Abstract
Infections with influenza viruses and respiratory bacteria each contribute substantially to the global burden of morbidity and mortality. Simultaneous or sequential infection with these pathogens manifests in complex and difficult-to-treat disease processes that need extensive antimicrobial therapy and cause substantial excess mortality, particularly during annual influenza seasons and pandemics. At the host level, influenza viruses prime respiratory mucosal surfaces for excess bacterial acquisition and this supports increased carriage density and dissemination to the lower respiratory tract, while greatly constraining innate and adaptive antibacterial defences. Driven by virus-mediated structural modifications, aberrant immunological responses to sequential infection, and excessive immunopathological responses, co-infections are noted by short-term and long-term departures from immune homoeostasis, inhibition of appropriate pathogen recognition, loss of tolerance to tissue damage, and general increases in susceptibility to severe bacterial disease. At the population level, these effects translate into increased horizontal bacterial transmission and excess use of antimicrobial therapies. With increasing concerns about future possible influenza pandemics, the past decade has seen rapid advances in our understanding of these interactions. In this Review, we discuss the epidemiological and clinical importance of influenza and respiratory bacterial co-infections, including the foundational efforts that laid the groundwork for today’s investigations, and detail the most important and current advances in our understanding of the structural and immunological mechanisms underlying the pathogenesis of co-infection. We describe and interpret what is known in sequence, from transmission and phenotypic shifts in bacterial dynamics to the immunological, cellular, and molecular modifications that underlie these processes, and propose avenues of further research that might be most valuable for prevention and treatment strategies to best mitigate excess disease during future influenza pandemics.
Introduction
Respiratory infections are common, distributed across all social and economic strata, and encompass both pneumonia, the single most important disease state resulting in mortality of children younger than 5 years globally, and otitis media, the primary cause of childhood physician visits and prescription of antibiotic therapy in middle-income and high-income countries.1,2 In 2011, 120·4 million pneumonia cases in children included 14·11 million severe episodes (11·7%) and 1·26 million childhood deaths (18% of all-cause mortality), with a case-fatality rate of 0·01.1 In adults, incidence of community-acquired pneumonia (CAP) across Europe is estimated at 1·07–1·2 per 1000 person-years and 14 per 1000 person-years in elderly people.3 Pneumonia is the fourth leading cause of death globally and the leading infectious cause.4 In the upper respiratory tract, otitis media affects 80% of all children within the first 3 years of life and 40% of children have more than six recurrences by age 7 years, which has consequences for antibiotic resistance in pathogens.2
A major contributor to both pneumonia and otitis media, influenza viruses rank among the most important pathogens to affect human health and cause disease and mortality.1 With relatively low case-fatality rates, influenza takes its toll through annual epidemic waves that infect hundreds of millions of people, causing severe infections in 3 million to 5 million people and 25 0000–500 000 deaths annually, 99% of which occur in low-income countries.5 Although pneumonia deaths are primarily of bacterial causes, particularly Streptococcus pneumoniae (32·7%) and Haemophilus influenzae (15·7%), influenza viruses add substantially, accounting for 7% of all severe pneumonia episodes and 10·9% of pneumonia deaths.1
Because of rapid mutation and gene segment reassortment, between the late 19th and mid 20th centuries, at least four major influenza pandemics transpired.6,7 Three of these pandemics—the H3N8 Russian pandemic of 1889, the H2N2 Asian flu of 1957, and the H3N2 Hong Kong pandemic of 1968—were of a considerably milder nature relative to the much more devastating H1N1 Spanish influenza pandemic of 1918–19.7 Infecting a third of the global population in 1918, and with estimates of 20 million to 50 million deaths, the 1918 influenza pandemic is the most deadly known pandemic in the history of humankind.7
Although the 1918 influenza virus was extraordinary in transmissibility and virulence, only seldom did acute respiratory distress and death follow viral infection alone. Current evidence suggests that mortality during the 1918 pandemic was primarily a result of an extraordinary capacity of the virus to enhance susceptibility to bacterial infections, particularly in adults aged 20–40 years.6,8 An analysis of more than 8000 autopsy reports showed evidence of bacterial invasion in 92% of fatal 1918 influenza cases.8 Streptococcus pneumoniae (the pneumococcus) predominated, while β-aemolytic Streptococcus, Staphylococcus aureus, and Haemophilus influenzae were also detected.8 More recently, a double-blind, randomised, placebo-controlled trial of the nine-valent pneumococcal conjugate vaccine (PCV9) showed 41% efficacy against influenza associated non-bacteraemic pneumonias.9 Coupled with recent results from a PCV13 trial suggesting around 45% efficacy of PCV13 against non-bacteraemic pneumonia,10 a similar role for bacterial infections in 90% of influenza pneumonia cases during the aforementioned PCV9 trial can be inferred. Specimens from the Asian influenza pandemic of 1956–57 and more recently the 2009 H1N1 pandemic (pH1N1) also show conclusive evidence of bacterial lung infections in 30–50% of fatal cases.8,11,12 Positive blood cultures in 40% of fatal 1918 epidemic cases also showed a shift in cause of pneumococcal bacteraemia towards inclusion of less invasive strains that are more often associated with asymptomatic carriage than disease.13
Although death from severe influenza alone usually occurs between 2 and 4 days after the onset of flu-like symptoms, only 5% of deaths in 1918 took place within this timeframe, with the majority following timecourses that were more akin to those of fatal pneumococcal pneumonias in the pre-antibiotic era (6–10 days).13
Influenza–bacterial co-infections have been known about for much of the 20th century. In 1931, Richard Shope, referring to the filterable influenza virus, concluded that “the disease induced by [the] filtrable infectious agent…was definitely not typical swine influenza and will be referred to hereafter as ‘filtrate disease’”.14 Medical reports by physicians and bacteriologists (most notably Richard Friedrich Johannes Pfeiffer) from as early as 1892 suggested that Bacillus influenzae (now H influenzae) was responsible for the mortality associated with pandemic influenza. Unable to culture the same bacteria as Pfeiffer, others believed that severe influenza resulted from a pathogen with low virulence working synergistically with a pneumonia-causing bacterial agent.15
The first conclusive reports of influenza-bacterial co-infections date back to 1931 (2 years before Smith and Laidlaw’s discovery of influenza A virus in human beings) when Shope, at the Rockefeller Institute, and his mentor, Paul Lewis, showed that sequential infection of pigs with swine influenza virus and H influenzae together induced far greater disease than either pathogen alone.14 These experiments reconciled, at least in part, Pfeiffer’s H influenzae theory and the postulation by Olitsky and Gates in 1921 that the pathogen causing influenza was of viral origin.15
Several subsequent animal studies throughout the early to mid-20th century also support the idea of influenza–bacterial synergy.8,16–18
Laboratory and epidemiological evidence from pandemics and interpandemics of the 20th century have left little room to debate the importance of bacterial secondary infections. Thus, modern research has emphasised efforts to elucidate the structural and immunological mechanisms and resulting dynamics of co-infections. In the remainder of this Review we discuss the most important recent advances in our understanding of the effect of infection with influenza viruses on bacterial infections. In view of their known clinical importance, we focus on the unidirectional effects that influenza viruses have on bacterial disease. However, evidence suggests that the relationship is bidirectional, whereby bacterial infection modulates virus dynamics and disease, an important issue that has been recently reviewed.19 Further, we limit our discussion to influenza viruses while acknowledging an increasing body of work showing that influenza is but one of a number of viral respiratory pathogens that include (in order of importance) respiratory syncytial virus, parainfluenza virus, human metapneumovirus, and rhinovirus, which are important in development of severe viral–bacterial co-infections.20
The complex nature of the interactions between influenza and bacterial infections yield mechanisms of disease that can be described at distinct phenotypic, cellular, molecular, and immunological levels, which might be tissue specific. Thus, as best as possible, we aim to review what is known in sequential order, from transmission and upper respiratory tract carriage, to diseases of the upper and lower respiratory tract, and from the macroscopic to the microscopic: from influenza-induced changes in bacterial transmission, colonising dynamics, and windows of susceptibility to disease (table 1; figure 1); structural mechanisms of enhanced carriage and invasion; and finally to disruptions in antibacterial inflammatory processes and cellular immune defences (figure 2; tables 2 and 3).
Table 1.
