Abstract
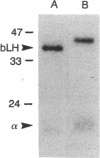
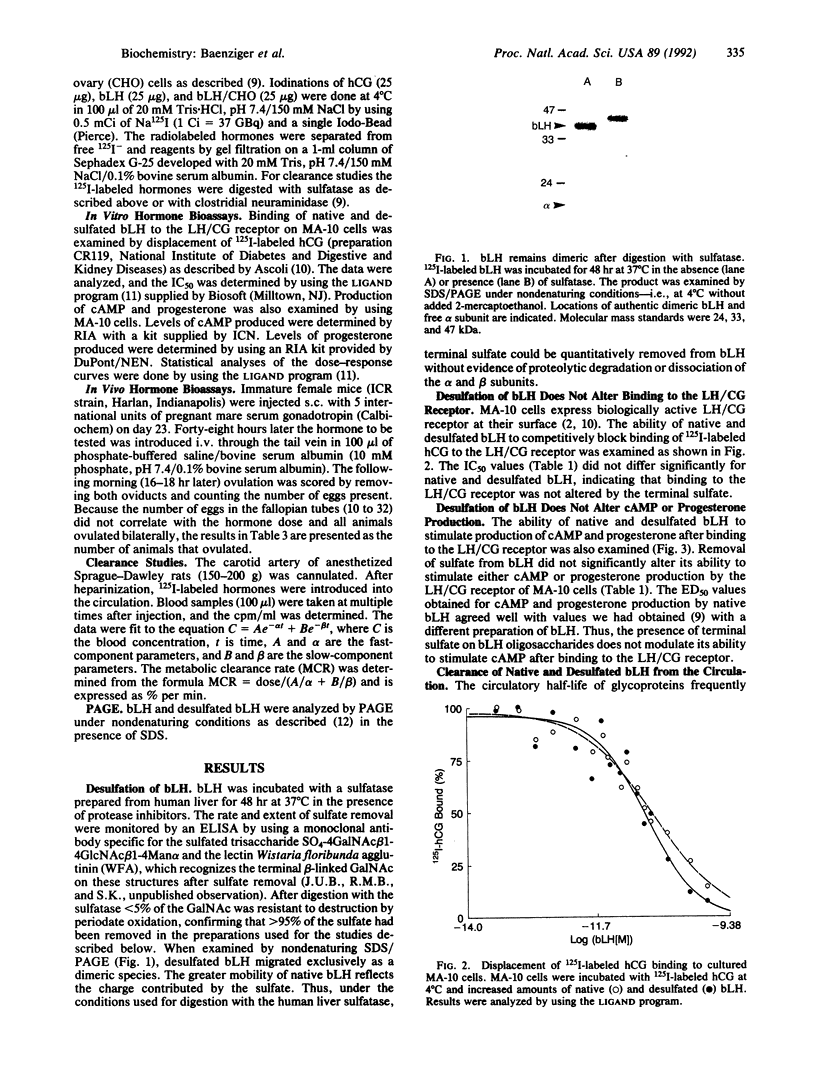
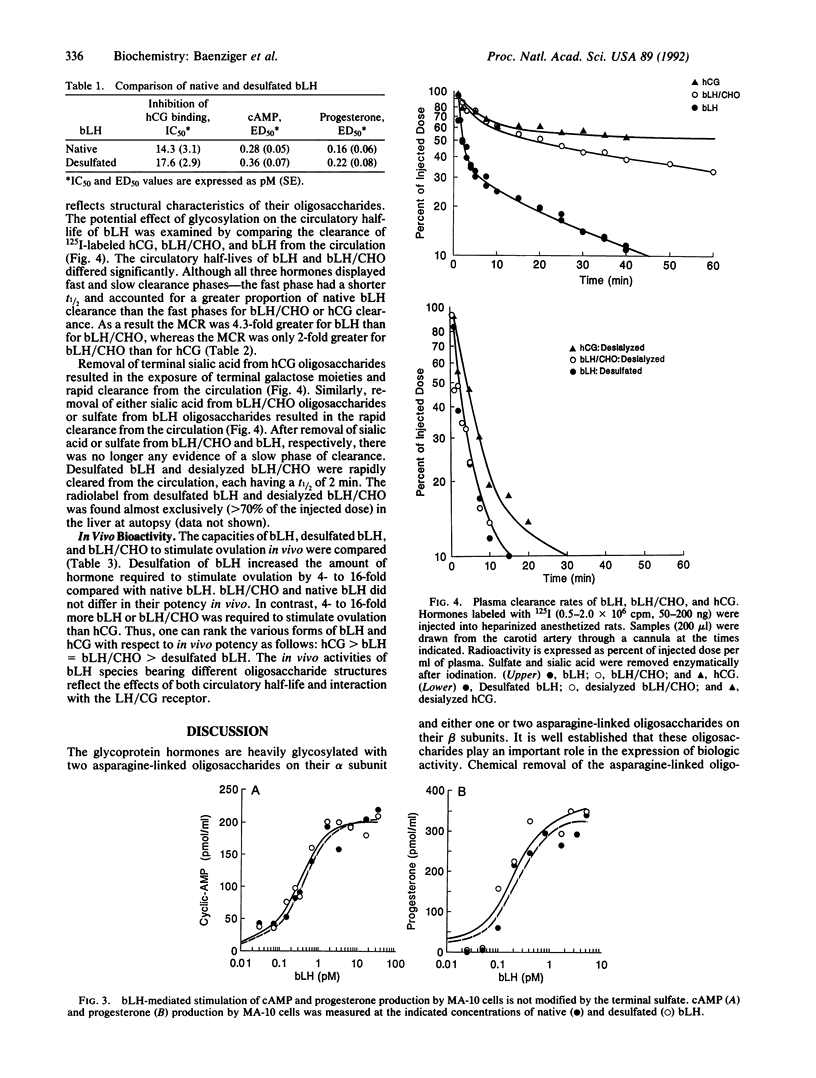
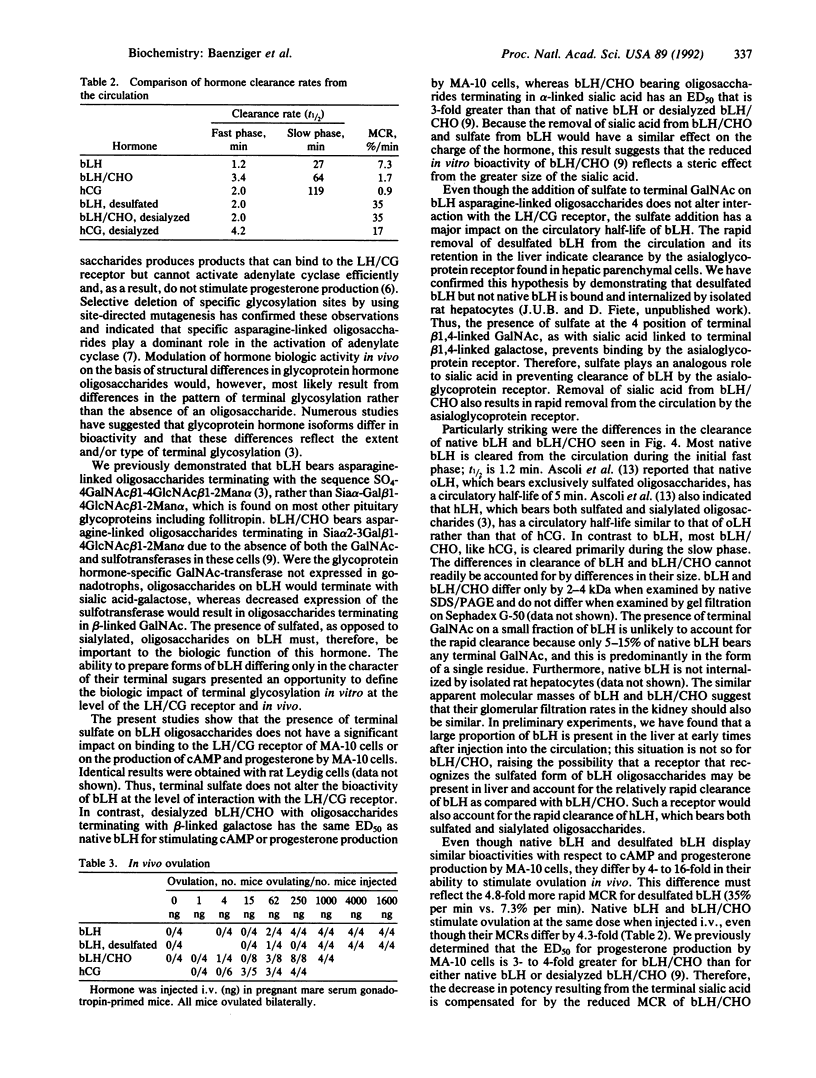
Certain of the glycoprotein hormones, including bovine lutropin (bLH), bear asparagine-linked oligosaccharides terminating with the sequence SO4-4GalNAc beta 1-4GlcNAc beta 1-2Man alpha. To establish the biologic significance of these sulfate-bearing oligosaccharides we have compared properties of native bLH, desulfated bLH, recombinant bLH produced in Chinese hamster ovary cells that bears asparagine-linked oligosaccharides terminating with sialic acid alpha 2- 3Gal beta 1-4GlcNAc beta 1-2Man alpha rather than sulfated oligosaccharides (bLH/CHO), and desialyzed bLH/CHO. Using cultured MA-10 cells, a Leydig cell tumor line expressing the lutropin/chorionic gonadotropin receptor, we have found no differences in binding, cAMP production, or progesterone production between native and desulfated bLH. Sulfation of bLH oligosaccharides does not, therefore, modulate bLH bioactivity at the level of the lutropin/chorionic gonadotropin receptor. Removal of sulfate from bLH oligosaccharides and sialic acid from bLH/CHO oligosaccharides results in rapid clearance from the circulation by the hepatocyte asialoglycoprotein receptor. Thus sulfate, like sialic acid, prevents clearance from the circulation by the asialoglycoprotein receptor. The rapid removal of desulfated bLH from the circulation causes a 4- to 16-fold increase in the amount of bLH required to stimulate ovulation compared with native bLH. Particularly striking were differences in the metabolic clearance rates for native bLH and bLH/CHO, 7.3% per min and 1.7% per min, respectively. These differences were unexpected because bLH