intravenous) nmol/h. Likely because of the significant time in the colon, the mean apparent absorption across the colon was 46%.
Conclusions: Folate is absorbed across the colon in humans with an undisturbed microbiome. This finding and previous observations of the size of the colonic depot of folate and its potential for manipulation by diet (eg, dietary fiber, oligosaccharides, and probiotics) suggest that an individual’s dietary folate requirement may differ depending on the consumption of dietary constituents that affect the size and composition of their gastrointestinal microbiota. In addition, a systematic investigation of the role of colonic folate on gastrointestinal development and the prevention of colorectal cancer is warranted. This trial was registered at clinicaltrials.gov as NCT00941174.
INTRODUCTION
A growing body of evidence suggests a potential dual modulatory role of folate in health and disease where both inadequate and excessive intakes are associated with undesirable consequences (1). Suboptimal folate status, particularly in countries that do not have a folic acid–fortified food supply, is associated with risk of neural tube defects, cleft lip with or without cleft palate, colorectal cancer, and, to a lesser degree, stroke and neuropsychiatric disorders (2, 3). However, some individuals in countries with a folic acid–fortified food supply who also consume folic acid supplements have supraphysiologic concentrations of circulating folate, which have been proposed by some authors to negatively modify health risks (4–6). The literature in this area is often conflicting and has been polarizing.
To set dietary guidance for folate intake that strikes the right balance between the known benefits of addressing suboptimal intake and potential risks associated with very high intakes of folate, a better understanding of the input side of folate nutrition is required. To our knowledge, on the input side of the equation, only oral intakes of folate have been considered. Another potential source of folate is the folate pool produced by microorganisms in the colon. Many bacterial species, including several in the colon, are capable of synthesizing folate by a process that involves the condensation of paraaminobenzoic acid with dihydropterin (7, 8). The size of the colonic depot of folate is significant and was shown to approach or exceed the Recommended Dietary Allowance (400 ¼g) for adults (9, 10). Work in animal models that used a [3H]paraaminobenzoic acid tracer indicated that folate produced by intestinal bacteria can be absorbed across the colon of mammals (11, 12). More recently, after cecal infusion of 684 nmol [13C5]5-formyltetrahydrofolate after bowel cleansing, we observed the labeled metabolite, [13C5]5-methyltetrahydrofolate in plasma, which showed that folate can be absorbed across the colon in humans (13). Although the folate-specific transporter [eg, proton-coupled folate transporter (PCFT)5] is expressed in the colon, mechanisms by which folate absorption occurs across the colon remain unclear because it is uncertain whether the pH at the absorptive surface is in the range that would allow for appreciable PCFT activity (14–17).
Although we previously showed that preformed folate can be absorbed across the colon after cecal infusion in humans during colonoscopy, we do not know what impact bowel cleansing in preparation for this procedure had on our findings. In addition, the percentage of bioavailability of folate from the colon of humans remains unknown. Therefore, the primary objective of the current study was to evaluate folate absorption across the human colon with an intact microbiome by using specially designed enteric-coated caplets to deliver 855 nmol [13C5]5-formyltetrahydrofolate past the ileocecal junction.
SUBJECTS AND METHODS
Study population
Healthy adults between the ages of 18 and 65 y of age were recruited between September and October 2008 by public advertisement from the University of Toronto community. During an initial screening visit in the Clinical Investigation Unit at The Hospital for Sick Children, individuals provided a venous blood sample to confirm normal red blood cell (RBC) folate concentrations and determine their 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C>T genotype. Individuals shown to be homozygous for the T allele were excluded from participating in the study to minimize potential intersubject differences in the way folate was metabolized. Blood samples were also analyzed in the Core Laboratory Facilities at The Hospital for Sick Children to ensure subjects had normal blood chemistries (serum electrolytes and complete blood counts) and vitamin B-12 and pyridoxal-5-phosphate concentrations. During screening, potential subjects were also excluded if they reported a history of any chronic disease (eg, inflammatory bowel or celiac disease), recent gastrointestinal surgery, or medication use known to interfere with folate absorption, folate metabolism, intestinal motility, or intestinal pH (eg, dilantin, metformin, antacids, laxatives, or antibiotics). Individuals who consumed >1 alcoholic drink/d or were smokers were also excluded. Women were screened for pregnancy (Clearview hcG II; Wampole Laboratories), and those with a positive pregnancy test, planning a pregnancy, breastfeeding, or taking high-dose estrogen (eg, hormone replacement therapy) were also excluded. All subjects gave written informed consent. Subjects who met eligibility requirements were asked to refrain from the use of vitamin or mineral supplements ≥2 wk before study initiation and avoid alcohol within 24 h of receiving the folate test dose. Anthropometric measurements were determined according to standardized procedures (18). The study protocol was approved by the Human Research Ethics Board at The Hospital for Sick Children, and regulatory approval was received from the Therapeutic Products Directorate, Health Canada.
Test compound and dose formulations
The isotopically labeled test compound [glutamyl-13C5]-[6S]-5-formyltetrahydrofolic acid was synthesized by Merck Eprova AG and purchased in powdered form as a calcium salt. The chemical purity of the compound was confirmed to be 98% pure by the manufacturer by using HPLC and infrared spectroscopy. [13C5]5-Formyltetrahydrofolate was mixed with barium sulfate, which is a radioopaque substance, to allow for the monitoring of the transit of the test compound through the gastrointestinal transit via serial fluoroscopic imaging as we have described previously (19). This mixture was pressed into a caplet and coated with 2 different pH-sensitive acrylic copolymer products in a 3:1 mass ratio (Eudragit L100 and Eudragit S100; Evonik Industries AG). We previously reported that this combination of Eudragit products was the most efficacious in ensuring caplets did not start to disintegrate before the ileocecal junction but did so in the colon (19). Caplets in the current study were produced by the Toronto Institute of Pharmaceutical Technology following good manufacturing practices, and except where indicated, all ingredients used to prepare caplets were purchased from Sigma. Each compressed caplet core was formulated to contain 855 nmol [13C5]5-formyltetrahydrofolate (400 ¼g), 64% (wt:wt) barium sulfate, 7% (wt:wt) polyvinyl pyrillidone K90, 13% (wt:wt) microcrystalline cellulose, and 4% (wt:wt) sodium starch glycolate (JRS Pharma) to a target weight of 2220 mg (19). Caplets were coated by first dispersing Eudragit L100 (4% wt:wt) and S100 (1% wt:wt) in water and adding a plasticizer (triethylcitrate) (4% wt:wt), glidant (talc) (2% wt:wt), and a neutralizing agent (potassium hydroxide) (0.1% wt:wt). The final aqueous coating mixture was filtered to eliminate sediment or agglomerates and sprayed on barium sulfate caplet cores [Laboratory Development Coating System 5 (LDCS-5) Hicoater; Vector Corp] to achieve an increase in caplet mass ~12.6%. Caplets were stored in the dark at 4°C.
