Abstract
Background
Endothelial dysfunction is regarded as the early stage of atherosclerosis and associated with cardiovascular (CV) events. This study was designed to determine whether the assessment of coronary endothelial function (CEF) is safe and can re-classify risk in patients with early coronary artery disease beyond the Framingham risk score (FRS).
Methods and Results
CEF was evaluated using intracoronary acetylcholine in 470 patients who presented with chest pain and non-obstructive coronary artery disease. CV events were assessed after a median follow-up of 9.7 years. The association between CEF and CV events was examined, and net reclassification index (NRI) used to compare incremental contribution of CEF when added to FRS.
Mean age was 53 years and 68% of the patients were women with a median FRS of 8. Complications (coronary dissection) occurred in 3 (0.6 %) and CV events in 61 (13%) patients. In univariate analysis, microvascular CEF (HR 0.85, 95% CI 0.72 – 0.97, P=0.032) and epicardial CEF (HR 0.73, 95% CI 0.59 – 0.90; P=0.01) were significant predictors of CV events, while FRS was not (HR 1.05, 95% CI 0.85 – 1.26; P=0.61). When added to the FRS, microvascular CEF correctly reclassified 11.3% of the patients, NRI 0.11 (95% CI, 0.019 – 0.21); epicardial CEF correctly reclassified 12.1% of the patients, NRI 0.12 (95% CI, −0.02 – 0.26) and the combined microvascular and epicardial CEF correctly reclassified 22.8% of the patients, NRI 0.23 (95% CI, 0.08 – 0.37).
Conclusion
Coronary endothelial function testing is safe and adds value to the FRS, with superior discrimination and risk stratification compared to FRS alone in patients presenting with chest pain or suspected ischemia.
Introduction
Globally, cardiovascular (CV) diseases are the number one cause of death and a leading cause of morbidity [1]. Traditional CV risk factors based on the Framingham study have been used to estimate an individual global CV risk [2]. Practice guidelines use the Framingham risk score (FRS) to classify individuals as low, intermediate or high risk and determine their target cholesterol levels for primary prevention [3].
However, these traditional risk factors as identified in the Framingham study have been inconsistent in predicting CV events when applied to different populations and correctly assigned the risk of the development of coronary heart disease only in half of the cases [4]. This underscores the complex interplay between traditional CV risk factors, genetic predisposition and other atheroprotective factors prevalent in individuals of different populations in predicting CV events.
Functional vascular abnormalities such as endothelial dysfunction are considered a key event in the initiation, progression and the complications of coronary artery disease. Endothelial dysfunction is a systemic disorder affecting multiple vascular beds and can be regarded as the integrated index of both the overall cardiovascular risk factor burden and the sum of genetic risk factors and environmental factors [4].
Previous studies have shown an association between the presence of both coronary and systemic endothelial dysfunction and an increased risk of future CV events [5–8]. We have previously summarized the role of endothelial function testing in predicting CV events [9] No published studies, however, have evaluated how measures of coronary endothelial function affect discrimination of the FRS.
Invasive measurement of the change in coronary blood flow (CBF) in response to an intracoronary infusion of acetylcholine (ACh) is considered the reference standard in coronary endothelial function testing [10]. However, its invasive nature and potential safety concerns limit its use. [10] To our knowledge no previous study has compared the efficacy of FRS to invasive coronary endothelial function in predicting CV events. This study was designed to test the hypothesis that coronary epicardial and microvascular endothelial function testing is safe and improves the predictive accuracy and classification of the FRS in patients with non-obstructive coronary artery disease.
Methods
Study Design
This study is a prospective single center cohort study. The study was approved by the Mayo Clinic Institutional Review Board, and informed consent obtained from all patients.
Study Population
The study group consisted of 470 patients with chest pain who were referred to the cardiac catheterization laboratory for evaluation of coronary artery disease, found to have non-obstructive disease, and had comprehensive coronary physiology study, including the assessment of endothelial dependent and independent function.
Exclusion criteria included: significant coronary artery stenosis (>40%), ejection fraction <45%, unstable angina, previous acute coronary syndrome, significant systemic disease, and pregnancy. Medications that may affect cardiovascular hemodynamics were discontinued for at least 48 hours before the study.
Study Protocol
At baseline, diagnostic coronary angiography and determination of endothelium-dependent changes in coronary blood flow (CBF) and endothelium-independent coronary flow reserve were performed as previously described. [11, 12] [13, 14]A Doppler guide wire (0.014-in diameter, FloWire, Volcano Incorporated) within a 2.2F coronary infusion catheter (Ultrafuse, SciMed Life System) was advanced and positioned in the middle portion of the left anterior descending coronary artery (LAD). Intracoronary bolus injections of incremental doses (18 to 72 µg) of adenosine (Fujisawa), an endothelium-independent vasodilator (primarily of the microcirculation), [15] were administered into the guiding catheter until maximal hyperemia was achieved.