Major characteristics of influenza effect on bacterial co-infections
| Effect (maximum) |
Window (maximum effect) |
Order or timing of infection |
Primary mechanisms | Evidence in human beings? |
|
|---|---|---|---|---|---|
| Carriage density21–29 | Increase (100 000 times) |
4–10 days after influenza |
Influenza infection up to 28 days before, or during, bacterial colonisation |
Increased adherence Reduced mucociliary clearance Excess inflammation Reduced antibacterial innate defences (table 2) |
Yes |
| Carriage duration24,28 | Increase (at least doubled) |
NA | Influenza infection up to 28 days before, or during, bacterial colonisation |
Increased adherence Reduced mucociliary clearance Excess inflammation Reduced antibacterial innate defences (table 2) |
Yes |
| Transmission26,27,29 | Increased (at least 1 m) |
3–7 days after influenza |
Influenza 3–7 days before, or during, active bacterial colonisation |
Increased carriage density Influenza symptoms and aerosolised secretions Shift in carriage from nasopharynx to anterior nares Increased dissemination from biofilms |
No |
| Acquisition26,27,29,30 | Increased (at least 3 m from transmitter) |
3–7 days after influenza |
Influenza before contact with colonised donor |
Increased adherence Reduced mucociliary clearance Excess inflammation Reduced antibacterial innate defences (table 2) |
Yes |
| Otitis media (susceptibility)23,31,32,33 |
Increased (at least three times increased incidence) |
3–7 days after influenza |
Influenza 3–7 days before, or during, bacterial colonisation |
Virus-mediated middle ear epithelial tissue inflammation Ab-mediated NETosis Reduced antibacterial innate defences (table 2) |
Yes |
| Pneumonia and invasive disease (susceptibility)*8,13,28,34,35,36 |
Increased (100 times; possible reduction when bacteria precedes virus) |
Around 7 days after influenza |
Influenza infection 3–14 days before bacterial inoculation |
Increased carriage density Increased dissemination from biofilms Reduced mucociliary clearance Aberant innate immune defences (table 2) Excess immunopathological and tissue damage |
Yes |
NA=not applicable. m=metres. Ab=antibody.
See main text for further references.
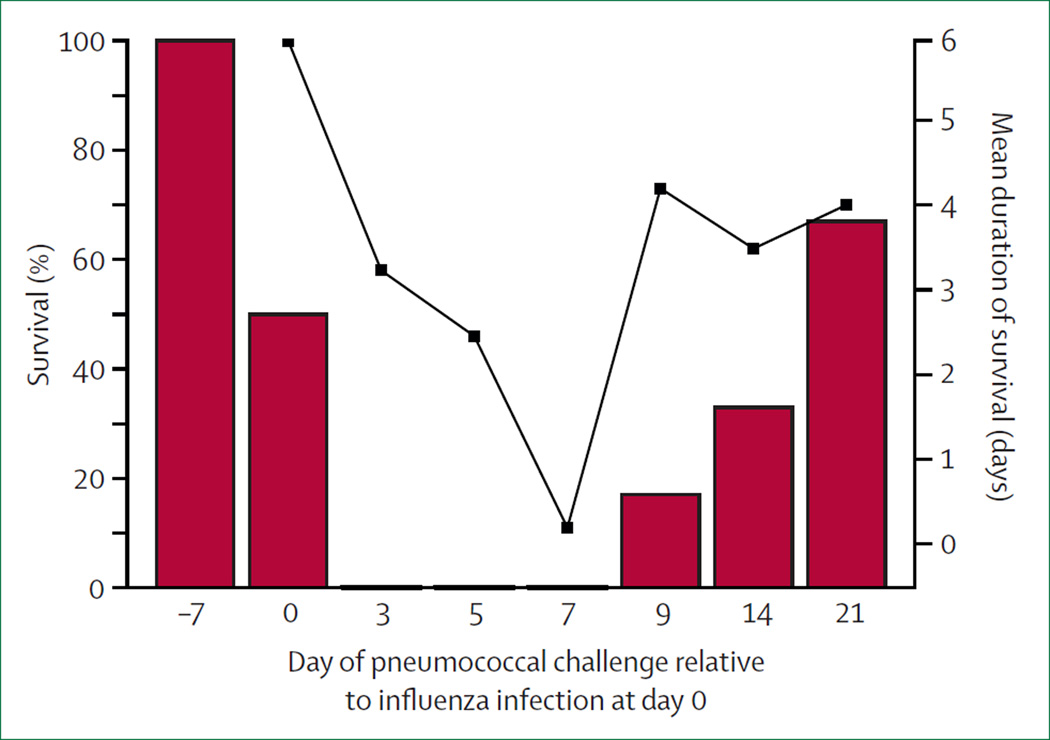
Figure 1. Timing of synergism between influenza and pneumococcal infection.
Groups of mice were challenged with Pneumococcus at different times relative to influenza infection at day zero of infection. Bars=percentage survival after pneumococcal inoculation. Line with black squares=mean duration of survival (only for mice that died). Adapted with permission from McCullers and Rehg.35
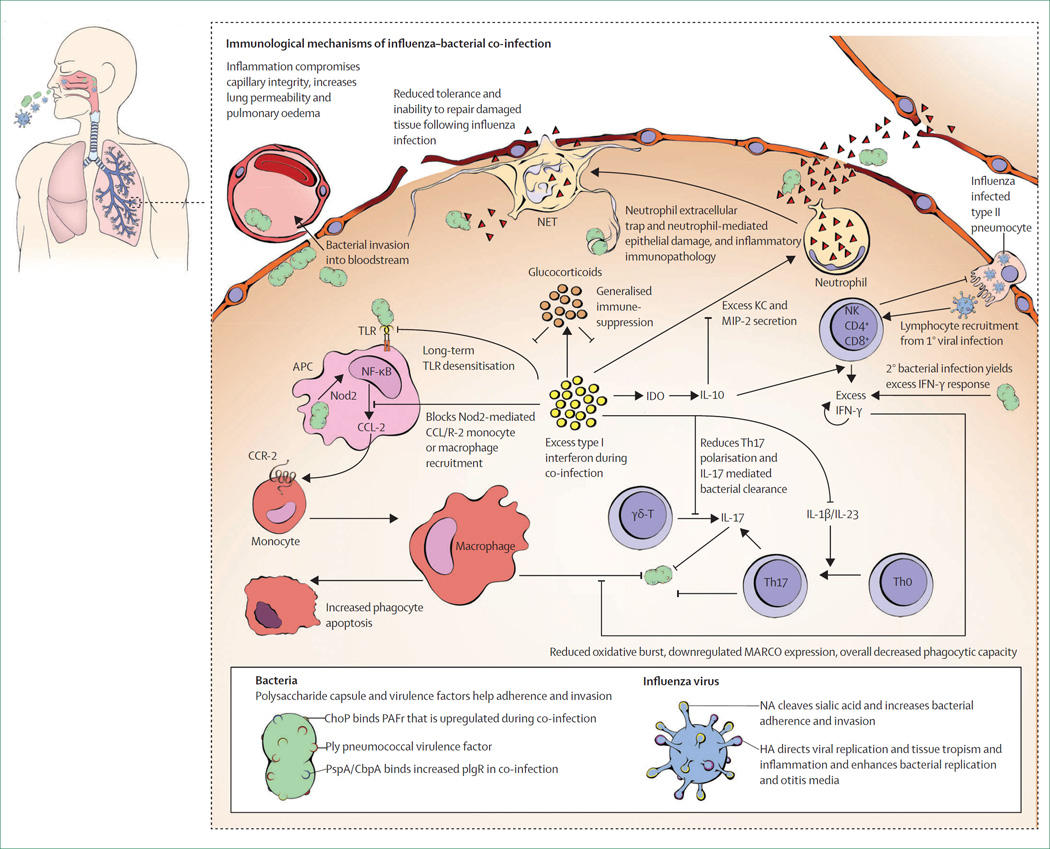
Figure 2. Mechanisms of influenza–bacterial co-infection.
Influenza viruses and respiratory bacteria enter into, infect, or colonise the cells of the upper respiratory tract. Increased bacterial adherence after influenza infection results from an increased number of PAFr and plgR receptors, viral neuraminidase cleavage of sialic acids, and epithelial denudation that exposes basement membrane components; each enables enhanced binding by bacterial adherence factors (eg, ChoP, cbpA, and PspA) with increased bacterial replication and carriage. Viral-induced inflammation enhances expression of bacterial virulence factors (eg, pneumolysin) and increases release of bacteria from biofilms in the upper respiratory tract to a planktonic state, which in turn increases bacterial dissemination to the lower respiratory tract. Primary influenza infection followed by secondary bacterial inoculation yields excess cytokine and chemokine production with numerous downstream consequences, as depicted within a single alveolus and described within the main text of this Review. Excess type I interferon secretion yields overabundant, mixed, immature and mature neutrophil recruitment, which leads to severe immunopathology, particularly because of neutrophil ROS secretion and development of neutrophil extracellular traps, which further increases the inflammatory response. Excess type I and II interferons reduce recruitment of monocytes or macrophages by blunting Nod2 signalling and enhancing anti-inflammatory IL-10 secretion, which could also increase production of type II interferons, with reduced macrophage function and increased apoptosis. Excess inflammation is exacerbated by viral-mediated reduced secretion of amphiregulin and other factors important for tissue regeneration, which adds to the reduced alveolar and endothelial integrity and leads to capillary leakage, pulmonary oedema, and bacterial bloodstream invasion. Cytokine storm or bacterial overgrowth often result in irreparable damage to the lower respiratory tract and alveolar sacs, which results in severe pneumonia, sepsis, and often death. PAFr=platelet-activating-factor receptors. plgR=polymeric immunoglobulin receptors. ChoP=phosphorylcholine. cbpA= choline-binding protein A. PspA=pneumococcal surface protein A. ROS= reactive oxygen species. Nod2=nucleotide-binding, oligomerisation domain-containing protein 2. IL=interleukin. NA=neuraminidase. HA=haemagglutinin. APC=antigen-presenting cell. IFN=interferon. CCR-2=chemokine (C-C motif) receptor 2. CCL-2= monocyte chemotactic protein 1/chemokine ligand 2. NET=neutrophil extracellular traps. MARCO=macrophage receptor with collagenous structure. Th=T helper. KC= keratinocyte chemoattractant CXCL1. MIP-2=macrophage inflammatory protein CXCL2. NK=natural killer cell. IDO=indoleamine 2,3-dioxygenase. TLR=toll-like receptor. Ply=pneumolysin. γδT=γδ T cell.