For the intravenous test dose, [13C5]5-formyltetrahydrofolate (214 nmol; 100 ¼g) was dissolved in physiologic saline (pH 7.0; 74 ¼g folate/mL) under aseptic conditions in the Department of Pharmacy at The Hospital for Sick Children. After testing for sterility and bacterial endotoxins, intravenous solutions were stored in the dark at 4°C for up to 1 mo. Folate concentrations of intravenous solutions and caplets were confirmed by microbial assay to be ±10% of the targeted folate concentration.
Study protocol
On study day 1, a fasting baseline venipuncture blood sample (5 mL) was collected. On day 2, at ~0600, subjects consumed the test caplet that contained 855 nmol [13C5]5-formyltetrahydrofolate with as much water as desired. A standard breakfast that consisted of puffed rice cereal and a nondairy rice beverage was provided. This breakfast was previously assessed to provide low amounts of folate (direct analysis in our laboratory), energy, and residue (food-composition tables and product labels) (19). Approximately 2 h later, the location in the gastrointestinal tract and disintegration profile of each caplet was determined by a qualified radiation technologist by using a fluoroscope (Infinix; Toshiba America Medical Systems Inc) in the Image Guided Therapy Unit at The Hospital for Sick Children (19). Imaging proceeded at ~60-min intervals and concluded at 1800 h or earlier if complete caplet disintegration was observed. Each image required an average of 2–3 s fluoroscopic exposure and delivered an approximate radiation entrance dose of 20 mRem/image. In total, subjects received an average (±SD) of 127 ± 22-mRem exposure, which was comparable to one-tenth of the effective dose for an adult abdominal X-ray. In the event that the caplet had not completely dissolved by 1800, the next stool was collected. Between imaging sessions, subjects were able to move around freely but were asked to abstain from vigorous exercise. All fluoroscopic images were independently reviewed by one of the authors, a radiologist (BC), and a doctoral student to confirm the anatomical location where caplets disintegrated and determine caplet transit and disintegration times. The caplet disintegration initiation time was defined as the time when clear erosion of the caplet edges could be observed, and complete caplet disintegration time was defined as the time when a solid caplet core was no longer discernible.
Peripheral blood samples (5 mL) were collected hourly via an indwelling catheter after the caplet had passed through the pyloric sphincter and until 2000 and again at 24 and 48 h postcaplet ingestion. Once the caplet had exited the stomach, lunch and snacks were provided ad libitum from a selection of low-folate–containing foods that investigators provided.
After ≥4 wk, subjects returned to the Clinical Investigation Unit to complete the intravenous arm of the study. After an overnight fast, blood samples were collected from each subject at ~0800. Thereafter, subjects were immediately injected thereafter with 214 nmol [13C5]5-formyltetrahydrofolate in 1.35 mL sterile saline. We previously showed that a lower dose of [13C5]5-formyltetrahydrofolate was necessary to produce a detectable plasma folate response than with the administration of the dose through the colon (13). Blood samples (5 mL) were collected 15 min postinjection and at 30-min intervals thereafter for 4 h via an indwelling catheter inserted in the arm that was not used for injection of the test dose. During blood collection, low-folate snacks and beverages were provided ad libitum.
All foods and beverages consumed while subjects were in the Clinical Investigation Unit were recorded by one of the authors (AL) during both caplet and intravenous injection arms of the study. Each participant received written and oral instructions on how to record their food intakes by using household measures after they went home and until the 48-h blood draw during the caplet arm of the study was completed. Subjects were also asked to limit foods high in folate during this time and were provided with food lists. Total energy, macronutrient, and folate contents of foods consumed were determined from food records and a food-composition database (Food Processor SQL version 10.2.0; Esha Research).
Biochemical and mass spectrometry analyses
Blood samples for folate analyses were collected into EDTA-treated tubes and processed as described previously (13). The total folate concentration of whole blood and plasma was determined by using standard microbial assay with the test organism Lactobacillus rhamnosus and folic acid to generate the standard curve (ATCC7649; American Type Tissue Culture Collection) (20). The RBC folate concentration was calculated by subtracting plasma folate from whole-blood folate with correction for the packed red cell volume. A whole-blood reference standard (certified value of 29.5 nmol/L, 95/528; National Institute of Biological Standards and Control) was used to assess the reproducibility and accuracy of our microbial assay. During this study, the analysis of the whole-blood reference standard yielded a folate concentration of 29.7 ± 1.8 nmol/L and an interassay CV of 6% (n = 36). The genotyping of MTHFR C677T (CC, CT, and TT) was determined by DNA extracted from the buffy coat of whole blood after centrifugation and processed by an allele-specific real-time polymerase chain reaction by using the TaqMan SNP Genotyping for MTHFR (Applied Biosystems) (21).