Assessment of the endothelium-dependent changes in vascular diameter and CBF was performed by selective infusion of ACh into the LAD. ACh (Iolab Pharmaceuticals) 10−6, 10−5, and 10−4 mol/L was infused at 1 mL/min for 3 minutes. [12, 16] Hemodynamic data (heart rate and mean arterial pressure), Doppler measurements, and coronary angiography were obtained after each infusion. Endothelium-independent epicardial vasodilation was assessed with an intracoronary bolus injection of nitroglycerin (200 µg, Abbott Laboratories). [17]
Epicardial coronary endothelial function
Coronary artery diameter was analyzed by quantitative coronary angiograms (QCA) from digital images with the use of a modification of a previously described technique from this institution. [11, 12] The LAD was divided into proximal, middle, and distal segments. For each segment, the measurements were performed in the region where the greatest change had occurred during the acetylcholine infusion. An angiographically smooth segment of the proximal, middle, and distal LAD, free of any overlapping branch vessels, was identified in each patient and served as the reference diameter for the calculation of diameter stenosis. End-diastolic cine frames that best showed the segment were selected, and calibration of the video and cine images was done, identifying the diameter of the guide catheter. Quantitative measurements of the coronary arteries were obtained using a computer-based image analysis system. Segment diameters were determined at baseline and after both ACh and nitroglycerin administration. The proximal segment was not exposed to ACh and thus served a control segment.
Microvascular coronary endothelial function
Doppler flow velocity spectra were analyzed online to determine the time-averaged peak velocity. Volumetric CBF was determined from the following relation: CBF=cross-sectional area × average peak velocity × 0.5. [18] Endothelium-dependent microvascular function was calculated as % ∆ CBF in response to ACh as previously described [19].
Follow-Up
Long-term follow-up was performed by a detailed questionnaire inquiring about occurrence and dates timing of CV events. Vital status of the patients was determined using the National Death Index. For subjects experiencing more than one CV event, only the first event was considered in the analysis. All CV events were confirmed by a review of the hospital records.
A composite endpoint of cardiovascular death, acute myocardial infarction, stroke, coronary artery bypass surgery (CABG), repeat coronary angiography and percutaneous coronary intervention and other vascular surgeries (endarterectomy, repair of abdominal aortic aneurysm or peripheral bypass surgeries) was assessed during the follow-up.
Statistical analysis
Data are displayed as means ± SD or count and percentage as appropriate. Variables with heavily skewed distribution are reported as medians with first and third quartiles in parenthesis. The statistical analysis was performed by an independent statistician.
Survival Analysis
The starting point for all survival analysis was date of coronary endothelial function angiogram. The Composite of CV events was the primary endpoint and the patient’s survival time was the interval from endothelial function angiogram date to date of first event. For patients who did not have an event, event free survival time was the interval from the endothelial function angiogram to December 2010.
FRS was then calculated for each patient using the original variables of the 10-year FRS (age, sex, cigarette smoking status, blood pressure, antihypertensive medication use, total cholesterol level, HDL and presence of DM) [2]. The FRS score was then refitted using a multivariable Cox proportional-hazards model. This baseline model was then extended to three other models by including microvascular CEF (%∆ CBF), then epicardial CEF (%∆ CAD) and finally both microvascular and epicardial CEF. For each of the models the 10 year absolute risk to develop the CV events was calculated and used to classify the patients into as low (<10%), intermediate10% – 20%) and high risk (>20%) based on ATP III classification [3].
Cox proportional hazards multivariable regression analysis was used to determine the univariate and multivariable relationships between FRS, microvascular CEF, epicardial CEF and CV events during the follow-up period. All probability values were 2-tailed, and a p-value of 0.05 was considered statistically significant.
Net Reclassification Improvement (NRI)
The NRI was calculated to assess whether microvascular CEF, epicardial CEF or combined microvascular and epicardial CEF improved discrimination of the FRS using methods developed by Pencina et al [20]. The NRI calculates the percentage of correct movement across categories for those with and without events. NRI assesses the probability of being correctly reclassified to a higher risk category for patients with events minus the probability of being incorrectly reclassified to a lower risk category for patients with events plus the probability of being correctly reclassified to a lower risk category of patients with no events minus the probability of being incorrectly classified to a higher risk category for patients with no events. As the risk prediction models were based on a time to event data, the NRI was calculated using methods that took survival time into account [21, 22]. The corresponding 95% confidence intervals were obtained with bootstrapping.
Results
Study Cohort
We studied 470 patients with a mean ± SD age of 53 ± 12 years, 68% of whom were women. The FRS median (IQR) was 8 (4.2 – 15.2).
The % ∆ CBF (mean ± SD) was 61.5 ± 164.6 and % ∆ CAD was −15.2 ± 24.8. The baseline characteristics of the cohort at the time of coronary vascular testing are shown in Table 1 and 2.