Table 2.
Mechanisms and mediators of influenza–bacterial interactions
| Main infection vs bacterial co-infection |
Effect during co-infection | Known phenotypic or disease effect |
Mechanism or cytokine effect | Notes | |
|---|---|---|---|---|---|
|
Structural alterations or surface protein expression | |||||
| Epithelial desquamation35,37–39,40 |
Increased | Increases bacterial adherence at sites of desquamation and tissue regeneration |
Increases carriage density, duration, and sinusitis Primes for increased acquisition Increases pneumonia |
Exposes basement membrane components, fibrinogen, hyaline, and other extracellular matrix proteins for bacterial binding |
·· |
| Platelet-activating factor receptor (PAFr)35,41–43 |
Upregulated on activated epithelial and endothelial cells |
Increases bacterial adherence, replication, and invasion Helps excess macrophage or neutrophil recruitment Reduces bacterial lung titres Increases bloodstream invasion |
Increases carriage density and duration Primes for increased acquisition Increases pneumonia Increases bacteraemia Reduces pneumonia Reduces mortality Increases bacteraemia |
Binds phosphorylcholine embedded in bacterial cell walls (ie, ChoP) Enhances TNF-α, IL-6, and KC expression Reduces TNF-α, IL-1β, IL-6, KC, and MIP-1a Helps bacterial traversal of epithelial and endothelial layers to enter bloodstream |
High viral doses Unencapsulated bacteria Infection 7 days after virus Low viral doses Encapsulated bacteria Infection 14 days after virus PAFr not needed for secondary pneumonia but possibly for bacteraemia |
| Polymeric immunoglobulin receptor (pIgR) 44,45,46 |
Upregulated on epithelial cells of mucosal surfaces |
Increases bacterial adherence to epithelial cells Enables transcytosis of epithelial barriers |
Synergistic increase in bacterial carriage density Increases pneumonia and bacteraemia Increased mortality |
Influenza-mediated interferon γ production increases expression of pIgR pIgR binds choline binding proteins on bacterial surface (eg, PspA and cbpA) Binding increases adherence and facilitates epithelial transcytosis |
pIgR facilitiates transcellular transport of IgA and IgM across the epithelial or mucosal layers. Bacteria exploit this function to move across the barriers of mucosal tissue |
| Ciliary beat frequency and coordination47,48 |
Reduced | Reduces bacterial clearance from respiratory tract |
Increases bacterial carriage density Increases pneumonia Increased mortality |
Viral haemagglutinin inhibits Caz+/Na+ channels via phospholipase C and proteinase kinase C activation |
·· |
|
Innate immune cytokines, signalling, and systemic responses | |||||
| Type I interferon (interferon α and interferon β)21,49,50–53 |
Synergistic increase |
Reduces bacterial clearance mediated by monocytes, macrophages, and neutrophils from the nasopharynx and lungs |
Increases bacterial carriage density and duration Enhances bacterial pneumonia and mortality Development of immunopathology |
Excess type I interferon signalling through IFNAR inhibits: Nod2/CCl2 recruitment of monocytes/ macrophage to URT KC and MIP-2 in lungs, needed for neutrophil recruitment Th-17 polarisation and expression of IL-17, IL-22, IL-23, IL-1β, and MCP-1 needed for clearance mediated by Th-17 and macrophages γδT–cell production of IL-17 Induces granulocyte apoptosis in bone marrow |
Excess type I interferon probably shuts down multiple antibacterial innate defences to prevent host-tissue injury Distinct URT and LRT effects show that there are clear anatomical differences in immune mechanisms |
| Type II interferon (interferon γ)34,54 |
Synergistic increase |
Reduces alveolar macrophage phagocytosis Increases pIgR-mediated bacterial adherence (see pIgR above) |
Enhances bacterial lung titres Increased pneumonia mortality Increases bacterial colonisation |
Induced by excess IL-12 Blunts beneficial pro-inflammatory cytokine secretion from macrophages Increases levels of oxidative radicals in macrophages Reduces MARCO expression on surface of AMs required for proper bacterial detection and phagocytosis |
Excess IL-12 mediated by interferon γ could be beneficial during primary bacterial infection55 Excess interferon γ might be downstream of increased type I interferon response (known to increase production of IL-12p70 from dendritic cells56) |
| IL-1254–56 | Increased | Increases type II interferon | Increases bacterial lung titres Increased pneumonia mortality |
Similar to effects with type II interferon | Similar to effects with type II interferon |
| IL-1057,58 | Increased | Inhibits appropriate inflammatory response Inhibits neutrophil recruitment to lungs |
Enhances bacterial lung titres Increased pneumonia mortality |
Possible induction by excess indoleamine 2,3-dioxygenase Inhibition of neutrophil recruitment and activity, which prevents early neutrophil- mediated bacterial clearance |
Could also increase interferon γ58 with similar effects as noted for type II interferon |
| TLR signalling59 | Reduced | Prevents initiation of appropriate cytokine and cellular response pathways for bacterial clearance |
Increased bacterial lung titres and mortality |
Sustained desensitisation of TLR-2, TLR-4, and TLR-5 to bacterial ligands reduces monocyte and macrophage recruitment to lungs Blunts TLR activation of NF-κB in alveolar macrophages (required for expression of KC-mediated and MIP-2-mediated neutrophil recruitment to lungs) Possibly due to increased immunosuppressive activity of alternatively activated macrophages (see AAM below in this table) |
Might last as long as 6 months after influenza infection TLR desensitisation might be crucial to reduce excess immunopathology60 |
| Glucocorticoids61 | Increased during systemic bacterial co-infection |
Overall immune suppression Prevention of bacterial clearance |
Increased systemic bacterial titres Reduce overall mortality from systemic bacterial infections |
Generalised suppression of innate and adaptive immune mechanisms Reduce neutrophil, macrophage, and adaptive responses for bacterial clearance Reduce excess inflammation and prevent immunopathological reactions |
Substantial immunopathological role as a main cause of death during post-influenza bacterial secondary infections |
|
Cellular innate immunity | |||||
| Alveolar macrophage | Reduced activity |
Inefficient bacterial phagocytosis |
Increased bacterial lung titres Increased pneumonia mortality |
Excess interferon γ production reduces MARCO expression on AM macrophage cell surface that is needed for clearance of many lung pathogens |
Similar effects to type II interferon, and alveolar macrophage apoptosis |
| Alternatively activated macrophage (AAM)62 |
Increased | Abrogates proper bacterial clearance by classic activated macrophages |
Increases bacterial lung titres Increases pneumonia mortality |
AAMs produce arginase-1, which competes with bactericidal effects of iNOS produced by classic-activated macrophages Might inhibit TLR-signalling or increase CD200-CD200R ligation |
Excess AAM could last for weeks in the lungs after influenza infection, which is important for tissue remodelling, homoeostasis, and injury repair |
| Reduced neutrophils31,35,41,49,53,54,57,59,63,64 |
Reduced | Increased bacterial replication because of reduced neutrophil function |
Increased bacterial lung titres Increased pneumonia mortality Excess immunopathological reactions |
Excess type I interferon reduces KC and MIP-2 expression and neutrophil recruitment |
When secondary infection occurs <4 days after viral infection |
| Increased neutrophils31,35,41,49,53,54,57,59,63,64 |
Increased | Excess neutrophils lead to inflammation and immunopathology |
Increased bacterial lung titres Increased pneumonia mortality Excess immunopathology |
Excess IL-10 expression reduces neutrophil bactericidal function (ROS generation) Retains immunopathological responses Excess KC and MIP-2 secretion recruit mixed pool of mature and immature neutrophils |
When secondary infection occurs >4 days after viral infection |
| Neutrophil extracellular traps (NETs or NETosis)33,65 |
Increased | Reduced bacterial clearance and increased immunopathology |
Increased bacterial acute otitis media Increased bacterial pneumonia |
Bacterial Abs increase production of NETs Bacterial endonucleases degrade NETs NET degradation induce endothelial damage, sepsis, small vessel vasculitis, and alveolar capillary damage Excess immunopathological reactions |
Direct injection of DNAse into middle ear to decrease NETosis reduced bacterial replication, which suggests therapeutic potential |
| Alveolar macrophage apoptosis66,67 |
Increased | Reduces alveolar macrophage-mediated clearance and increases immunopathological reactions |
Increased bacterial pneumonia Increased immunopathological reactions |
Increased AM FADD expression increases caspase-3 and caspase-8 >90% AM apoptosis Increases severe lung inflammation and damage |
·· |
|
Systemic mechanisms | |||||
| Tolerance to tissue damage68,69 |
Reduced | No effect on bacterial titres or infection |
Increases severe disease and mortality |
Substantial loss of epithelial tissue regeneration Reduced ability to cope with severe tissue damage during secondary infection |
Particularly important for systemic bacterial infections 3–6 days after influenza infection |
| Hyperthermia and stress response70,71 |
Increased | Increases bacterial invasion and dissemination to lungs |
Increases bacterial carriage density Increased acute otitis media Increased pneumonia Increased bacteraemia |
Hyperthermia increases expression of bacterial virulence genes Increases bacterial dissemination from biofilms Stress response increases host glucose and ATP production (helps bacterial replication and invasion) |
·· |
ChoP=phosphorylcholine. TNF-α=tumour necrosis factor-α. IL=interleukin. KC=keratinocyte chemoattractant CXCL1. MIP=macrophage inflammatory protein CXCL2. PspA=pneumococcal surface protein A. cbpA=choline-binding protein A. IFNAR=type I interferon receptor. Nod2=nucleotide-binding, oligomerisation domain-containing protein 2. CC=chemokine. URT=upper respiratory tract. AM=alveolar macrophage. Th=T helper. MCP-1=monocyte chemoattractant protein I. TLR=toll-like receptor. LRT=lower respiratory tract. MARCO=macrophage receptor with collagenous structure. iNOS=inducible nitric oxide synthase. Abs=antibodies. FADD=fas-associated protein with death domain. ROS=reactive oxygen species.