The plasma enrichments of the administered test dose of [13C5]5-formyltetrahydrofolate and its metabolite [13C5]5-methyltetrahydrofolate were determined by using liquid chromatography–tandem mass spectrometry (LC-MS/MS) at the CDC as described in detail previously (13, 22). Briefly, folates were extracted from plasma by using phenyl solid-phase extraction 96-well plates, and extracts were analyzed with the use of LC-MS/MS by using reversed-phase chromatographic separation with an isocratic mobile phase. Mass-to-charge ratios of transitions of interest [(M + 0) and (M + 5)] were monitored in positive-ion mode via turbo ion electrospray on an AB Sciex 5500 triple quadrupole mass spectrometry system (Applied Biosystems). Plasma samples collected immediately after intravenous injection showed a mean (±SEM ) enrichment with [13C5]5-formyltetrahydrofolate of 11.6 ± 1.5%.
Quantification of plasma folate response
In addition to reporting data as molar ratios of 5-formyltetrahydrofolate and 5-methyltetrahydrofolate, they are also presented as the sum of peak areas as nanomoles of folate per person. The quantification of the plasma response was done with a number of important assumptions detailed previously (13). Most importantly, to calculate the sum of all peak areas, we added the peak areas for labeled (M + 5) and unlabeled (M + 0) 5-formyltetrahydrofolate and 5-methyltetrahydrofolate and folic acid. To account for the difference in the LC-MS/MS signal of 5-formyltetrahydrofolate, 5-methyltetrahydrofolate, and folic acid, we adjusted peak areas for 5-formyltetrahydrofolate (divided by 0.92) and folic acid (divided by 0.7). Second, to quantify the total concentration of labeled (M + 5) 5-formyltetrahydrofolate or 5-methyltetrahydrofolate (nmol folate/L plasma), we multiplied the peak area for each labeled metabolite by the baseline total plasma folate concentration determined by using a microbial assay for each subject at each treatment period and divided this value by the sum of all peak areas as we have done previously (13). Finally, to express results on a whole-body basis (ie, convert from nmol/L to nmol/person), each individual subject’s total blood volume was estimated by using the standard value of 75 mL/kg for men and 66.5 mL/kg for women (23). The plasma volume was calculated from the estimated whole blood volume by using the packed red cell volume.
Statistical analysis
Our sample size was based on our previous work in this area that facilitated a prediction of the percentage of specially coated caplets that would release their test dose quantitatively in the colon and the number of subjects required to produce a robust estimate of the mean rate of [13C5]5-methyltetrahydrofolate appearance in plasma (13, 19). Descriptive statistics were generated with SAS for Windows software (version 9.3; SAS Institute Inc). We analyzed the change in molar ratios of formyltetrahydrofolate or 5-methyltetrahydrofolate or in the total plasma folate concentration (ie, nmol/L or nmol/person) over time by using repeated-measures ANOVA (PROC MIXED; SAS Institute Inc) with the sample as the main effect and quadratic sample or cubic sample as necessary. The baseline RBC folate concentration was included as a covariate in these statistical models.
The individual rate of appearance of [13C5]5-methyltetrahydrofolate in plasma over time after caplet ingestion was determined from the linear slope of the ascending portion of each plasma response curve and with GraphPad Prism version 4.00 for Windows software (GraphPad Software). The estimated apparent plasma half-life (one-phase exponential decrease over time) of [13C5]5-formyltetrahydrofolate after the intravenous injection for each subject was determined from the slope of the descending portion of each plasma response curve.
RESULTS
Subject characteristics
Eleven adults responded to the poster advertisement about the research project, attended the screening visit, and provided a blood sample to determine their eligibility for the study. One individual was excluded because the person was homozygous for the MTHFR 677C>T allele, and one person withdrew after the screening visit but before any study intervention because of other commitments. All 9 remaining individuals completed both the caplet and intravenous injection arms of the study. Characteristics of these 6 men and 3 women (22–26 y old) are summarized in Table 1. Screening RBC folate concentrations varied but were well above the cutoff of 360 nmol/L associated with liver folate depletion (18). Blood indexes of vitamin B-12 status and vitamin B-6 status were within common normative ranges (18); however, one subject’s pyridoxal-5-phosphate concentration exceeded the group mean by >3 SDs and, therefore, was not included in the calculation of the baseline mean value. All other blood vitamin concentrations for this subject were included in the calculation of baseline means. Four subjects, including the individual with the very high pyridoxal-5-phosphate concentration, consumed supplements that contained folic acid and vitamin B-6 before the screening visit, but all participants discontinued supplementation ≥2 wk before the study intervention. The BMI (in kg/m2) of each subject was <30. During the period of serial blood sample collection after caplet ingestion (14 h) and the intravenous injection (4 h), mean (±SD) dietary intakes of folate were 47.9 ± 8 and 12 ± 1.2 ¼g dietary folate equivalents, respectively.
TABLE 1.
Subject characteristics1
| Value | Range | |
| Sex (F/M) (n) | 3/6 | — |
| Age (y) | 25 ± 12 | 22–26 |
| Weight (kg) | 73.2 ± 17.2 | 47.8–103.6 |
| BMI (kg/m2) | 24.6 ± 3.6 | 18.8–29.9 |
| Plasma volume (L)3 | 2.91 ± 0.7 | 1.87–4.07 |
| MTHFR 677C>T | ||
| CC | 5 | — |
| CT | 4 | — |
| Red blood cell folate (nmol/L) | 1108 ± 437 | 604–1988 |
| Total plasma folate (nmol/L) | 41.7 ± 17.7 | 14.9–68.5 |
| Vitamin B-12 (pmol/L) | 331 ± 188 | 151–583 |
| Pyridoxal-5-phosphate (nmol/L)4 | 53 ± 17 | 34–82 |
| Ethnicity (n) | ||
| Middle Eastern | 1 | — |
| White | 5 | — |
| Chinese | 2 | — |
| South Asian | 1 | — |
| Dietary intake of folate during blood sampling (¼g DFE) | ||
| After intravenous injection | 12 ± 1.2 | 7.3–25 |
| After caplet ingestion | ||
| 0–14 h | 47.9 ± 8.0 | 2.47–65.9 |
| 15–24 h | 48.0 ± 19.4 | 0–162.3 |
| 25–48 h | 327.3 ± 74.9 | 26.6–677.2 |
n = 9 unless otherwise indicated. DFE, dietary folate equivalent.