Table 1.
Baseline Patient Characteristics by tertiles of Framingham Risk Score (FRS)
| Variables N (%) |
Low 277 (59) |
Inter- mediate 117 (25) |
High 76 (16) |
P |
|---|---|---|---|---|
| Age, years ±SD | 48 ± 11 | 57 ± 9 | 62 ± 8 | <0.001 |
| Female, N (%) | 228 (82) | 66 (56) | 26 (34) | <0.001 |
| BMI, kg/m2 | 28 ± 6 | 29 ± 6 | 29± 6 | 0.27 |
| Hypertension, N (%) | 80 (29) | 58 (50) | 49 (64) | <0.001 |
| Diabetes, N (%) | 10 (4) | 11 (9) | 19 (25) | <0.001 |
| Hypercholesterolemia, N (%) | 139 (50) | 84 (72) | 57 (75) | <0.001 |
| Family history CAD, N (%) | 167 (61) | 82 (71) | 48 (65) | <0.2 |
| Aspirin, N (%) | 121 (44) | 62 (53) | 40 (52) | 0.15 |
| ACE inhibitor, N (%) | 33 (12) | 31 (27) | 10 (13) | <0.001 |
| B- Blockers, N (%) | 81 (29) | 34 (29) | 21 (28) | 0.96 |
| Ca2+ Channel blocker, N (%) | 80 (29) | 48 (41) | 28 (37) | 0.04 |
| Lipid lowering, N (%) | 102 (37) | 55 (47) | 29 (38) | 0.16 |
| Oral hypoglycemic, N (%) | 6 (2) | 4 (3) | 11 (14) | <0.001 |
| Insulin use, N (%) | 5 (2) | 5 (4) | 3 (4) | 0.31 |
| % ΔCBF | 69 ± 193 | 47 ± 118 | 57 ± 98 | 0.48 |
| % ΔCAD | −14.8 ± 25 | −16.3 ± 25 | −15.2 ± 21 | 0.85 |
| CFR | 2.95 ± 0.7 | 2.7 ± 0.7 | 2.9 ± 0.6 | <0.001 |
Values are mean ± SD or N (%). BMI indicates body mass index, % ΔCBF indicates percent change in coronary blood flow to acetylcholine, % ΔCAD indicates percent change in coronary artery diameter to acetylcholine, and CFR indicates coronary flow reserve.
Table 2.
Baseline Patient Characteristics by Microvascular Endothelial Function
| Variables N (%) |
Microvascular Endothelial dysfunction 265 (56) |
Normal Microvascular Endothelial Function 117 (25) |
P |
|---|---|---|---|
| Age, years ±SD | 54 ± 12 | 52 ± 12 | 0.03 |
| Female, N (%) | 186 (70) | 134 (65) | 0.27 |
| BMI, kg/m2 | 29 ± 6 | 28 ± 6 | 0.48 |
| Hypertension, N (%) | 114 (43) | 73 (46) | 0.09 |
| Diabetes, N (%) | 25 (9) | 15 (7) | 0.41 |
| Hypercholesterolemia, N (%) | 157 (59) | 123 (60) | <0.85 |
| Family history CAD, N (%) | 178 (69) | 119 (60) | <0.04 |
| Aspirin, N (%) | 138 (52) | 85 (41) | 0.02 |
| ACE inhibitor, N (%) | 42 (15) | 32 (16) | 0.94 |
| B- Blockers, N (%) | 80 (30) | 56 (27) | 0.50 |
| Ca2+ Channel blocker, N (%) | 94 (35) | 62 (30) | 0.25 |
| Lipid lowering, N (%) | 118 (45) | 68 (33) | 0.01 |
| Oral hypoglycemic, N (%) | 13 (5) | 8 (4) | 0.6 |
| Insulin use, N (%) | 8 (3) | 5 (2) | 0.70 |
| % ΔCBF | −10.9 ± 36 | 155 ± 212 | <0.001 |
| % ΔCAD | −25.4 ± 21 | −2.1 ± 23 | <0.001 |
| CFR | 2.78 ± 0.66 | 2.98 ± 0.7 | <0.001 |
Values are mean ± SD or N (%). BMI indicates body mass index, % ΔCBF indicates percent change in coronary blood flow to acetylcholine, % ΔCAD indicates percent change in coronary artery diameter to acetylcholine, and CFR indicates coronary flow reserve.
Patients were enrolled into this study between December 1st 1992 and May 31st 2009. After a follow period of median (Q1, Q3): 9.7 years (6.1, 14.0), 61 patients (13%) experienced a CV event, including cardiac death, myocardial infarction, repeat angiogram/angioplasty, stroke, CABG, and other vascular surgeries (carotid endarterectomy and peripheral vascular bypass (Table 3 and 4).
Table 3.