Table 3.
Respiratory bacterial and influenza virus components that support secondary bacterial infections
| Effect | Mechanism | |
|---|---|---|
| Bacterial phosphorylcholine (ChoP*) | Increases bacterial adherence and replication Enhances bacterial invasion |
Binds host epithelial PAFr, which is upregulated during influenza infection (see table 2) |
| Bacterial surface proteins and choline binding proteins (PspA, cbpA*) |
Increases bacterial adherence and replication Enhances bacterial invasion |
Binds host pIgR, which is upregulated during influenza infection (see table 2) Helps bacterial transcytosis of epithelial and endothelial barriers to support bloodstream invasion |
| Influenza neuraminidase72,73 | Increases bacterial adherence and replication Increased bacterial pneumonia and mortality |
Cleaves sialic acids on epithelial cells: Exposes cryptic receptors for bacterial adherence Allows increased range and diffusion of bacterial adherence |
| Influenza haemagglutinin32,73,74 | Directs viral replication, and controls tissue-tropism and inflammation Increases bacterial replication and acute otitis media |
Influenza tropism for epithelial tissue is greater for H3 than H1 viruses Induces excess inflammation and primes cells for increased bacterial growth |
| Pro-apoptotic influenza A protein (PB1-F2)75,76 |
Substantial host-tissue damage and immunopathological reactions Increased activity increases bacterial replication, lung infections, pneumonia, and mortality |
Increased PB1-F2 activity in turn increases influenza virulence and induces inflammation Enhances aberrant innate immune responses (see table 2) allowing for bacterial replication and disease Pulmonary immunopathological reactions |
PAFr=Platelet- activating factor receptor. PspA=pneumococcal surface protein A. cbpA=choline-binding protein A. pIgR=polymeric immunoglobulin receptor.
See reference in table 2.
Influenza and bacterial dynamics
Colonisation and carriage
Bacterial carriage, although largely asymptomatic, is often considered a prerequisite for invasive disease, priming for tissue invasion or dissemination into the lower airways.44 Importantly, new acquisition events and increased carriage density are risk factors for invasion, thus influenza-mediated increases in these processes might be detrimental at both individual and population (ie, via transmission) levels.
Infection with influenza virus has potent effects on both the density and duration of bacterial carriage.21–25 In children, influenza is associated with an average 15-times increase in pneumococcal nasopharyngeal titre,25 an effect that has been corroborated in numerous animal studies.21,24,26–28 Bacterial density in the nasopharynx after influenza infection can be increased as much as 100 000 times compared with influenza-naive hosts, usually after attainment of peak viral titres, noted by a 3–4 day lag when influenza inoculation occurs in hosts that are already colonised.28 The duration of carriage is also significantly extended two times to five times. Although effects can last for weeks after influenza infection, the magnitude of the effect diminishes with time after the first 7–10 days after viral inoculation.28
Transmission and acquisition
Bacterial transmission is increased by influenza infections, and driven by increased carriage density in the transmitters and viral-priming for enhanced susceptibility to acquisition in recipients.26,27,29 Although increased density could alone be sufficient to enhance transmission,27 influenza-priming for acquisition is a particularly potent conductor of increased transmission, driving both the frequency and radius over which bacteria are transmitted.26 In a recent study in Peruvian children,30 influenza infection was associated with 2·2-times increased odds of pneumococcal acquisition relative to influenza-uninfected children.
Windows of susceptibility to disease
Infections with respiratory bacterial pathogens often begin as asymptomatic infections designated as carriage.44 Within a healthy host, bacterial replication and migration are maintained at subclinical levels through combinations of host epithelial and mucosal defences and innate and adaptive immune processes. Although almost all cases resolve with few if any clinical symptoms,44 occasionally bacteria replicate and disseminate to or invade surrounding tissue, causing a range of diseases such as sinusitis, otitis media, pneumonia, bacteraemia, sepsis, and meningitis. Although progression from carriage to disease remains a topic of investigation, it is clear that infection with an influenza virus could undermine normal immunological processes and provoke disease.
Bacterial otitis media
Influenza–bacterial co-infection in the upper respiratory tract is clinically realised as excess bacterial otitis media. The window of increased otitis media is coincident with peak viral titres or follows for up to 3–6 days thereafter—a pattern akin to that of excess colonising density.23,31 Although it is tempting to assume that excess otitis media is a product of bacterial spillover from increased carriage, it follows local influenza-mediated inflammation in the epithelial tissue of the middle ear, particularly by type 3 haemagglutinin-encoding influenza viruses with particular tropism for this tissue.23,32
Bacterial pneumonia and invasive disease
The window of heightened susceptibility to bacterial invasive disease and pneumonia is most pronounced within the first 7 days after influenza infection, particularly between days 3 and 7, during which peak bacterial lung and blood titres are at their highest and time-to-death might be less than 1 day (figure 1).34,35,72 Although the magnitude of the response (ie, morbidity and mortality) is dependent on experimental conditions, the trends displayed in figure 1 are generalisable across nearly every laboratory and epidemiological investigation describing lethal influenza–bacterial co-infections.13,34,36,66 Subtle effects of viral infection on bacterial invasion and replication might be retained for weeks, with one study suggesting that effects can last as long as 6 months.24,57,59 Of note, the window of susceptibility to lethal infection is distinct from that in the upper respiratory tract, because bacterial infection before influenza infection might reduce morbidity and improve survival from lethal influenza infections.35
Mechanisms
Desquamation and non-specific bacterial adherence
Excess bacterial acquisition and carriage could follow influenza-mediated bacterial adherence. As early as 1949, pathologists noted virus-mediated patches of desquamated epithelium where bacteria adhered and invaded with increased vigour.41 As noted by Hers in 196139 and Parker in 1963,38 influenza desquamation exposes basement membrane components ideal for bacterial attachment. Furthermore, epithelial regeneration yields excess hyalinisation and production of fibrinogen, fibronectin, and other matrix elements to which bacteria could bind.35,37,40,77 Similar effects have been described for non-influenza viruses that induce similar patterns of desquamation, which include respiratory syncytial virus, human parainfluenza virus type 3, and paramyxovirus.22
Specific bacterial adherence
Platelet-activating factor receptor and phosphorylcholine
Specific adhesion molecules expressed in excess during influenza-mediated inflammation, or exposed via alterations of epithelial surface proteins, also help bacterial adherence and invasion. After viral infection, epithelial and endothelial cells increase expression of the G-protein-coupled platelet-activating factor receptor (PAFr), which is able to bind phosphorylcholine (ChoP) embedded in the cell wall of many respiratory bacterial pathogens. PAFr-ChoP ligation helps pathogen docking to epithelial tissue and increases bacterial lung titres, bacteraemia, and mortality.35 Blockade of PAFr blunts the pro-inflammatory cytokine and cellular responses that are often associated with severe secondary infections.35,41,42 Although PAFr is not alone sufficient to account for the excess disease that occurs during secondary infections, it might be particularly important for bacterial bloodstream invasion, facilitating transmigration across the respiratory epithelial and endothelial layers.41–43
Pneumococcal surface protein A and polymeric immunoglobulin receptors
The pneumococcal surface protein A (PspA) is a choline-binding adhesion molecule that targets epithelial cell polymeric immunoglobulin receptors (pIgR), which are important for epithelial transcytosis of mucosal antibodies and excretion of antigen across mucosal surfaces. During primary pneumococcal infections, pIgR-PspA binding (and binding by other choline binding proteins such as choline-binding protein A [cbpA]) enables efficient pneumococcal adherence to host epithelial tissue. During influenza infection, upregulation of pIgR (potentially mediated by interferon γ) enhances bacterial adherence and, because of pIgR’s role in facilitation of epithelial transcytosis, provides a distinct pathway for bacterial migration across the epithelial barriers.44,45,46
Influenza neuraminidase and sialic acids