and bLH/CHO do not differ significantly in charge or size. The different metabolic clearance rates obtained for bLH and bLH/CHO indicate that the presence of sulfated rather than sialylated oligosaccharides on bLH results in a shorter circulatory half-life, which has a significant impact on in vivo bioactivity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M., Liddle R. A., Puett D. The metabolism of luteinizing hormone. Plasma clearance, urinary excretion, and tissue uptake. Mol Cell Endocrinol. 1975 Jul;3(1):21–36. doi: 10.1016/0303-7207(75)90029-5. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Green E. D. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim Biophys Acta. 1988 Jun 9;947(2):287–306. doi: 10.1016/0304-4157(88)90012-3. [DOI] [PubMed] [Google Scholar]
- Damm J. B., Hård K., Kamerling J. P., van Dedem G. W., Vliegenthart J. F. Structure determination of the major N- and O-linked carbohydrate chains of the beta subunit from equine chorionic gonadotropin. Eur J Biochem. 1990 Apr 20;189(1):175–183. doi: 10.1111/j.1432-1033.1990.tb15474.x. [DOI] [PubMed] [Google Scholar]
- Green E. D., Morishima C., Boime I., Baenziger J. U. Structural requirements for sulfation of asparagine-linked oligosaccharides of lutropin. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7850–7854. doi: 10.1073/pnas.82.23.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyves P. W., Gesundheit N., Thotakura N. R., Stannard B. S., DeCherney G. S., Weintraub B. D. Changes in the sialylation and sulfation of secreted thyrotropin in congenital hypothyroidism. Proc Natl Acad Sci U S A. 1990 May;87(10):3792–3796. doi: 10.1073/pnas.87.10.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Fraser H. M., Lincoln G. A., Martin G. B., McNeilly A. S. Hypothalamic pulse generators. Recent Prog Horm Res. 1985;41:369–419. doi: 10.1016/b978-0-12-571141-8.50013-5. [DOI] [PubMed] [Google Scholar]
