Abstract
Pneumocystis jiroveci pneumonia (PJP) is associated with high morbidity and mortality after hematopoietic stem cell transplantation (HSCT). Little is known about PJP infections after HSCT because of the rarity of disease given routine prophylaxis. We report the results of a CIBMTR study evaluating the incidence, timing, prophylaxis agents, risk factors, and mortality of PJP after autologous (auto) and allogeneic (allo) HSCT. Between 1995 and 2005, 0.63% allo recipients and 0.28% auto recipients of first HSCT developed PJP. Cases occurred as early as 30 days to beyond a year after allo HSCT. A nested case cohort analysis with supplemental data (n=68 allo cases, n=111 allo controls) revealed that risk factors for PJP infection included lymphopenia and mismatch after HSCT. After allo or auto HSCT, overall survival was significantly poorer among cases vs. controls (p=0.0004). After controlling for significant variables, proportional hazards model revealed that PJP cases were 6.87 times more likely to die vs. matched controls (p<0.0001). We conclude PJP infection is rare after HSCT but is associated with high mortality. Factors associated with GVHD and with poor immune reconstitution are among the risk factors for PJP and suggest that protracted prophylaxis for PJP in high-risk HSCT recipients may improve outcomes.
Keywords: hematopoietic stem cell transplantation, engraftment, pneumocystis, prophylaxis, PCP, PJP
Introduction
Pneumocystis jiroveci (formerly Pneumocystis carinii) pneumonia (PJP), a unicellular fungal infection, is a severe infectious complication in immunocompromised hosts, including hematopoietic stem cell transplant (HSCT) recipients, often leading to fulminant respiratory failure.1–3 In immunocompromised hosts, PJP disease confers increased mortality, with published historical rates as high as 34–62%4–7, although data in the current era are unknown due to the rarity of disease with the implementation of routine PJP prophylaxis. Prior to prophylaxis, PJP disease was reported in 5–37% of HSCT patients8, 9. However, since the advent of PJP prophylaxis, this incidence has decreased, with recent reports suggesting 1–6%4, 7, 9–13 and a few reports of no cases in as many as 120 high-risk HSCT recipients14. However, with this rarity of disease, the actual incidence of PJP after HSCT remains unknown, as the largest number of PJP cases in reports is often under 10, suggesting that these data may overestimate the true incidence due to reporting bias, since cohort studies are often undertaken in the setting of outbreaks.15–17
Multiple studies have suggested that trimethoprim/sulfamethoxazole (TMP/SMX) is the drug of choice to prevent PJP in immunocompromised patients due to its efficacy against PJP and concurrent prophylaxis against other opportunistic pathogens including Toxoplasma gondii, Nocardia, and susceptible bacteria (e.g. Staphylococcus, Streptococcus pneumoniae)1, 5, 8, 10, 18. However, it is not well tolerated in HSCT recipients, with reported intolerance as high as 55% requiring discontinuation of the drug due to rash, marrow suppression, and allergy19–23. For these TMP/SMX intolerant patients, PJP prophylaxis alternative agents include: aerosolized or intravenous pentamidine, dapsone, atovaquone, clindamycin and pyrimethamine, though clindamycin and pyrimethamine have been largely abandoned due to lack of efficacy.20, 22–25 However, there is no consensus regarding efficacy of these agents in HSCT patients, nor an appropriate algorithm for choosing among these second line agents. PJP breakthrough rates are as high as 9% for aerosolized pentamidine, 7.2% for dapsone, and not well categorized for intravenous pentamidine and atovaquone, with several anecdotal reports of failures with these agents, although these account for less than 300 total reported patients26–30. As a result of these sparse data, the most recent recommendation from the Cochrane Collaboration includes only TMP/SMX and suggests continuation for at least 6 months after HSCT and continuation until discontinuation of immune suppression. 10, 31
PJP disease risk and clearance relies upon recovery of lymphocyte numbers and function. Not surprisingly, published factors linked to PJP infection after HSCT are those associated with lymphocyte impairment, including: steroids, T cell depletion in vitro or in vivo, persistent lymphopenia, immunosuppression, graft vs. host disease (GVHD), and relapse.4, 11, 32–35. The highest period of risk for PJP is thought to be from day 80 through day 270 post HSCT due to impaired lymphocyte function during this timeframe, though very early and very late cases have been described.4, 18, 36–39 While these risk factors are likely determinants of PJP disease, there are conflicting reports, and small sample size limits interpretation.