Influenza neuraminidase might also increase bacterial adherence to epithelial tissues. Influenza neuraminidase cleaves sialic acid glycoconjugates on the epithelial cell surface and exposes greater numbers of cryptic receptors than bacterial neuraminidase can itself reveal, enabling bacterial adherence over a larger and more diffuse area of the epithelium than during single infection.72,78 Higher influenza neuraminidase activity has been associated with increased severity of secondary bacterial infections, and field data from the H3N2 pandemic of 1957, which was caused by a virus with uniquely potent influenza NA activity, showed substantially elevated rates of bacterial carriage and disease.73,79 Experimental treatment with the viral influenza neuraminidase inhibitor oseltamivir before influenza-bacterial co-infection improves survival—interestingly, however, not by reducing viral titres, but rather by reducing the number of lung bacteria.72
Influenza and mucociliary clearance
Increased bacterial carriage and dissemination might result, in part, from reduced mucociliary clearance mechanisms. Infection with influenza virus reduces ciliary beat frequency on respiratory epithelial cells for up to 28 days after infection, particularly at day 7—the time of greatest susceptibility to secondary infection. Reduced or uncoordinated ciliary beating decreases clearance velocity and allows increased bacterial density within hours of inoculation.47,77 One hypothesis posits that regenerated intact epithelium might follow influenza HA-mediated inhibition of calcium and sodium channels, which suggests that treatment with β agonists could improve outcomes. However, influenza has also been shown to downregulate β-receptor function, potentially limiting such benefits.48
Influenza and antibacterial innate immunity
Fisher and Ginsberg in 195680 and Walsh and colleagues in 195781 noted prominent reductions in leucocyte recruitment after influenza infection in guineapigs and in human beings, respectively, and Sellers and colleagues82 showed viral attenuation of lymphocytes as a cause of increased bacterial infections after influenza. These early investigations introduced the idea that secondary bacterial infections do not result from physical alterations in epithelial tissues alone, but arise from a complex system of aberrant and unstable immunological signalling cascades. Indeed, the past two decades have shown, overwhelmingly, a primary role for dysregulated innate and adaptive antibacterial immunity after viral infection. Although numerous individual cytokines, chemokines, and cell-mediated responses have been investigated, a number of key immunological processes might explain the myriad studies now represented in the literature (figure 2).
Type I interferons
Type I interferons include many interferon α proteins and one interferon β protein that signals through the common type I interferon receptor to elicit expression of several cytokines that are essential for interference with viral replication.21 Although type I interferons are traditionally associated with innate antiviral responses and polarisation of adaptive immunity, they are complicit as key mediators of post-influenza bacterial infections. In the absence of influenza, pneumococcal clearance from the nasopharynx is mediated by nucleotide-binding, oligomerisation domain-containing protein 2 (Nod2) recognition of pneumococcal peptidoglycans, which activates nuclear factor (NF)-κB to promote monocyte chemotactic protein 1/chemokine ligand 2 (CCL2)-chemokine (C-C motif) receptor 2 (CCR2 )ligation. Ligation aids antibacterial monocyte or macrophage recruitment and secretion of type I interferon, which requires the expression of the pneumococcal pore-forming toxin pneumolysin, presumably to allow Nod2 access to microbial ligands.21 Sequential influenza– bacterial infection induces excess type I interferon that reduces recruitment of monocytes and macrophages (but not neutrophils) to the upper respiratory tract (required for control of pneumococcal carriage) via blockade of Nod2-mediated expression of CCl2.21 Interestingly, although mediated by blockade of Nod2 signalling, deletion of Nod2 or pneumococcal pneumolysin (required for Nod2 detection of pneumococci) abrogates excess interferon production and returns carriage to normal levels. This suggests that accumulation of type I interferon above a specific threshold could shut down several antibacterial immune pathways, prioritising prevention of immunopathological responses and bystander tissue damage over immediate control of bacterial proliferation.
Contrasting with the processes in the upper respiratory tract, excess production of interferon α during co-infection might enhance bacterial pneumonia by inhibiting production of pulmonary keratinocyte cell-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2—potent neutrophil chemotactic signals) in the lower respiratory tract that are necessary for efficient bacterial clearance from the lungs.49,50 The distinct effects of excess type 1 interferon production in the nasopharyngeal versus lung tissue highlight the complexity and tissue-specific heterogeneity of immune processes within a single host.83
Excess secretion of type I interferon could also reduce bacterial clearance by inhibiting γδ-T-cell secretion of interleukin 17, which is crucial for efficient bacterial clearance.50,51 Furthermore, during S aureus co-infection, type I interferon reduces NF-κB-mediated production of interleukin 1β and interleukin 23, which are essential for proper T-helper type 17 (Th17) cell polarisation. Subsequent reductions in interleukin 17, interleukin 22, and monocyte chemoattractant protein-1 (MCP-1) reduce recruitment of monocytes and macrophages and clearance of S aureus.51,52 Supporting this mechanism, patients with hyper IgE syndrome who present with S aureus pneumonia often have mutations in signal transducer and activator of transcription 3 (STAT3), important for Th17 cell polarisation.52
Type II interferons
The type II interferon, interferon γ, is mainly produced by natural killer, CD4+ T helper, and CD8+ cytotoxic T lymphocytes. In co-infection, excess interferon concentrations peak when bacterial inoculation follows 7 days after influenza infection.34,54,57,58,84 Monocytes grown in vitro or in vivo in the presence of interferon γ, or during influenza-bacterial co-infection, display reduced phagocytosis associated with depressed pro-inflammatory cytokine secretion, increased concentrations of oxidative radicals, and, in alveolar macrophages, reduced expression of the scavenger receptor MARCO, which is important for efficient clearance of invaders from the lower airways.34 Although interleukin-12 production is beneficial in the regulation of inflammation, Th1 polarisation, and development of sterilising immunity against influenza virus, excess interleukin-12 production increases interferon γ concentrations, which might enhance susceptibility to pulmonary bacterial outgrowth and invasion. During primary bacterial infection, however, interleukin-12 induction of interferon γ helps pneumococcal clearance, which highlights the delicate balance of many immunological processes.55
Excess production of interferon γ during secondary infection might indirectly result from increased expression of interleukin 10, possibly a consequence of influenza induction of indoleamine 2,3-dioxygenase (IDO).58 Inhibition of interleukin-10 signalling substantially reduces secretion of interferon γ and improves bacterial lung clearance and survival.57,58 However, during primary bacterial infection, increased interleukin-10 secretion assumes its classic anti-inflammatory role and dampens the interferon γ response, antagonising bacterial clearance from the lungs.85 It is interesting to note that an exaggerated type II interferon response during secondary bacterial infection might also be downstream of an overzealous type I interferon response. Indeed, secretion of type I interferon during influenza virus infection increases interleukin 12p70, a major inducer of both interferon γ and Th1 polarisation.56
Toll-like receptor signalling
Toll-like receptors (TLRs) are pattern-recognition receptors that exist on and within several mucosal sentinel cells, and constitute an important family of sensors for detection of pathogens via their pathogen-associated molecular patterns (PAMPs). TLR-PAMP ligation initiates TLR signalling that is crucial for induction of innate immune cascades that cause cytokine or chemokine secretion and cellular recruitment for pathogen clearance.86
Common dogma posits that after infection, immune memory is relegated to the adaptive immune arm, while innate immunity returns to baseline within an appropriately short duration—often following a period of tightly regulated and dampened innate immunity, a period exploited during co-infection.86 Recent evidence, however, suggests that influenza infections yield sustained desensitisation of TLR to bacterial ligands that might increase susceptibility to bacterial infections for at least 6 weeks, but possibly as long as 6 months.53,59 Although not fully understood, TLR desensitisation, and the neutropenic states induced, might result from increased numbers of recruited alternatively activated macrophage cells that aid tissue regeneration and immune homoeostasis, but also have potent immunosuppressive effects.62
TLR signalling in excess might also enable bacterial proliferation secondary to overly abundant cytokine and neutrophil responses.60 Generally, a powerful and swift response to co-infection could reduce bacterial load and improve survival by preventing the need for a more aggressive, and often immunopathological response to control co-infection.87 If, however, inflammation is insufficient for swift bacterial clearance, then the host might fare better to prioritise immune regulation over pathogen control while handing over the task of bacterial clearance to the adaptive immune response.