Mean ± SD (all such values).
Whole blood volumes were calculated by using an estimate of 66.5 mL/kg for women and 75 mL/kg for men (23). The plasma volume was calculated from the estimated whole blood volume by using an adjustment for the packed cell volume.
n = 8.
Caplet transit and disintegration
No caplet began to disintegrate before the ileocecal junction in any subject as assessed by the intact caplet edges observed during fluoroscopic imaging (see Supplemental Table 1 under “Supplemental data” in the online issue). In 8 of 9 subjects, some initial disintegration of the caplet occurred in the colon during the 14-h observation period, and in 6 of 9 subjects, disintegration was complete. In the one remaining subject (subjects G), no disintegration of the caplet was observed in the colon. However, a complete disintegration of the caplet was noted in a subsequently collected stool sample. We observed plasma uptake of [13C5]5-methyltetrahydrofolate after caplet ingestion in all subjects but 2 subjects (subjects H and I; one subject with complete caplet disintegration, and one subjects without complete caplet disintegration. These 2 subjects were removed from subsequent analyses unless otherwise indicated.
Pharmacokinetics
Summaries of pharmacokinetic data from the caplet and intravenous injection arms of the study are shown in Tables 2 and 3, respectively. We did not detect [13C5]5-formyltetrahydrofolate in the plasma of any subject after test caplets were consumed; however, its metabolite [13C5]5-methyltetrahydrofolate was detected in the plasma of 7 subjects (subjects A–G). For 6 subjects (subjects A–F) in whom complete caplet disintegration was observed, the mean (±SEM) delay in appearance of [13C5]5-methyltetrahydrofolate in plasma after caplet ingestion was 6.6 ± 1.0 h and appeared at a rate of 0.33 ± 0.09 nmol/h that reached a maximal plasma concentration (Cmax) of 2.6 ± 0.6 nmol/person. We were unable to include subject G in the pharmacokinetic analysis because only trace amounts of [13C5]5-methyltetrahydrofolate appeared in plasma, and there was no discernible linear slope on the ascending portion of the plasma response curve (Figure 1).
TABLE 2.
Pharmacokinetic data from subjects (n = 6) who exhibited a linear plasma folate response after caplet ingestion of 855 nmol [13C5]5-formyltetrahydrofolate1
| Caplet disintegration | ||||||||
| Plasma volume | Sex | t delay | C max | t max | Rate of appearance | Initiation location | Completion location | |
| L | h | nmol | h | nmol/h | ||||
| Subject | ||||||||
| A | 2.21 | F | 5 | 3.7 | 6.0 | 0.72 | ICJ | Cecum |
| B | 4.07 | M | 8 | 4.0 | 10.0 | 0.37 | Cecum | Ascending |
| C | 1.87 | F | 4 | 1.6 | 5.5 | 0.31 | ICJ | Ascending |
| D | 3.54 | M | 6 | 3.7 | 7.1 | 0.33 | Cecum | Ascending |
| E | 2.77 | M | 6 | 0.5 | 10.1 | 0.05 | Cecum | ND2 |
| F | 2.38 | F | 7 | 2.0 | 10.0 | 0.21 | ICJ | Ascending |
| Mean ± SEM | 2.9 ± 0.3 | — | 6.6 ± 1.0 | 2.6 ± 0.6 | 8.7 ± 0.9 | 0.33 ± 0.09 | — | — |
Rate of appearance was determined from the ascending slope for the concentration of labeled [13C5]5-methyltetrahydrofolate in the plasma of 6 subjects with complete caplet disintegration. Cmax, maximal concentration; ICJ, ileocecal junction; ND, not detected; tdelay, time lag before detection of [13C5]5-methyltetrahyrofolate in plasma after caplet ingestion (baseline) in 6 subjects; tmax, time of maximal concentration.
Last image was taken in the transverse colon.
TABLE 3.
| 5-formyltetrahydrofolate | 5-methyltetrahydrofolate | |||
| C max | t 1/2 | C max | Rate of appearance | |
| nmol | h | nmol | nmol/h | |
| Subject | ||||
| A | 14.1 | 0.376 | 10.0 | 4.0 ± 1.0 |
| B | 7.5 | 0.279 | 5.0 | 4.2 ± 1.8 |
| C | 10.9 | 0.315 | 6.0 | 3.1 ± 0.8 |
| D | 15.2 | 0.300 | 5.8 | 5.3 ± 1.6 |
| E | 20.0 | 0.262 | 6.8 | 6.4 ± 1.1 |
| F | 10.2 | 0.330 | 6.6 | 6.2 ± 1.6 |
| G | 20.3 | 0.305 | 10.2 | 14.5 ± 7.7 |
| H | 7.1 | 0.244 | 3.1 | 2.9 ± 0.6 |
| I | 15.9 | 0.593 | 7.8 | 5.6 ± 0.2 |
| Mean ± SEM | 13.5 ± 1.6 | 0.3 ± 0.03 | 6.8 ± 0.8 | 5.8 ± 1.2 |
Rate of appearance was determined from the ascending slope over time of the concentration of labeled [13C5]5-methyltetrahydrofolate in plasma (n = 9). Cmax, maximal concentration; t1/2, apparent plasma half-life.
FIGURE 1.
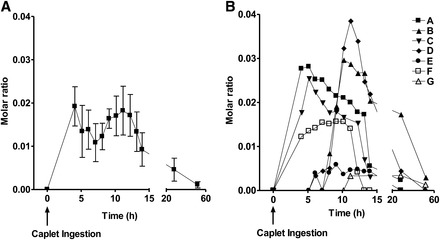
Mean (±SEM) (A) and individual subject (B) molar ratios of (M + 5) to (M + 0) for 5-methyltetrahydrofolate in plasma after initiation of disintegration of enteric-coated caplets containing 855 nmol [13C5]5-formyltetrahydrofolate. The change in the mean molar ratio was significant (P = 0.0250; repeated-measures ANOVA). No [13C5]5-formyltetrahydrofolate was observed in the plasma of any subject.