Distribution of Cardiovascular events in the patient population by tertiles of Framingham Risk Score (FRS
| Variable N=61 |
Low 277 (59) |
Inter- mediate 117 (25) |
High 76 (16) |
P |
|---|---|---|---|---|
| Cardiac death, n (%) | 1 (0.3) | 2 (1.7) | 2 (2.6) | 0.01 |
| Myocardial infarction, n (%) | 1 (0.3) | 2 (1.7) | 0 | 0.04 |
| Repeat angiogram/PCI, n (%) | 18 (6.4) | 14 (11.9) | 6 (7.8) | 0.11 |
| Stroke, n (%) | 3 (1) | 0 | 0 | 0.24 |
| CABG, n (%) | 3(1) | 4 (3.4) | 2 (2.6) | 0.26 |
| Other vascular surgery, n (%) | 1(0.3) | 2 (1.7) | 0 | 0.16 |
Values are n (%). CABG denotes coronary artery bypass surgery; other vascular surgery includes endarterectomy, and peripheral vascular bypass surgery.
Table 4.
Distribution of Cardiovascular events in the patient population by Microvascular Endothelial Function
| Variables N (%) |
Microvascular Endothelial dysfunction 265 (56) |
Normal Microvascular Endothelial Function 117 (25) |
P |
|---|---|---|---|
| Cardiac death, n (%) | 3 (1.2) | 2 (1.7) | 0.01 |
| Myocardial infarction, n (%) | 2 (0.8) | 1 (0.8) | 0.04 |
| Repeat angiogram/PCI, n (%) | 25 (9.4) | 13 (11.1) | 0.11 |
| Stroke, n (%) | 3 (1.2) | 0 | 0.24 |
| CABG, n (%) | 4(1.5) | 5 (4.3) | 0.26 |
| Other vascular surgery, n (%) | 1(0.3) | 2 (1.7) | 0.16 |
Values are n (%). CABG denotes coronary artery bypass surgery; other vascular surgery includes endarterectomy, and peripheral vascular bypass surgery.
Complications
The invasive coronary vasomotion testing was complicated by a procedure related coronary dissection in 3 (0.6%) out of 470 patients, all of which occurred prior to 2007. One of the dissections was mild and did not require stenting. The other two were managed with stenting and the patients did well after the procedure. There were no deaths or myocardial infarction associated with the procedure during the study period.
Framingham Risk Score (FRS)
In univariate analysis the FRS did not significantly predict the CV events (HR per 10% of predicted risk: 1.05, 95% CI 0.85 – 1.26, P=0.61). The estimated 10 year risk based on the FRS placed all the 470 patients into the intermediate risk category (10–20% risk) [3]. There was no difference in the incidence of CV events when patients were classified into low (<10%), intermediate10% – 20%) and high risk (>20%) based on FRS ATP III classification (P=0.08 by Kaplan Meier analysis; Figure 1).
Figure 1.
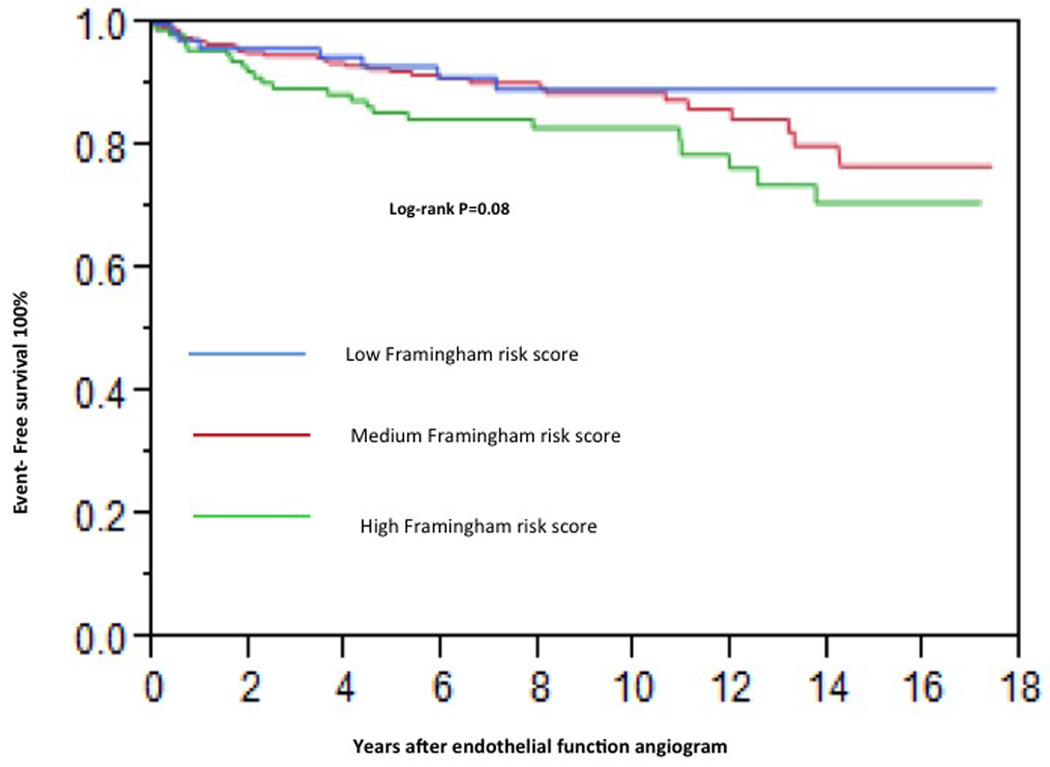
Kaplan-Meier curve showing cumulative proportion of patients without cardiovascular events during follow-up. Status of Framingham risk score is divided into low (<10%), intermediate10% – 20%) and high risk (>20%) based on ATP III classification.