- Matsui T., Sugino H., Miura M., Bousfield G. R., Ward D. N., Titani K., Mizuochi T. Beta-subunits of equine chorionic gonadotropin and lutenizing hormone with an identical amino acid sequence have different asparagine-linked oligosaccharide chains. Biochem Biophys Res Commun. 1991 Jan 31;174(2):940–945. doi: 10.1016/0006-291x(91)91509-b. [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Boime I. Mutagenesis and gene transfer define site-specific roles of the gonadotropin oligosaccharides. Biol Reprod. 1989 Jan;40(1):48–53. doi: 10.1095/biolreprod40.1.48. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Baenziger J. U. A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science. 1988 Nov 11;242(4880):930–933. doi: 10.1126/science.2460923. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Kaetzel D., Nilson J., Baenziger J. U. The sialylated oligosaccharides of recombinant bovine lutropin modulate hormone bioactivity. J Biol Chem. 1990 Jan 15;265(2):874–881. [PubMed] [Google Scholar]
- Thotakura N. R., Desai R. K., Bates L. G., Cole E. S., Pratt B. M., Weintraub B. D. Biological activity and metabolic clearance of a recombinant human thyrotropin produced in Chinese hamster ovary cells. Endocrinology. 1991 Jan;128(1):341–348. doi: 10.1210/endo-128-1-341. [DOI] [PubMed] [Google Scholar]
- Veldhuis J. D., Carlson M. L., Johnson M. L. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis J. D., Johnson M. L. A novel general biophysical model for simulating episodic endocrine gland signaling. Am J Physiol. 1988 Dec;255(6 Pt 1):E749–E759. doi: 10.1152/ajpendo.1988.255.6.E749. [DOI] [PubMed] [Google Scholar]