The mean Cmax of [13C5]5-formyltetrahydrofolate after intravenous injection was 13.5 ± 1.6 nmol. The concentration of labeled 5-formyltetrahydrofolate in plasma decreased from its maximum after intravenous injection with a half-life of 0.3 ± 0.03 h. The mean Cmax for [13C5]5-methyltetrahydrofolate was 6.8 ± 0.8 nmol/person.
Plasma labeled folate response
The molar ratios (M + 5:M + 0) for 5-methyltetrahydrofolate during the caplet and for 5-formyltetrahydrofolate and 5-methyltetrahydrofolate during the intravenous injection arms of the study are illustrated in Figure 1 and Figure 2, respectively. After caplet ingestion (n = 7), there was a statistically significant change in the molar ratios for 5-methyltetrahydrofolate (P = 0.0250). Similarly, after intravenous injection (n = 9), there was a significant change in molar ratios for 5-formyltetrahydrofolate and 5-methyltetrahydrofolate (P < 0.0001).
FIGURE 2.
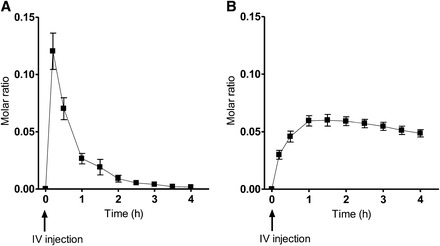
Mean (±SEM) molar ratios of (M + 5) to (M + 0) for 5-formyltetrahydrofolate (A) and 5-methyltetrahydrofolate (B) after IV injection of 214 nmol [13C5]5-formyltetrahydrofolate in 9 subjects. The change in the molar ratio for both [13C5]5-formyl- and [13C5]5-methyltetrahydrofolate was significant (P < 0.0001; repeated-measures ANOVA). IV, intravenous.
We converted LC-MS/MS (M + 5) and (M + 0) peak areas for5-formyltetrahydrofolate and 5-methyltetrahydrofolate to nanomoles per person in each arm of the study as illustrated in Figure 3. After caplet ingestion, there was a significant change in the total amount of labeled 5-methyltetrahydrofolate in plasma over time which returned to baseline within 24 h (P = 0.0192). After intravenous injection, there was a significant change in labeled 5-formyltetrahydrofolate, 5-methyltetrahydrofolate, and unlabeled 5-formyltetrahydrofolate over time (P < 0.0001).
FIGURE 3.
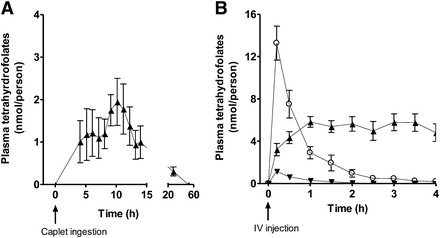
Mean (±SEM) plasma folate content of [13C5]5-formyltetrahydrofolate (circles, M + 5), its metabolite [13C5]5-methyltetrahydrofolate (upward triangles, M + 5), and unlabeled 5-formyltetrahydrofolate (downward triangles, M + 0) after administration of [13C5]5-formyltetrahydrofolate by an enteric-coated caplet (855 nmol; n = 7) (A) and IV injection (214 nmol; n = 9) (B). Note that y axes differ between panels. The change in [13C5]5-formyltetrahydrofolate after IV injection was significant (P < 0.0001) (B). The change in labeled [13C5]5-methyltetrahydrofolate was significant after caplet ingestion (P = 0.0192) (A) and IV injection (P < 0.0001) (B). The change in unlabeled 5-formyltetrahydrofolate was significant after IV injection (P < 0.0001) (B). Repeated-measures ANOVA statistical analyses were used to determine the change in the amount of plasma folate per person after administration of the test dose. IV, intravenous.
Calculation of apparent absorption
We intended to calculate the percentage bioavailability of the [13C5]5-formyltetrahydrofolate from the test caplets by comparing the AUC produced for [13C5]5-methyltetrahydrofolate in plasma after caplet ingestion with that generated after intravenous injection. However, as illustrated in Figure 2B, the molar ratio of 5-methyltetrahydrofolate in plasma after intravenous injection did not return to baseline, and thus, the calculation of the percentage of bioavailability by comparison of AUCs was not possible. Therefore, we elected to calculate the apparent absorption of [13C5]5-formyltetrahydrofolate from the test caplets by using the mathematical model for a simple one-compartment model for folate absorption across the small intestine described by Wright et al (24). In this set of 3 equations, C was defined as the plasma concentration of labeled 5-methyltetrahydrofolate, and M was the mass of the dose absorbed. The apparent volume of distribution for folate in the sample compartment was defined as V and estimated to be 387 mL/kg body weight (25). From the plasma labeled 5-methyltetrahydrofolate curve, tlag was defined as the length of time plasma enrichment remained at baseline before the first appearance of labeled folate in plasma (set at 1 h). In addition, tmax was defined as the time to peak labeled 5-methyltetrahydrofolate concentration, and T was calculated as the difference between tmax and tlag. The rate constant of elimination of labeled 5-methyltetrahydrofolate from the plasma compartment to body tissues or excretion was defined as k, whereby
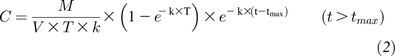 |
The mean absorption of the 855-nmol dose of [13C5]5-formyltetrahydrofolate was 46.2 ± 16.5%. This estimate of folate absorption did not consider the fraction of newly absorbed folate that may have been metabolized by colonocytes or sequestered by the liver.
DISCUSSION
[13C5]5-Formyltetrahydrofolate (855 nmol; 400 ¼g) delivered to the colon by enteric-coated caplets was absorbed across the colon of humans. This observation was consistent with earlier work that used the rodent or pig as an animal model (11, 12) and in which labeled folate was quantitatively infused into the cecum of humans after bowel cleansing (13). The enteric-coated caplets, which were specially designed for this study, allowed for the systematic investigation of folate absorption across the colon without disruption of the microbiome, which was a major strength of this work. This approach could be applied in future research to investigate the availability of other nutrients and bioactive components in the colon that are of interest to human health.