Since PJP is an uncommon event in the HSCT population, the incidence, timing, risk factors, and best prophylaxis regimens may only be addressed in a large registry study, which overcomes the limitation of disease rarity. The reported high mortality underscores the need for these data, to both determine the true mortality in a sufficiently large cohort, and reveal the population most at risk, for whom new interventions could be targeted. Thus, we interrogated the largest HSCT database, the Center for International Blood and Marrow Transplant Research (CIBMTR) registry, to identify the incidence of PJP, and then performed a nested case control study to assess risk factors and PJP-associated mortality, and to provide evidence-based data for choice of prophylaxis agents for HSCT recipients.
Methods
Data Source
The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive HSCTs to a Statistical Center located at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplantations consecutively; compliance is monitored by onsite audits. The CIMBTR maintains an extensive database of detailed patient-, transplant-, and disease-related information, and prospectively collects data longitudinally with yearly follow-ups. Observational studies conducted by the CIBMTR are performed in compliance with HIPAA regulations as a public health authority and also in compliance with all applicable federal regulations pertaining to the protection of human research participants, as determined by a continuous review by the Institutional Review Boards of NMDP and the Medical College of Wisconsin.
Patients
This study includes all patients, irrespective of age, who received HSCT for either malignant or non-malignant indications between 1995 and 2005 and identified with PJP infection within 2 years of transplantation. PJP infection was captured in the CIBMTR data forms either as an infection documented in the post-transplant period or listed as a primary or secondary cause of death. Centers report based upon organism identification and those cases reported as suspected fungal infection were excluded. Those with a history of PJP infection prior to HSCT were excluded. A subsequent analysis was performed to interrogate incidence only using the same inclusion and exclusion criteria from 2006–2012.
Analysis
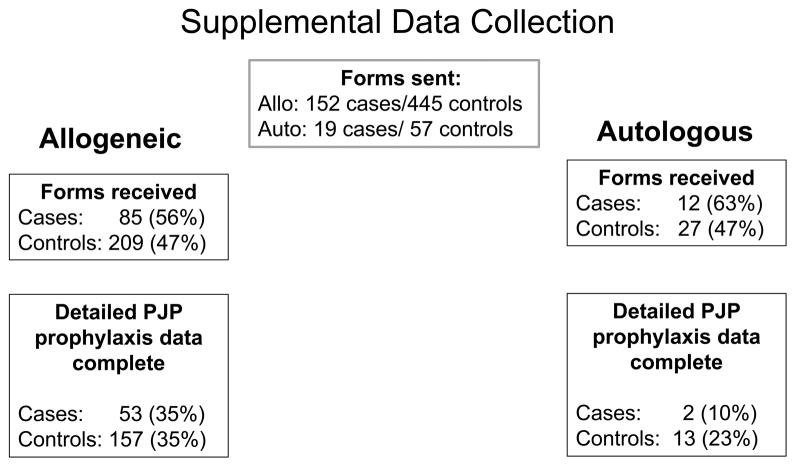
This is a nested case control cohort study to assess for clinical factors impacting development of PJP and outcomes. Controls were selected 3:1 based on 1) type of transplant (autologous or allogeneic), 2) the same duration of post-HSCT follow-up (ensuring controls are alive at time of case PJP diagnosis), and 3) the same disease indication for HSCT. A marginal proportional hazards model for clustered data was used for matching 40. Supplemental data forms were requested to evaluate PJP prophylaxis agents, concomitant neutrophil and lymphocyte counts, and methods of PJP diagnosis including autopsy, bronchoalveolar lavage, and methenamine silver. Beta-D glucan was not included as a diagnostic modality due to the study period evaluated. We received supplemental data on 97 cases (57%) and 236 controls (47%). Data forms were missing PJP prophylaxis data for 14 cases (14%) and 21 controls (9%), and the time of administration of prophylaxis was reported as unknown for 28 cases (29%) and 45 controls (19%). Therefore, detailed prophylaxis medication within the 30 days prior to the diagnosis of PJP was only available for 33% of cases and 34% of controls (Figure 1). Consequently, information on PJP prophylaxis is described but was unable to be analyzed as a risk factor for PJP.
Figure 1.
This chart summarizes the cases included in the supplemental data collection for allogeneic and autologous HSCT recipients.
The effect of PJP infection on overall survival of the entire study population was assessed using a proportional hazards model adjusting for the effect of other significant covariates including time dependent variables of PJP infection (main effect variable) and GVHD, GVHD was incorporated as a variable in the multivariable model, such that the GVHD event must have occurred prior to the PJP infection to be evaluable. This precludes studying GVHD as an outcome. Interactions between PJP infection and significant covariates were tested. Backward elimination was used to select significant covariates. Analysis was performed using SAS 9.2.