Microvascular coronary endothelial function
In univariate analysis microvascular CEF (% ∆ CBF) was a significant predictor of CV events (HR per 50% increase in Δ CBF: 0.85, 95% CI 0.72 – 0.97, P=0.032). The incidence of CV events was not different in patients with normal Microvascular function compared to those with Microvascular endothelial dysfunction (microvascular endothelial dysfunction was defined as ≤ 50% increase in coronary blood flow (CBF) in response to the maximal dose of ACh compared with baseline CBF) (P=0.36 by Kaplan Meier analysis; Figure 2). After adjusting for the FRS, microvascular CEF was no longer a significant predictor of CV events (HR per 50% increase in Δ CBF: 0.88, 95% CI 0.74 – 1.01). When added to the FRS, microvascular CEF correctly reclassified 11.3% of the patients NRI, 0.11 (95% CI, 0.019 to 0.21, P=0.02). Eleven percent of the patients with events were incorrectly classified in a lower category and 21% of patients with no events were correctly classified into lower risk category providing a net correct reclassification of 11.3% (Table 3).
Figure 2.
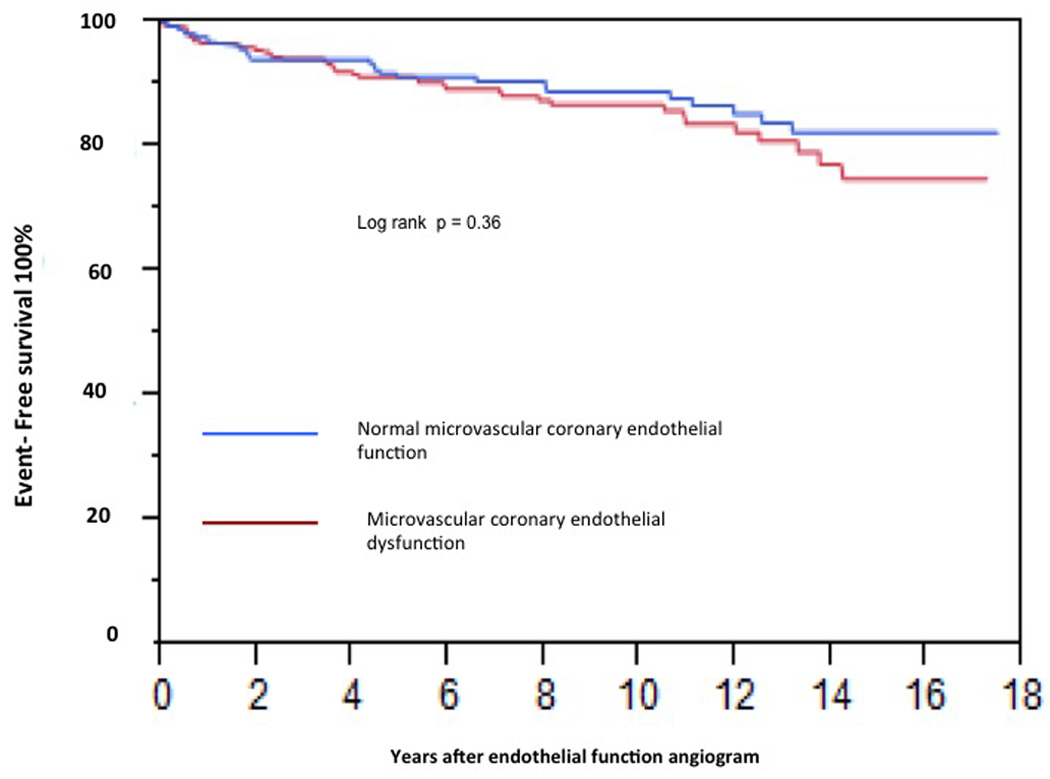
Kaplan-Meier curve showing cumulative proportion of patients without cardiovascular events during follow-up. Status of epicardial coronary endothelial function is divided into normal epicardial endothelial function and epicardial endothelial dysfunction.