In subjects who had a linear plasma folate response after caplet ingestion (n = 6), the mean (±SEM) rate of absorption assessed by the appearance of [13C5]5-methyltetrahydrofolate in plasma was 0.33 ± 0.09 nmol/h. We previously observed the rate of folate absorption after infusion of a bolus dose of [13C5]5-formyltetrahydrofolate (684 nmol; 320 ¼g) directly into the cecum to be 0.6 ± 0.02 nmol/h. A comparison of results from the 2 studies must be approached with caution. However, a significant difference in the rate of absorption between the 2 studies was shown (P = 0.02; unpaired t test). Nonetheless, the rate of absorption across the colon in both studies was significantly lower than that reported in the literature for the small intestine (24, 26). With the use of data derived from published reports of Wright et al (24, 26) in which the appearance of [13C5]5-methyltetrahydrofolate was measured after an oral bolus dose of [13C5]5-formyltetrahydrofolate (421–569 nmol), we estimated that the rate of folate absorption across the small intestine is ~34 nmol/h.
The difference in reported rates of folate absorption across the small intestine compared with the colon is consistent with the current understanding of how physiologic concentrations of the vitamin are absorbed throughout the gastrointestinal tract. Beyond the early postnatal period, folate must be in the monoglutamyl form to be absorbed, which is the form of the vitamin secreted into the lumen by folate synthesizing bacteria and lost during enterohepatic circulation (27–29). In the colon, the lysis of prokaryotes and eukaryotic cells yields a source of polyglutamylated folates. Folate absorption across the apical side of the enterocyte in the small intestine is thought to occur mainly by PCFT (optimum pH of 5.5). This transporter is also present in the colon at lower concentrations (17). More work needs to be done to ascertain whether the pH at the absorptive surface of the colon would allow for an appreciable PCFT activity.
Available evidence, primarily from in vitro and animal studies, has suggested that many factors influence the expression of folate transporters and or folate absorption (2, 16, 30, 31). These factors include the folic acid concentration in cell culture media, mix of folates consumed (monoglutamylated compared with polyglutamylated) fiber intake (soluble or insoluble), genetic mutations in folate metabolism or transport, and the use of proton-pump inhibitors. Although the concentration of bacteria in the small intestine is considerably lower than the colon, it has been shown that folate synthesized by bacteria in the small intestine can similarly be absorbed (32). Whether these factors or differences in microbial community composition in the colon account for the wide subject-to-subject variability in the current study is not clear.
Although the rate of folate absorption is much slower in the colon than small intestine, the residence time in the colon is up to 20 times longer (33, 34). A longer transit time allows for greater opportunity for folate absorption to occur and likely accounts for the estimated 46% bioavailability of the [13C5]5-formyltetrahydrofolate dose in the current study. With the use of the same assumptions as used in the current study, Wright et al (24) reported an apparent absorption of 38 ± 6% after a 500-nmol orally administered test dose of [13C5]5-formyltetrahydrofolate in the small intestine. If the 46% apparent rate of absorption across the colon estimated herein is taken into account and with the assumption of the total folate content in the aqueous fraction of colonic evacuates that we previously reported in a sample of South Africans (699 ± 131 ¼g) and white Americans (860 ± 129 ¼g), we estimated that ~322–396 ¼g colon-derived folates could be potentially absorbed daily (10). These estimates do not take into consideration that many folates are polyglutamylated and, hence, differ in their bioavailability compared with that of monoglutamylated folate used as a test dose in the current study, but these study results emphasize the potential contribution of colonic folate to whole-body folate metabolism and colonic health (2). Previous research has suggested at least one-half of folates in stools of infants and piglets were short-chain folates with a significant fraction comprised of monoglutamylated folates (9). The hydrolysis of the polyglutamate chain of folate occurs in the brush border of the small intestine of humans by glutamate carboxypeptidase II (17). Although glutamate carboxypeptidase II transcripts are present in the colon, they have been shown at much-lower concentrations than in the small intestine (35). Similarly, we know that bacteria themselves contain folate conjugase, usually at low amounts (36). The impact of these sources of folate conjugase on conversion of polyglutamylated folate to the bioavailable monoglutamyl form is unknown.
We acknowledge the limitations of this study and offer suggestions for future research. As described, we intended to calculate the [13C5]5-formyltetrahydrofolate bioavailability from test caplets by comparing the AUC produced for [13C5]5-methyltetrahydrofolate in plasma after caplet disintegration with that generated after intravenous injection. However, the molar ratio of methyltetrahydrofolate in plasma after intravenous injection did not return to baseline; hence, this approach was not possible. We observed this same phenomenon in our previous colonoscopy study after 4 h of blood sampling; therefore, we suggest that blood samples be collected for a longer period of time after intravenous injection in future studies (13).
Second, to formulate the enteric coating on caplets to ensure that they remained intact until after the ileocecal junction, in 3 of our 9 subjects, caplets did not appear to completely disintegrate in the colon. In an earlier study that assessed the efficacy of the enteric coating on these caplets, Aimone et al (19) noted, in 2 of 10 subjects, that there was no observable dissolution of caplets in the colon. These observations suggest that, in future work that uses these caplets to investigate the colonic absorption of folate, other nutrients, or bioactive components, some type of imaging will be required to confirm in which subjects caplets dissolved. In addition, in 2 subjects, one in whom the caplet appeared to completely disintegrate and one in whom it did not, there was no observed plasma folate response after caplet ingestion. Our estimate of the [13C5]5-formyltetrahydrofolate bioavailability from the colon did not include these 2 subjects. If we assume the true bioavailability of the test dose in these 2 subjects was zero and included them in the analysis, the estimated (±SEM) bioavailability in our sample would have been 34.7 ± 14.3%. Future studies should examine the availability of polyglutamylated folates across the colon because they comprise a significant fraction of colonic folate. Finally, we acknowledge that our sample size of 6 subjects was small, and hence, the generalization of the rate of folate absorption across the colon or the percentage of the total dose of folate absorbed to the larger population must be approached with caution.