Results
Incidence and timing of PJP infection after HSCT
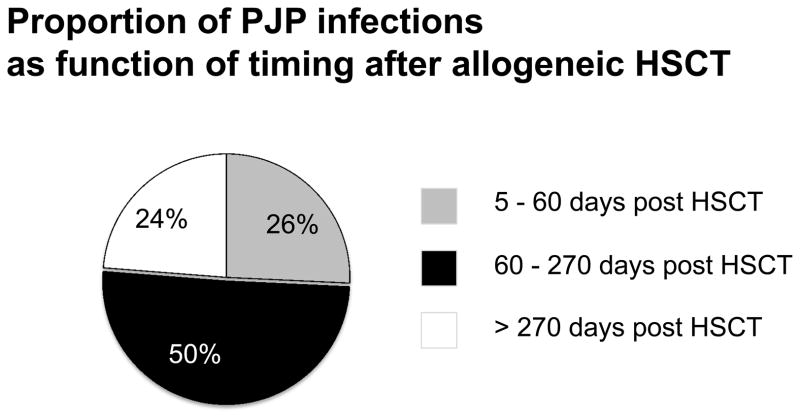
Between 1995 and 2005, the incidence of PJP was 0.63% in allogeneic recipients (n=177 cases of 27,934 total) and 0.28% in autologous recipients (n=52 cases of 18,525 total). This has not changed with time as the incidence was 0.53% in allo-recipients and 0.32% in auto-recipients from 2006 – 2012. PJP developed both early (between day 0 – 60) and late (beyond day 270) after allo-HSCT. PJP occurred at a median of 120 days (range, 2 – 620 days), with 26% of cases occurring early (median 31, range 5–56 days), 50% occurring between 60 and 270 days after HSCT (median 120, range 60–265 days), and 24% occurring late (median 342, range 279–587 days) (Figure 2). After auto HSCT, although the numbers were small, a similar trend was observed with 18% prior to day 60, 55% between day 60 and 270, and 27% after day 270. Of those that completed the secondary forms, bronchoalveolar lavage was the most common method of diagnosis for allogeneic and autologous recipients, for allogeneic recipients, this totaled 74%, though autopsy (2%), biopsy (4%), sputum (9%), imaging (9%), and methanamine silver (2%) were also reported.
Figure 2. Timing of PJP after allogeneic HSCT.
This chart shows the relationship between time after allogeneic HSCT and development of PJP disease.
Risk factors for PJP infection after HSCT
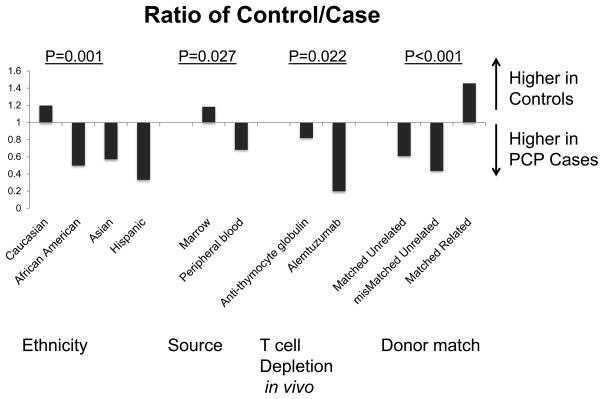
Cases and controls were similar in terms of gender, age, Karnofsky score, co-existing endocrine or pulmonary diseases, disease status at the time of transplant, and prior autologous HSCT for both autologous and allogeneic recipients (Table 1). For allogeneic recipients, sex mismatch, intensity of conditioning regimen, and CMV mismatch were not significantly different between cases and controls by univariate analysis. After auto HSCT, coexisting autoimmune disease (p<0.05) and lymphopenia (p<0.04) were more likely in cases as compared to controls. Lymphocyte number for the cases was 622 (range 0–1260) versus 1170 in controls (range 0–4758). While recent steroid use was more prevalent in cases than in controls after auto HSCT (16% vs. 2% respectively), this did not achieve statistical significance. After allo HSCT, lymphopenia (p<0.001) and use of immunosuppressive agents (p<0.001) were significantly greater in PJP cases versus controls. Lymphocyte numbers were 400 in cases (range 0–7000) versus 810 (range 0–6270) in controls. Relapse was also evaluated as a risk factor after allo HSCT; however, only 14% of patients with malignant indications for HSCT developed PJP after relapse suggesting that this was not a significant factor for the development of PJP disease. Collectively, these data suggest that PJP disease is most likely associated with impaired lymphocyte reconstitution after auto or allo HSCT. In addition, non-Caucasian (p=0.001), peripheral blood stem cell source (p=0.027), administration of GVHD prophylaxis other than tacrolimus/methotrexate (p< 0.001) and/or alemtuzumab/ATG (p=0.022), more recent year of transplant (p<0.001), and greater HLA mismatch (p<0.001) were significantly associated with PJP infection compared to controls after allo HSCT (Figure 3).