Epicardial coronary endothelial function
In univariate analysis, epicardial CEF (% ∆ CAD) significantly predicted CV events (HR per 20% increase in Δ CAD: 0.73, 95% CI 0.59 – 0.90, P=0.01). The incidence of CV events was significantly greater in patients with epicardial endothelial dysfunction that in those who vasodilated with ACh (P=0.02 by Kaplan Meier analysis; Figure 3). After adjusting for the FRS, epicardial CEF was remained a significant predictor of CV events (HR per 20% increase in Δ CAD: 0.76, 95% CI 0.61 – 0.96). When added to the FRS, epicardial CEF correctly reclassified 12.1% of the patients NRI, 0.12 (95% CI, −0.02 to 0.26, P=0.09), but did not reach statistical significance. Five percent of the patients with events were incorrectly classified in a lower category and 23 % of patients with no events were correctly classified into lower risk category providing NRI of 12.1% (Table 3).
Figure 3.
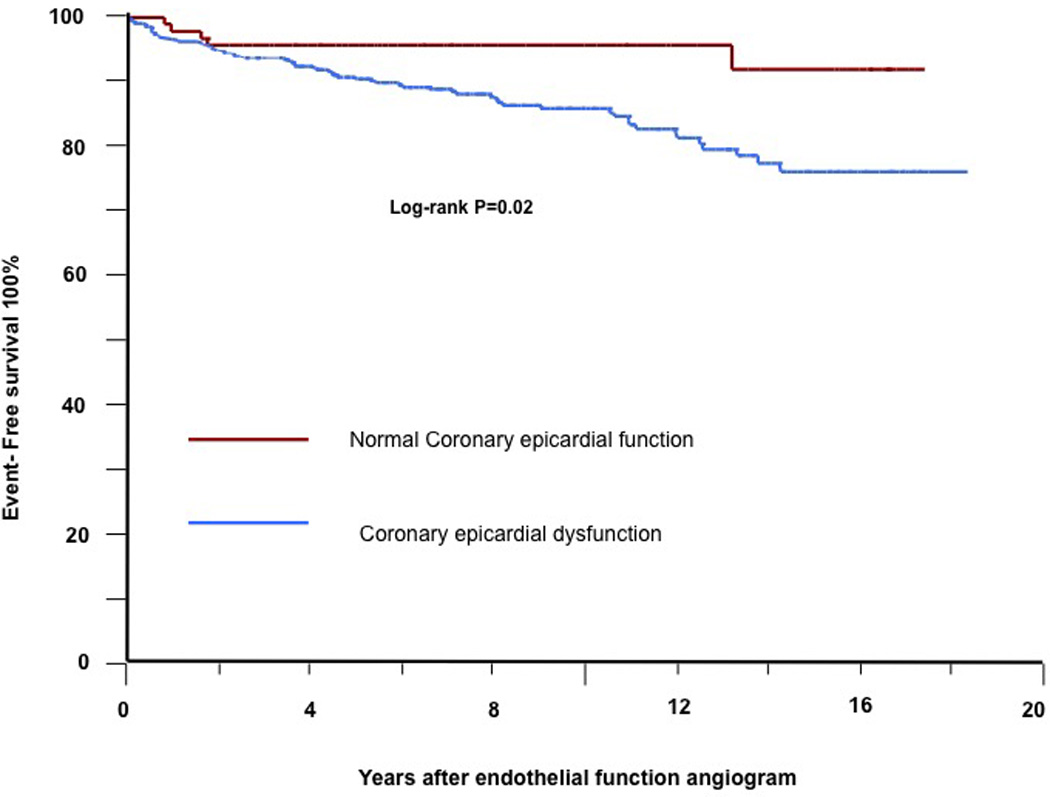
Kaplan-Meier curve showing cumulative proportion of patients without cardiovascular events during follow-up. Status of microvascular coronary endothelial function is divided into normal microvascular endothelial function and microvascular endothelial dysfunction.
When added to the FRS, the combined microvascular and epicardial CEF correctly reclassified 22.8% of the patients NRI, 0.23 (95% CI, 8% to 36.7%, P=0.001). Only 3 % of the patients with events were incorrectly classified in a lower category and 26 % of patients with no events were correctly classified into lower risk category providing a net correct reclassification of 22.8% (Table 3).
Subanalysis in Diabetics
To assess if the FRS and CEF could predict the risk of CV events differently in patients with diabetes mellitus, we assessed separately for an interaction between diabetes and FRS, microvascular CEF and epicardial CEF. No test for interaction yielded a significant result (p value FRS, 0.68; p value microvascular CEF; 0.56 and p value epicardial CEF; 0.79) suggesting that the risk of CV events predicted by the FRS, microvascular CEF and epicardial CEF did not vary significantly between patients with and without diabetes.