In conclusion, results from the current study suggest that a physiologic dose of folate delivered to the colon by means of enteric-coated caplets is absorbed. We estimate an apparent rate of absorption of 46% compared with 38% reported previously for the small intestine by using similar methodology. Previous studies used to establish the Recommended Dietary Allowance for folate included participants with an intact microbiome (2). However, because of the large quantity of folate present in the colon and its potential for manipulation by diet (eg, dietary fiber increasing the bacterial load and folate synthesis), we speculate that an individual’s dietary requirement for folate may differ depending on the consumption of dietary constituents that influence the size and composition of the gastrointestinal microbiome. Although there has been some evidence from animal and human studies that suggested that systemic folate concentrations are associated with bacterial folate biosynthesis, more research is required before changes to dietary recommendations should be considered (37–40). In addition, the current research suggests that a systematic investigation of the role of colonic folate on gut health, including gastrointestinal development during infancy and the prevention of colorectal cancer, is warranted.
Supplementary Material
Acknowledgments
We are grateful to our subjects for their contribution of time. Also, we thank the Toronto Institute of Pharmaceutical Science (Surrinder Chaudhary, Jamie Lugtu-Pe, and Frank Martinuzzi) for the development and manufacturing of caplets. We appreciate Albert Aziza and Sally Gopaul-Mattook from the Image Guided Therapy Department at The Hospital for Sick Children as well as the medical radiology staff for accommodating our study. We thank Karen Chapman, Roberta Gardiner, and nurses from the Clinical investigation Unit at the Hospital for Sick Children for their ongoing assistance. We are grateful to Aneta Plaga, Ashley Aimone, and Dubraiicka Pichardo for assisting with study logistics and Desta Ramlackhansingh for helping with the Health Canada submission. Finally, we thank Mark Bedford for support in preparing the test solutions and providing guidance on meeting regulatory requirements
The authors’ responsibilities were as follows—SA, JFG, and DLO: designed the study; AL and SA: conducted the experiment and collected data; ZF and CMP: were responsible for the LC-MS/MS analysis; PBP and BC: provided medical support and safety oversight; BC: oversaw the fluoroscopic imaging; AL and DLO: wrote the manuscript; and ZF, SA, JFG, CMP, PBP, and BC: contributed to the editing of the manuscript. None of the authors had a conflict of interest.
ABBREVIATIONS
- Cmax
maximal plasma concentration
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- PCFT
proton-coupled folate transporter
- RBC
red blood cell
FOOTNOTES
Funding agencies had no role in the design or conduct of the research study, statistical analysis, or data interpretation or writing of the manuscript. Findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the CDC/Agency for Toxic Substances and Disease Registry.
Supported and a trainee award through the Canadian Institutes of Health Training Program in Clinical Research (STP 53889; to AL).
REFERENCES
- 1. Kim YI.. Folic acid fortification and supplementation–good for some but not so good for others. Nutr Rev 2007;65:504–11. [DOI] [PubMed] [Google Scholar]
- 2. Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. 10th ed. Washington, DC: National Academy Press, 1998. [PubMed] [Google Scholar]
- 3. Li S, Chao A, Li Z, Moore CA, Liu Y, Zhu J, Erickson JD, Hao L, Berry RJ.. Folic acid use and nonsyndromic orofacial clefts in China: a prospective cohort study. Epidemiology 2012;23:423–32. [DOI] [PubMed] [Google Scholar]
- 4. Colapinto CK, O’Connor DL, Tremblay MS.. Folate status of the population in the Canadian Health Measures Survey. CMAJ 2011;183:E100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith AD, Kim YI, Refsum H.. Is folic acid good for everyone? Am J Clin Nutr 2008;87:517–33. [DOI] [PubMed] [Google Scholar]
- 6. Yang Q, Cogswell ME, Hamner HC, Carriquiry A, Bailey LB, Pfeiffer CM, Berry RJ.. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003-2006. Am J Clin Nutr 2010;91:64–72. [DOI] [PubMed] [Google Scholar]
- 7. Brody T, Shane B, Stokstad EL.. Folic acid In: Machlin L, ed. Handbook of vitamins: nutritional, biochemical and clinical aspects. New York, NY: Dekker, 1984:459–96. [Google Scholar]
- 8. Shiota T.. Biosynthesis of folate from pterin precursors In: Blakely RL, Benkovic S, eds. Folate and pterins: chemistry and biochemistry of folates. New York, NY: Wiley, 1984:121–34. [Google Scholar]
- 9. Kim TH, Yang J, Darling PB, O’Connor DL.. A large pool of available folate exists in the large intestine of human infants and piglets. J Nutr 2004;134:1389–94. [DOI] [PubMed] [Google Scholar]
- 10. O’Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T.. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr 2009;139:2044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asrar FM, O’Connor DL.. Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J Nutr Biochem 2005;16:587–93. [DOI] [PubMed] [Google Scholar]
- 12. Rong N, Selhub J, Goldin BR, Rosenberg IH.. Bacterially synthesized folate in rat large intestine is incorporated into host tissue folyl polyglutamates. J Nutr 1991;121:1955–9. [DOI] [PubMed] [Google Scholar]
- 13. Aufreiter S, Gregory JF 3rd, Pfeiffer CM, Fazili Z, Kim YI, Marcon N, Kamalaporn P, Pencharz PB, O’Connor DL.. Folate is absorbed across the colon of adults: evidence from cecal infusion of (13)C-labeled [6S]-5-formyltetrahydrofolic acid. Am J Clin Nutr 2009;90:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dudeja PK, Torania SA, Said HM.. Evidence for the existence of a carrier-mediated folate uptake mechanism in human colonic luminal membranes. Am J Physiol 1997;272:G1408–15. [DOI] [PubMed] [Google Scholar]