Influenza-induced glucocorticoids
Contrary to co-infection with more common respiratory bacterial pathogens, reduced bacterial clearance of post-influenza Listeria monocytogenes infection results from generalised systemic suppression of innate and adaptive immune processes after induction of systemic glucocorticoid secretion—known for their pleiotropic immunosuppressive effects.61 Interestingly, although glucocorticoids suppress antibacterial innate immunity, sustained secretion could ultimately benefit the host through reduced immunopathological responses.61
Neutrophils
The role of neutrophils might be either beneficial or detrimental during post-influenza pneumococcal infection. Many studies have shown either reduced41,49,53,54,59 or increased35,57,63,64 neutrophil recruitment during such infections. When pneumococcal infection occurs within 3 days of influenza inoculation, neutrophils help bacterial lung clearance and survival, whereas co-infection 6–10 days after influenza yields overly abundant but inefficient responses that are unable to control bacterial infection.54 At these later timepoints, bactericidal function is compromised, possibly because of increased interleukin-10 secretion57 or excess chemotaxis that recruits a mixed pool of both mature and immature neutrophils—the immature ones unable to elicit appropriate antibacterial defences.63 In support of this theory, excess intact pneumococci can be detected localised to neutrophil infiltrate in the middle ear during post-influenza pneumococcal otitis media.31 Conversely, reduced neutrophil recruitment during secondary infection could be coincident with reduced KC and MIP-2 expression, perhaps following, as mentioned previously, an excessive type I interferon response or blunted TLR signalling in the post-influenza state.49,53,54,59,
Neutrophil extracellular traps
Neutrophil extracellular traps (NETs) were initially identified for their unique extracellular bactericidal activity whereby neutrophils undergo a form of cell death, described as NETosis, releasing chromatin bound to neutrophil granules and cytoplasmic proteins into the immediate extracellular space, forming net-like structures able to trap and kill bacteria.65 Pneumococcal co-infection increases both NET formation and degradation. NET formation might be an antibody-dependent process, as shown during secondary bacterial otitis media;33 NET degradation is mediated by bacterial endonucleases that enable bacterial release from NET entanglement.65 Although NETosis is beneficial to control bacterial infection during uncomplicated infections, during co-infection it drives epithelial and endothelial damage that increases lung and middle ear inflammation, sepsis, and small vessel vasculitis, ultimately harming alveolar capillary surfaces in the lungs.
Immune-cell apoptosis
Although leucopenia during secondary infection might result from reduced chemokine-mediated recruitment, it also results from increased apoptosis, through which more than 90% of alveolar macrophages could be lost for up to 14 days after influenza infection, noted by alveolar macrophage expression of fas-associated protein with death domain (FADD), an activator of caspase-8 and caspase-3 after secondary, as opposed to primary, bacterial infection.66,67 Findings from a non-influenza model of lymphocytic choriomeningitis virus (LCMV)– bacterial co-infection showed reduced neutrophil recruitment and increased secondary bacterial infections, by many bacterial species, after bone marrow granulocyte apoptosis.53
Resistance versus tolerance to tissue damage
Understanding of the consequences of pathogen infection traditionally places emphasis on pathogen virulence and host resistance to the pathogen. However, even when resistance to a secondary non-virulent invader is fully maintained, inadequate tolerance to host tissue damage could still result in disease progression.68 When bacterial infection with Legionella pneumophila is properly controlled and bacterial virulence attenuated, and the host made entirely incapable of mounting a cytokine storm, excess death from secondary infection versus single infection remains. Interestingly, the effect is shortlived, occurring only when bacterial infection is between 3 and 6 days after influenza. Afterwards no excess mortality is noted—presumably resulting from sufficient tissue repair before bacterial infection.68 Similarly, secondary pneumococcal infections after pandemic H1N1 infection are mediated, at least in part, by loss of epithelial cell reproliferation and tissue repair mechanisms.69
Influenza-induced hyperthermia, stress, and bacterial dissemination
Bacterial colonisation and biofilm formation are helped by stringent downregulation of virulence factors that enable immune evasion by reduced induction of epithelial pro-inflammatory cytokine responses.70 During an influenza infection, however, hyperthermia from pyrogenic cytokines induces expression of bacterial virulence genes and increases release of bacteria from the biofilms that colonise the nasopharynx, permitting microaspiration and invasion. Furthermore, influenza increases concentrations of glucose, ATP, and noradrenaline, which, although important for lymphocyte activation and viral clearance, induce excess bacterial carriage, pneumonia, and otitis media in otherwise stably colonised mice.70,71
Influenza genotype influences bacterial co-infection
Haemagglutinin and neuraminidase
The influenza genotype might have profound effects on the mechanisms and phenotypes of bacterial co-infection. Results from epidemiological74 and animal studies32,73 suggest that H3N2 viruses are more potent inducers of pneumococcal disease than H1N1 viruses, a finding that could be mediated by increased H3 tropism for human epithelial cells. Indeed, inflammation after H3 versus H1 viruses is associated with elevated bacterial titres in the middle ear during otitis media.32 Similarly, viruses with increased neuraminidase activity show an increased capacity to support secondary bacterial pneumonia.72,73 Furthermore, whether an influenza virus is particularly pathogenic in a given host, or increases susceptibility to bacterial infection, could be affected by whether the initial influenza viruses that an individual was exposed to early in life contained homosubtypic versus hetero-subtypic internal (eg, nucleoprotein) or external (ie, haemagglutinin and neuraminidase) proteins to the virus of interest—heterosubtypic proteins would result in enhanced susceptibility to disease.6
PB1-F2
PB1-F2 is a pro-apoptotic influenza A protein that contributes substantially to the virulence and pathogenicity of influenza viruses, and affects the extent of the inflammation driven by the viral infection.75 Viruses with increased PB1-F2 virulence, which was elevated in the 1918 influenza virus, result in particularly severe secondary infections.76 Interestingly, viruses carrying truncated PB1-F2 proteins with very low virulence predispose to only mild bacterial disease despite similarly elevated bacterial titres, reinforcing the important role of host tissue damage and tolerance during co-infection.76
Prevention and treatment strategies
Detection
Central to the prevention of influenza–bacterial co-infections is an understanding of the bacterial species that are important in these processes. Although most work in this field has, quite rightly, focused on culturable pathogens, recent advances in 16S ribosomal RNA (rRNA) sequencing has enhanced our ability to detect changes in most of the microbial flora of the respiratory tract that is non-culturable. The use of 16S sequencing in clinical settings will provide a more complete picture of the effects of influenza, and other respiratory viruses, on commensal and pathogenic bacteria. However, as detection of such broad arrays of microbial species increases through these newer technologies, care should be taken to not bias clinical acumen or assume the causes, because the sensitivity of these technologies will detect alterations in bacterial species existing in sufficiently low densities so as to be unlikely to cause disease.88
Furthermore, it should be noted here that detection should include both agonistic as well as antagonistic relationships between bacterial species and influenza viruses. Indeed, elucidation of antagonistic interactions that exist between influenza viruses and bacteria, as have been recently shown for the atypical bacteria Mycoplasma pneumoniae and Chlamydophila pneumoniae,89 could prove useful in the discovery of mechanisms or development of improved therapeutics to combat co-infections.
Vaccines
Influenza and pneumococcal infections are both largely preventable through vaccination. Vaccination is the best strategy for prevention of secondary bacterial infections. As discussed previously, the PCV9 vaccine prevented 41% of influenza virus-associated pneumonias,9 and PCV has also been shown to reduce influenza hospitalisations by 48% in young children.90 Influenza vaccination might also prevent secondary bacterial infection by abrogating the primary viral infection.24 In the immediate post-influenza state, early vaccination with a live, attenuated influenza virus (LAIV) has been shown to be superior to PCV to prevent excess pneumococcal carriage,24 although both vaccines reduce mortality.
Although influenza vaccination is undoubtedly beneficial to reduce influenza and secondary bacterial infections, we recently reported28 an unexpected effect of LAIV (but not inactivated influenza vaccine) on increased bacterial carriage for up to 28 days after vaccination. Importantly, however, LAIV had no detrimental effect on bacterial infections of the lower respiratory tract.28 Thus, although LAIV is beneficial to reduce influenza and influenza-mediated secondary disease, it might be important to consider the level of risk of bacterial acquisition during the weeks after vaccination when deciding between LAIV and inactivated vaccines.