Discussion
The present study demonstrates that coronary endothelial function testing is safe and adds significant value to the FRS, providing greater discrimination power for risk stratification of patients without obstructive coronary artery disease. The combined effect of microvascular and epicardial coronary endothelial function provided the greatest value to FRS with more than one in five of individuals being correctly reclassified. Microvascular and epicardial endothelial function when used alone also provided modest value to the FRS with 12 % and 11%, respectively, of individuals being correctly reclassified. The model incorporating epicardial CEF alone did not reach statistical significance. Thus, the current study further supports the clinical utility of the individual assessment of endothelial dysfunction in the risk stratification.
Traditional CV risk factors are not sufficient to correctly assign risk of development of CV disease in up to 50% of cases. [23] Indeed, the FRS when applied to different populations has shown inconsistent predictive value. [24, 25] For example, the first 20-year mortality data in 12 cohorts of six countries showed that traditional risk factors are associated with risk only within certain cohorts. [26] Only age and mean blood pressure were universal predictors of coronary heart disease. In the current study all patients were classified as intermediate risk when their 10-year absolute risk was calculated using the FRS alone. In univariate and multivariable models the FRS indeed was not a significant predictor of CV events. Thus, there is a need for a more comprehensive method to assess the risk in these individuals without CAD.
Several studies have tested different novel risk marker for improving CV risk assessment especially in individual with intermediate risk [27, 28], by providing greater discrimination of higher and lower risk patients within the intermediate risk group. [28] The AHA practice guidelines for assessment of CV risk in asymptomatic adults gives recommendations on appropriate test modalities to further define the risk of patients with intermediate risk. [29] In this study we show that coronary endothelial function testing in patients presenting with chest pain and found to have non-obstructive coronary artery disease on diagnostic angiogram correctly reclassifies 22% of the patients with intermediate risk by the FRS with potential implications on risk management of these individuals.
Administration of intracoronary ACh is considered the reference standard in the assessment of epicardial and microvascular endothelial function. [10] Its use in clinical practice however still remains limited, possibly due to safety concerns related to its invasive nature. In the recent guidelines of the European Society of Cardiology on the management of stable coronary artery disease, the use of intracoronary ACh for assessment of coronary endothelial function was endorsed and labeled as class IIa indication [30], underscoring the need for better risk stratification in these patients. In the present study we demonstrate that comprehensive intracoronary physiology assessment to determine endothelial function is safe when performed by experienced operators. Our findings are consistent with a previous report demonstrating the safety of the assessment of endothelial function in women. [31] Additional barriers to adoption of this test are the lack of standardization of the testing protocol, and difficulty interpreting the results. Moreover, although several strategies aimed at reducing CV risk factors are associated with improvement in endothelial health, no single agent to date has been approved for the treatment of endothelial dysfunction per se. [32] Nevertheless, recent studies have shown that treatment of CV risk factors that leads to improvement of peripheral endothelial function (versus persistent endothelial dysfunction) is associated with improved CV prognosis. [33, 34]
Several studies have shown an association between endothelial dysfunction (both in the coronary and systemic circulation) as a marker of atherosclerotic risk and CV prognosis in patients with and without coronary artery disease [5, 7, 8, 35, 36]. Endothelial dysfunction can be regarded as a functional expression of the inherent atherosclerotic risk representing an integrated index of both the overall CV risk factor burden and the sum of all vasculoprotective factors in an individual. It may provide the link between CV risk factors and the progression of atherosclerotic disease. [37]
Previous studies have shown that most atherosclerotic plaques responsible for future acute coronary syndromes occur in lesions associated with minimal luminal stenosis. [38, 39] Lesion-related characteristics are thus crucial in determining vulnerable plaques in mild stenotic lesions and by extension identifying patients at risk of cardiac events (vulnerable patients). We have previously shown that segments of the coronary epicardial arteries with endothelial dysfunction are associated with plaque characteristics typical of vulnerable plaques [40] and with the presence of lipid core [14]. Moreover, segments with endothelial dysfunction are also those that ultimately progress to atherosclerotic plaques [41]. Thus, endothelial dysfunction may be an integral element of the evolution of the vulnerable plaque. Vulnerable plaque may be a potential mechanism by which coronary endothelial dysfunction cause increase in CV events in vulnerable patients and thus its detection may aid in more accurate risk stratification of patients those classified as having intermediate risk.
Study limitations
There were several limitations in our study. First, CV events were determined by questionnaires filled by the patients. A reviewer verified the events that occurred in our institution. We were however limited in verifying events that occurred elsewhere. Furthermore, our response rate for our questionnaires was 43% which is comparable to that of other studies but is still a limitation of this study.
Second, we did not have IVUS data on all the patients to ascribe the site of endothelial dysfunction to the culprit coronary arteries. This would have provided for a stronger link between segmental epicardial endothelial dysfunction and site coronary CV events.