- 15. Kumar CK, Moyer MP, Dudeja PK, Said HM.. A protein-tyrosine kinase-regulated, pH-dependent, carrier-mediated uptake system for folate in human normal colonic epithelial cell line NCM460. J Biol Chem 1997;272:6226–31. [DOI] [PubMed] [Google Scholar]
- 16. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID.. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006;127:917–28. [DOI] [PubMed] [Google Scholar]
- 17. Visentin M, Diop-Bove N, Zhao R, Goldman ID.. The intestinal absorption of folates. Annu Rev Physiol 2014;76:251–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibson RS.. Principles of nutritional assessment. 2nd ed. New York, NY: Oxford University Press, 2005. [Google Scholar]
- 19. Aimone AM, Connolly B, Chaudhary S, Lugtu-Pe J, Martinuzzi F, Pencharz P, O’Connor DL.. A combination of pH-sensitive caplet coatings may be an effective noninvasive strategy to deliver bioactive substances, nutrients, or their precursors to the colon. Appl Physiol Nutr Metab 2009;34:893–900. [DOI] [PubMed] [Google Scholar]
- 20. Molloy AM, Scott JM.. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 21. Eny KM, Wolever TM, Fontaine-Bisson B, El-Sohemy A.. Genetic variant in the glucose transporter type 2 is associated with higher intakes of sugars in two distinct populations. Physiol Genomics 2008;33:355–60. [DOI] [PubMed] [Google Scholar]
- 22. Fazili Z, Whitehead RD Jr, Paladugula N, Pfeiffer CM.. A high-throughput LC-MS/MS method suitable for population biomonitoring measures five serum folate vitamers and one oxidation product. Anal Bioanal Chem 2013;405:4549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snyder WS.. Report of the Task Group on Reference Man: ICRP publication 23. New York, NY: Pergamon Press, 1975. [Google Scholar]
- 24. Wright AJ, Finglas PM, Dainty JR, Wolfe CA, Hart DJ, Wright DM, Gregory JF.. Differential kinetic behavior and distribution for pteroylglutamic acid and reduced folates: a revised hypothesis of the primary site of PteGlu metabolism in humans. J Nutr 2005;135:619–23. [DOI] [PubMed] [Google Scholar]
- 25. Loew D, Eberhardt A, Heseker H, Kubler W.. Plasma kinetics and elimination of folic acid. Klin Wochenschr 1987;65:520–4 (in German). [DOI] [PubMed] [Google Scholar]
- 26. Wright AJ, Finglas PM, Dainty JR, Hart DJ, Wolfe CA, Southon S, Gregory JF.. Single oral doses of 13C forms of pteroylmonoglutamic acid and 5-formyltetrahydrofolic acid elicit differences in short-term kinetics of labelled and unlabelled folates in plasma: potential problems in interpretation of folate bioavailability studies. Br J Nutr 2003;90:363–71. [DOI] [PubMed] [Google Scholar]
- 27. Rossi M, Amaretti A, Raimondi S.. Folate production by probiotic bacteria. Nutrients 2011;3:118–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shane B, Stokstad EL.. Transport and metabolism of folates by bacteria. J Biol Chem 1975;250:2243–53. [PubMed] [Google Scholar]
- 29. Sybesma W, Starrenburg M, Tijsseling L, Hoefnagel MH, Hugenholtz J.. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl Environ Microbiol 2003;69:4542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM.. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 2007;86:159–66. [DOI] [PubMed] [Google Scholar]
- 31. Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB.. The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiol Gastrointest Liver Physiol 2010;298:G248–54. [DOI] [PubMed] [Google Scholar]
- 32. Camilo E, Zimmerman J, Mason JB, Golner B, Russell R, Selhub J, Rosenberg IH.. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology 1996;110:991–8. [DOI] [PubMed] [Google Scholar]
- 33. Ghoshal UC, Sengar V, Srivastava D.. Colonic transit study technique and interpretation: can these be uniform globally in different populations with non-uniform colon transit time? J Neurogastroenterol Motil 2012;18:227–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SK.. Small intestine transit time in the normal small bowel study. Am J Roentgenol Radium Ther Nucl Med 1968;104:522–4. [DOI] [PubMed] [Google Scholar]
- 35. Devlin AM, Ling EH, Peerson JM, Fernando S, Clarke R, Smith AD, Halsted CH.. Glutamate carboxypeptidase II: a polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum Mol Genet 2000;9:2837–44. [DOI] [PubMed] [Google Scholar]
- 36. Oe H, Kohashi M, Iwai K.. Radioassay of the folate-hydrolyzing enzyme activity, and the distribution of the enzyme in biological cells and tissues. J Nutr Sci Vitaminol (Tokyo) 1983;29:523–31. [DOI] [PubMed] [Google Scholar]
- 37. Houghton LA, Green TJ, Donovan UM, Gibson RS, Stephen AM, O’Connor DL.. Association between dietary fiber intake and the folate status of a group of female adolescents. Am J Clin Nutr 1997;66:1414–21. [DOI] [PubMed] [Google Scholar]
- 38. Krause LJ, Forsberg CW, O’Connor DL.. Feeding human milk to rats increases Bifidobacterium in the cecum and colon which correlates with enhanced folate status. J Nutr 1996;126:1505–11. [DOI] [PubMed] [Google Scholar]
- 39. Pompei A, Cordisco L, Amaretti A, Zanoni S, Raimondi S, Matteuzzi D, Rossi M.. Administration of folate-producing bifidobacteria enhances folate status in Wistar rats. J Nutr 2007;137:2742–6. [DOI] [PubMed] [Google Scholar]
- 40. Wolever TMS, Assiff L, Basu T, Chiasson J, Boctor M, Gerstein HC, Hunt JA, Josse RG, Lau D, Leiter LA et al. . Miglitol, an α-glucosidase inhibitor, prevents the metformin-induced fall in serum folate and vitamin B12 in subjects with type 2 diabetes. Nutr Res 2000;20:1447–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