Antibiotics and antivirals
Although the Spanish influenza virus resulted in an extraordinary pandemic unlike any other, the 1918 pandemic occurred long before the discovery of antibiotics in the 1930s. Had antibiotics been available during the Spanish influenza pandemic, mortality rates might have been reduced by as much as 50%.91 Antibiotics such as linezolid or some macrolides that have immunomodulatory properties could be par ticularly beneficial in the setting of co-infection.92,93 Quinolones might also be of use, because they not only have immunoregulatory properties, but also could increase the synthesis of colony-stimulating factor (ie, GM-CSF), which has been shown to improve lung tissue repair through induction of amphiregulin during co-infection.94,95
In the context of secondary bacterial infections, however, not all antibiotics are necessarily beneficial, and some might inadvertently increase disease. β-lactams are first-line antibiotics for the treatment of bacterial lung infections and vancomycin is recommended for influenza– staphylococcal co-infections. However, in the highly unstable inflammatory environment of the co-infected lung, bacterial lysis by bactericidal antibiotics might have the adverse effect of enhancing the inflammatory processes through release of high concentrations of bacterial PAMPs and excessive TLR stimulation.96 In view of this potential consequence, bacteriostatic protein-synthesis inhibitors such as clindamycin and azithromycin might be better suited to improve survival, particularly azithromycin for its known immuno modulatory effects.96 β-lactams in combination with macrolide treatment have been shown to be effective for treatment of complicated community-acquired pneumonia.97
Antibiotics in combination with synthetic corticosteroids could also improve survival, particularly during severe infections.98 Other less conventional antibiotic approaches have been investigated. For example, purified bacteriophage cell wall hydrolases or lysins are useful for eradication of nasal carriage, and possibly treatment of otitis media that has resulted from Gram-positive bacteria.99
Antiviral agents too have been considered for prevention of secondary infections. In particular, treatment with neuraminidase inhibitors abrogates excess bacterial carriage and reduces mortality from co-infections, even when treatment is initiated as late as 5 days after influenza infection.100
Conclusions
In a time of unprecedented opportunity for influenza reassortment and global transmission, increasing resistance to antibiotics, and exponential growth in data to understand the interactions between host and pathogens, the importance of and capacity to gain a firm grasp on the mechanisms underlying influenza and bacterial co-infections has never been greater. Nearly a century ago, the 1918 influenza pandemic showed the devastation that can be wreaked by a perfect storm of influenza virus genotypes, previous influenza exposures, and bacterial pathogens. Improvement and development of new vaccines will be integral towards the first lines of defence. However, with ever-changing viral and bacterial genomes and shifting distributions of bacterial subtypes, vaccines could be far from a foolproof plan. In-depth under standing of the mechanisms underlying post-influenza bacterial infection is crucial for development of improved therapeutics to care for the combined infections that remain difficult, and in some cases impossible, to treat. It is clear that a primary cause of severe disease and death during post-influenza bacterial infection is an overzealous and uncoordinated immune response, coincident with an inability to balance pathogen clearance with prevention of host-tissue damage. Thus, development of immunomodulatory therapies might prove to be more beneficial than conventional antimicrobial agents to treat complicated co-infections. Increased understanding of the use of combination therapies of antimicrobials and immunomodulatory agents will be important for improvement of treatment outcomes and prevention of excess mortality during future influenza seasons and global pandemics.
Key messages.
Influenza virus infection predisposes patients to complicated and difficult-to-treat bacterial secondary infections or co-infections
Bacterial co-infections are a major cause of mortality during influenza epidemics and pandemics, implicated in 30–90% of fatal influenza cases
Excess disease begins with viral-mediated increases in bacterial carriage density and duration of colonisation, which might also increase bacterial transmission
Aberrant immunological processes during co-infection cause reduced antibacterial immune defences, but death often follows the cytokine storm and the immunemediated pathology
Future treatment regimens should focus on antibiotic therapy in combination with potent anti-inflammatory and immunomodulatory agents
Search strategy and selection criteria.
We identified references for this Review through searches of PubMed and Google Scholar for English-language articles including in their titles or abstracts the terms “influenza” with any of the following terms: “bacteria”, “coinfection”, “secondary infection”, “1918”, “synergy”, “Streptococcus pneumoniae”, “pneumococcus”, “Staphylococcus aureus”, “Haemophilus”, “mycoplasma”, “tuberculosis”, or “atypical bacteria.” More citations were identified from references in these initial reports. References for the epidemiology of pneumonia and respiratory infection were identified by searching Google scholar for the terms “global”,”epidemiology”, and “burden”, plus one of the following terms: “pneumonia”, “bacteria”, “respiratory tract infections”, “otitis media”, or “antibiotic therapy.”
Acknowledgments
We thank Ms Sarah Storrer for her substantial assistance in developing the artwork for this manuscript.
Footnotes
Contributors
MJM and KPK contributed equally to the conception of this Review. The manuscript was written by MJM and edited by MJM and KPK. MJM illustrated and contributed figure 2.
Declaration of interests
We declare no competing interests.
References
- 1.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 3.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. [accessed Feb 4, 2014];Influenza (seasonal) 2009 http://www.who.int/mediacentre/factsheets/fs211/en/index.html.
- 6.Worobey M, Han GZ, Rambaut A. Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1324197111. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfizer. [accessed April 2, 2014];Detailed results from landmark Community-Acquired Pneumonia immunization Trial In Adults (CAPiTA) evaluating efficacy of Prevenar 13. 2014 http://www.pfizer.com/news/press-release/press-release-detail/pfizer_presents_detailed_results_from_landmark_community_acquired_pneumonia_immunization_trial_in_adults_capita_evaluating_efficacy_of_prevenar_13.
- 11.Centers for Disease Prevention. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR. 2009;58:1071–1074. [PubMed] [Google Scholar]
- 12.Hers JF, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 13.Chien YW, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med. 2009;361:2582–2583. doi: 10.1056/NEJMc0908216. [DOI] [PubMed] [Google Scholar]
- 14.Shope RE. Swine influenza: III. Filtration experiments and etiology. J Exp Med. 1931;54:373–385. doi: 10.1084/jem.54.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olitsky PK, Gates FL. Experimental studies of the nasopharyngeal secretions from influenza patients: III. Studies of the concurrent infections. J Exp Med. 1921;33:373–383. doi: 10.1084/jem.33.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brightman IJ. Streptococcus infection occurring in ferrets inoculated with human influenza virus. Yale J Biol Med. 1935;8:127–135. [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab JL, Blubaugh FC, Woolpert OC. The response of mice to the intranasal inoculation of mixtures of Streptococcus hemolyticus and influenza virus. J Bacteriol. 1941;41:59–60. [Google Scholar]
- 18.Wilson HE, Saslaw S, Doan CA, Woolpert OC, Schwab JL. Reaction of monkeys to experimental mixed influenza and Streptococcus infections: an analysis of the relative roles of humoral and cellular immunity, with the description of an intercurrent nephritic syndrome. J Exp Med. 1946;85:199–215. doi: 10.1084/jem.85.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Short KR, Habets MN, Hermans PW, Diavatopoulos DA. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol. 2012;7:609–624. doi: 10.2217/fmb.12.29. [DOI] [PubMed] [Google Scholar]
- 20.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avadhanula V, Rodriguez CA, Devincenzo JP, et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltola VT, Boyd KL, McAuley JL, Rehg JE, McCullers JA. Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect Immun. 2006;74:2562–2567. doi: 10.1128/IAI.74.5.2562-2567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mina MJ, Klugman KP, McCullers JA. Live attenuated influenza vaccine, but not pneumococcal conjugate vaccine, protects against increased density and duration of pneumococcal carriage after influenza infection in pneumococcal colonized mice. J Infect Dis. 2013;208:1281–1285. doi: 10.1093/infdis/jit317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu HT, Yoshida LM, Suzuki M, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30:11–18. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]
- 26.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202:1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short KR, Reading PC, Wang N, Diavatopoulos DA, Wijburg OL. Increased nasopharyngeal bacterial titers and local inflammation facilitate transmission of Streptococcus pneumoniae. MBio. 2012;3:e00255. doi: 10.1128/mBio.00255-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mina MJ, McCullers JA, Klugman KP. Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. MBio. 2014;5:e01040. doi: 10.1128/mBio.01040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diavatopoulos DA, Short KR, Price JT, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J. 2010;24:1789–1798. doi: 10.1096/fj.09-146779. [DOI] [PubMed] [Google Scholar]