Third, our study was not designed to measure if improvement in endothelial function with treatment improves the prognosis of future CV events. Patients diagnosed with endothelial dysfunction may have been provided with treatment (at their physician’s discretion), which might have improved endothelial function and led to regression to the mean of our results.
Fourth, there may have been a selection bias in the study as the patients included in the study had clinical indication for a cardiac catheterization and may potentially be different from the general population for whom FRS was intended.
Conclusion
We demonstrated that the assessment of coronary endothelial function is safe and adds value to the FRS and correctly reclassifies patients in the intermediate risk category in patients presenting with chest pain or suspected ischemia. This potentially has implications on risk management and treatment of these individual patients and could be used in further risk stratification of patients especially in the intermediate risk category with implications on risk reduction strategies.
Table 5.
Net Reclassification Improvement (NRI) for CV events with addition of CEF to FRS (N=470)
| Variable | % Reclassified |
Low <10% |
Intermediate 10% – 20% |
High >20% |
% Net correct Reclassification | NRI |
|---|---|---|---|---|---|---|
| FRS plus Microvascular CEF | ||||||
| Events | 11.5% | 7 | 54 | 0 | 0 | 0.11 |
| Non events | 21.5% | 87 | 321 | 1 | 21.2% | |
| FRS plus Epicardial CEF Events |
27.9% | 10 | 44 | 7 | −4.9% | 0.12 |
| Non events | 30.3% | 110 | 285 | 14 | 23.5% | |
| FRS plus Microvascular & Epicardial CEF Events |
26.2% | 9 | 45 | 7 | −3.3% | 0.228 |
| Non events | 31.5% | 119 | 280 | 10 | 26.6% | |
Abbreviations: CEF, Coronary endothelial function; FRS, Framingham risk score; CV, cardiovascular; NRI, Net reclassification improvement.
Acknowledgments
Sources of Funding
The study was supported by grants from the NIH: NIH K24 HL-69840, NIH R01 HL-63911, HL121561, DK100081, DK104273, HL123160,, DK 73608, and the Mayo Foundation.
Footnotes
Disclosures
None
References
- 1.Anderson JL, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(23):e179–e347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 2.D'Agostino RB, Sr, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 3.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 4(3):351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halcox JP, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 6.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 7.Suwaidi JA, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 8.Targonski PV, et al. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107(22):2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis. 2014;25(8):713–724. doi: 10.1097/MCA.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osto E, et al. Restoring the dysfunctional endothelium. Curr Pharm Des. 2007;13(10):1053–1068. doi: 10.2174/138161207780487566. [DOI] [PubMed] [Google Scholar]
- 11.Reriani M, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122(10):958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasdai D, et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96(10):3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 13.Reriani M, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 122(10):958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon MH, et al. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013;168(2):1316–1321. doi: 10.1016/j.ijcard.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori M, Kitakaze M. Adenosine, the heart, and coronary circulation. Hypertension. 1991;18(5):565–574. doi: 10.1161/01.hyp.18.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Lerman A, et al. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92(9):2426–2431. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 17.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87(5):1461–1467. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 18.Doucette JW, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85(5):1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 19.Ofili EO, Labovitz AJ, Kern MJ. Coronary flow velocity dynamics in normal and diseased arteries. Am J Cardiol. 1993;71(14):3D–9D. doi: 10.1016/0002-9149(93)90128-y. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 21.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011;30(1):22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4(3):351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brindle P, et al. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. Bmj. 2003;327(7426):1267. doi: 10.1136/bmj.327.7426.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong J, et al. Predictors of postmenopausal osteoporosis: study methods and analysis require clarification. BMJ. 2003;327(7411):392. doi: 10.1136/bmj.327.7411.392. author reply 392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menotti A, et al. Seven Countries Study. First 20-year mortality data in 12 cohorts of six countries. Ann Med. 1989;21(3):175–179. doi: 10.3109/07853898909149929. [DOI] [PubMed] [Google Scholar]
- 27.Araujo AB, et al. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol. 2010;55(4):350–356. doi: 10.1016/j.jacc.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeboah J, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland P, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 30.Jneid H, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60(7):645–681. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Qian J, et al. Safety of intracoronary Doppler flow measurement. Am Heart J. 2000;140(3):502–510. doi: 10.1067/mhj.2000.109221. [DOI] [PubMed] [Google Scholar]
- 32.Deanfield J, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Kitta Y, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53(4):323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 34.Modena MG, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40(3):505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 35.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the "vulnerable" patient. Circulation. 2004;110(14):1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 36.von Mering GO, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 37.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34(3):631–638. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 38.Ambrose JA, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12(1):56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 39.Glaser R, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111(2):143–149. doi: 10.1161/01.CIR.0000150335.01285.12. [DOI] [PubMed] [Google Scholar]
- 40.Lavi S, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95(18):1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon MH, et al. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