- 30.Grijalva CG, Griffin MR, Edwards KM, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect Dis. 2014;58:1369–1376. doi: 10.1093/cid/ciu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short KR, Diavatopoulos DA, Thornton R, et al. Influenza virus induces bacterial and nonbacterial otitis media. J Infect Dis. 2011;204:1857–1865. doi: 10.1093/infdis/jir618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Short KR, Reading PC, Brown LE, et al. Influenza-induced inflammation drives pneumococcal otitis media. Infect Immun. 2013;81:645–652. doi: 10.1128/IAI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Short KR, von Kockritz-Blickwede M, Langereis JD, et al. Antibodies mediate formation of neutrophil extracellular traps in the middle ear and facilitate secondary pneumococcal otitis media. Infect Immun. 2014;82:364–370. doi: 10.1128/IAI.01104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 35.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 36.Shrestha S, Foxman B, Weinberger DM, Steiner C, Viboud C, Rohani P. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci Transl Med. 2013;5:191ra84. doi: 10.1126/scitranslmed.3005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harford CG, Leidler V, Hara M. Effect of the lesion due to influenza virus on the resistance of mice to inhaled pneumococci. J Exp Med. 1949;89:53–68. doi: 10.1084/jem.89.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker RG. The pathology of uncomplicated influenza. Postgrad Med J. 1963;39:564–566. doi: 10.1136/pgmj.39.456.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hers JF, Mulder J. Broad aspects of the pathology and pathogenesis of human influenza. Am Rev Resp Dis. 1961;83:84–89. doi: 10.1164/arrd.1961.83.2P2.84. [DOI] [PubMed] [Google Scholar]
- 40.Hirano T, Kurono Y, Ichimiya I, Suzuki M, Mogi G. Effects of influenza A virus on lectin-binding patterns in murine nasopharyngeal mucosa and on bacterial colonization. Otolaryngol Head Neck Surg. 1999;121:616–621. doi: 10.1016/S0194-5998(99)70068-9. [DOI] [PubMed] [Google Scholar]
- 41.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am J Physiol Lung Cell mMol Physiol. 2006;290:L194–L199. doi: 10.1152/ajplung.00050.2005. [DOI] [PubMed] [Google Scholar]
- 42.Rijneveld AW, Weijer S, Florquin S, et al. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis. 2004;189:711–716. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- 43.Seki M, Kosai K, Hara A, et al. Expression and DNA microarray analysis of a platelet activating factor-related molecule in severe pneumonia in mice due to influenza virus and bacterial co-infection. Jpn J Infect Dis. 2009;62:6–10. [PubMed] [Google Scholar]
- 44.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 45.King QO, Lei B, Harmsen AG. Pneumococcal surface protein A contributes to secondary Streptococcus pneumoniae infection after influenza virus infection. J Infect Dis. 2009;200:537–545. doi: 10.1086/600871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenhut M. Influenza virus amplifies interaction of polymeric immunoglobulin receptor with pneumococcal surface protein A, which mediates invasion by pneumococcus. J Infect Dis. 2010;201:1272–1273. doi: 10.1086/651432. [DOI] [PubMed] [Google Scholar]
- 47.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010;42:450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenhut M. Inhibition of epithelial sodium channels and reduction of ciliary function in influenza. Am J Respir Cell Mol Biol. 2012;46:414. doi: 10.1165/ajrcmb.46.3.414. [DOI] [PubMed] [Google Scholar]
- 49.Shahangian A, Chow EK, Tian X, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol. 2012;86:12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. JClin Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navarini AA, Recher M, Lang KS, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci USA. 2006;103:15535–15539. doi: 10.1073/pnas.0607325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007;75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuri T, Sorensen AS, Thomas S, et al. Influenza A virus-mediated priming enhances cytokine secretion by human dendritic cells infected with Streptococcus pneumoniae. Cell Microbiol. 2013;15:1385–1400. doi: 10.1111/cmi.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 58.van der Sluijs KF, Nijhuis M, Levels JH, et al. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006;193:214–222. doi: 10.1086/498911. [DOI] [PubMed] [Google Scholar]
- 59.Didierlaurent A, Goulding J, Patel S, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blok DC, van der Sluijs KF, Florquin S, et al. Limited anti-inflammatory role for interleukin-1 receptor like 1 (ST2) in the host response to murine postinfluenza pneumococcal pneumonia. PLoS One. 2013;8:e58191. doi: 10.1371/journal.pone.0058191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamieson AM, Yu S, Annicelli CH, Medzhitov R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe. 2010;7:103–114. doi: 10.1016/j.chom.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen WH, Toapanta FR, Shirey KA, et al. Potential role for alternatively activated macrophages in the secondary bacterial infection during recovery from influenza. Immunol Lett. 2012;141:227–234. doi: 10.1016/j.imlet.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S pneumoniae following influenza A infection in mice. J Virol Methods. 2001;94:173–186. doi: 10.1016/s0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 64.Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med. 2007;57:82–89. [PMC free article] [PubMed] [Google Scholar]
- 65.Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191:1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosai K, Seki M, Tanaka A, et al. Increase of apoptosis in a murine model for severe pneumococcal pneumonia during influenza A virus infection. Jpn J Infect Dis. 2011;64:451–457. [PubMed] [Google Scholar]
- 68.Jamieson AM, Pasman L, Yu S, et al. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340:1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kash JC, Walters KA, Davis AS, et al. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. MBio. 2011;2:e00172. doi: 10.1128/mBio.00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiser JN. The pneumococcus: why a commensal misbehaves. J Mol Med. 2010;88:97–102. doi: 10.1007/s00109-009-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio. 2013;4:e00438. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 73.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005;192:249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou H, Haber M, Ray S, Farley MM, Panozzo CA, Klugman KP. Invasive pneumococcal pneumonia and respiratory virus co-infections. Emerg Infect Dis. 2012;18:294–297. doi: 10.3201/eid1802.102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McAuley JL, Hornung F, Boyd KL, et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2:240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weeks-Gorospe JN, Hurtig HR, et al. Naturally occurring swine influenza A virus PB1-F2 phenotypes that contribute to superinfection with Gram-positive respiratory pathogens. J Virol. 2012;86:9035–9043. doi: 10.1128/JVI.00369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Amer Rev Respir Dis. 1986;134:1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 78.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(suppl 1):S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 79.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 80.Fisher TN, Ginsberg HS. The reaction of influenza viruses with guinea pig polymorphonuclear leucocytes II. The reduction of white blood cell glycolysis by influenza viruses and receptor-destroying enzyme (RDE) Virology. 1956;2:637–655. doi: 10.1016/0042-6822(56)90044-7. [DOI] [PubMed] [Google Scholar]
- 81.Walsh JJ, Dietlein LF, Low FN, Burch GE, Mogabgab WJ. Bronchotracheal response in human influenza Type A, Asian strain, as studied by light and electron microscopic examination of bronchoscopic biopsies. Arch Intern Med. 1961;108:376–388. doi: 10.1001/archinte.1961.03620090048006. [DOI] [PubMed] [Google Scholar]
- 82.Sellers TF, Jr, Schulman J, Bouvier C, McCure R, Kilbourne ED. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J Exp Med. 1961;114:237–256. doi: 10.1084/jem.114.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mina MJ, Klugman KP. Pathogen replication, host inflammation, and disease in the upper respiratory tract. Infect Immun. 2013;81:625–628. doi: 10.1128/IAI.01460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seki M, Yanagihara K, Higashiyama Y, et al. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur Respir J. 2004;24:143–149. doi: 10.1183/09031936.04.00126103. [DOI] [PubMed] [Google Scholar]
- 85.van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 86.Didierlaurent A, Goulding J, Hussell T. The impact of successive infections on the lung microenvironment. Immunol. 2007;122:457–465. doi: 10.1111/j.1365-2567.2007.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka A, Nakamura S, Seki M, Fukudome K, et al. Toll-like receptor 4 agonistic antibody promotes innate immunity against severe pneumonia induced by coinfection with influenza virus and Streptococcus pneumoniae. Clin Vaccine Immunol. 2013;20:977–985. doi: 10.1128/CVI.00010-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6:e17035. doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mina MJ, Burke RM, Klugman KP. Estimating the prevalence of coinfection with influenza virus and the atypical bacteria Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. Eur J Clin Microbiol Infect Dis. 2014 doi: 10.1007/s10096-014-2120-0. published online May 1. http://dx.doi.org/10.1007/s10096-014-2120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dominguez A, Castilla J, Godoy P, et al. Benefit of conjugate pneumococcal vaccination in preventing influenza hospitalization in children: a case-control study. Pediatr Infect Dis J. 2013;32:330–334. doi: 10.1097/INF.0b013e318280a34b. [DOI] [PubMed] [Google Scholar]
- 91.Chien YW, Levin BR, Klugman KP. The anticipated severity of a “1918-like” influenza pandemic in contemporary populations: the contribution of antibacterial interventions. PLoS One. 2012;7:e29219. doi: 10.1371/journal.pone.0029219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu X, He Y, Xiao K, White JR, Fusco DN, Papanicolaou GA. Effect of linezolid on clinical severity and pulmonary cytokines in a murine model of influenza A and Staphylococcus aureus coinfection. PLoS One. 2013;8:e57483. doi: 10.1371/journal.pone.0057483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karlstrom A, Heston SM, Boyd KL, Tuomanen EI, McCullers JA. Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis. 2011;204:1358–1366. doi: 10.1093/infdis/jir522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subramaniam R, Barnes PF, Fletcher K, et al. Protecting against post-influenza bacterial pneumonia by increasing phagocyte recruitment and ROS production. J Infect Dis. 2014;209:1827–1836. doi: 10.1093/infdis/jit830. [DOI] [PubMed] [Google Scholar]
- 95.Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3:359–371. doi: 10.1016/s1473-3099(03)00658-3. [DOI] [PubMed] [Google Scholar]
- 96.Karlstrom A, Boyd KL, English BK, McCullers JA. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis. 2009;199:311–319. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghoneim HE, McCullers JA. Adjunctive corticosteroid therapy improves lung immunopathology and survival during severe secondary pneumococcal pneumonia in mice. J Infect Dis. 2014;209:1459–1468. doi: 10.1093/infdis/jit653. [DOI] [PubMed] [Google Scholar]
- 99.McCullers JA, Karlstrom A, Iverson AR, Loeffler JM, Fischetti VA. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:e28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190:519–526. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]