Table 1.
Risk Factors associated with PJP disease after Allo and Auto HSCT
| Variable | Case N (%) | Controls N (%) | p-value |
|---|---|---|---|
| ALLOGENEIC HSCT | |||
|
| |||
| Number of patients | 152 | 445 | |
| Gender, male | 96 (63) | 252 (57) | 0.159 |
| Age, median (range), years | 33 (<1 – 70) | 30 (<1 – 72) | 0.361 |
| Race | 0.001 | ||
| Caucasian | 107 (70) | 372 (84) | |
| African-American | 6 (4) | 9 (2) | |
| Asian | 22 (14) | 35 (8) | |
| Hispanic | 14 (9) | 14 (3) | |
| Native American | 1 (<1) | 1 (<1) | |
| Other | 2 (1) | 14 (3) | |
| Graft Type | 0.027 | ||
| Bone Marrow | 90 (59) | 311 (70) | |
| Peripheral Blood | 57 (38) | 116 (26) | |
| Cord Blood | 5 (3) | 18 (4) | |
| Sex Match (Donor/Recipient) | 0.115 | ||
| Male-Male | 50 (33) | 160 (36) | |
| Male-Female | 30 (20) | 101 (23) | |
| Female-Male | 44 (29) | 90 (20) | |
| Female-Female | 25 (16) | 91 (20) | |
| Missing | 3 (2) | 3 (<1) | |
| CMV Match (Donor/Recipient) | 0.201 | ||
| Neg/Neg | 37 (24) | 139 (31) | |
| Pos/Neg | 16 (11) | 50 (11) | |
| Neg/Pos | 35 (23) | 82 (18) | |
| Pos/Pos | 52 (34) | 155 (33) | |
| Missing | 12 (8) | 19 (4) | |
| Conditioning Intensity | 0.414 | ||
| Myeloablative | 117 (77) | 353 (79) | |
| Non-Myeloablative/Reduced | 30 (20) | 85 (19) | |
| Missing | 5 (3) | 7 (2) | |
| Degree of HLA Match | <0.001 | ||
| HLA-identical sibling | 73 (48) | 310 (70) | |
| Other related | 10 (7) | 12 (3) | |
| Well matched unrelated | 28 (18) | 51 (11) | |
| Partially matched unrelated | 24 (16) | 32 (7) | |
| Mismatched unrelated | 14 (9) | 29 (7) | |
| Missing | 3 (2) | 11 (2) | |
| Immunosuppressive agents within 30 days of PJP diagnosis* | <0.001 | ||
| None | 10 (7) | 44 (10) | |
| Yes, Steroid containing | 52 (34) | 78 (18) | |
| Yes, Non-Steroid containing | 21 (15) | 84 (19) | |
| Missing | 2 (1) | 3 (<1) | |
| GVHD Prophylaxis | <0.001 | ||
| Ex vivo T-cell depletion alone | 8 (5) | 23 (5) | |
| Ex vivo T-cell depletion + post- HSCT immune suppression | 5 (3) | 37 (8) | |
| CD34 selection alone | 2 (1) | 0 | |
| CD34 selection + post-HSCT immune suppression | 5 (3) | 5 (1) | |
| FK506 + MMF ± others | 3 (2) | 4 (<1) | |
| FK506 + MTX ± others (− MMF) | 27 (18) | 11 (2) | |
| FK506 + others (− MTX, MMF) | 3 (2) | 4 (<1) | |
| CSA + MMF ± others (− FK506) | 3 (2) | 21 (5) | |
| CSA + MTX ± others (− FK506, MMF) | 68 (45) | 259 (58) | |
| CSA + others (− FK506, MTX, MMF) | 22 (14) | 67 (15) | |
| Other GVHD prophylaxis | 6 (4) | 14 (3) | |
| Antithymocyte globulin/Alemtuzamab | 0.022 | ||
| Antithymocyte globulin only | 43 (28) | 102 (23) | |
| Alemtuzamab only | 7 (5) | 5 (1) | |
| Neither | 102 (67) | 337 (76) | |
| Missing | 0 | 1 (<1) | |
| Year of HSCT | <0.001 | ||
| 1995 – 1996 | 39 (26) | 139 (13) | |
| 1997 – 1998 | 28 (18) | 103 (23) | |
| 1999 – 2000 | 26 (17) | 54 (12) | |
| 2001 – 2002 | 20 (13) | 77 (17) | |
| 2003 – 2004 | 29 (19) | 71 (16) | |
| 2005 | 10 (7) | 1 (<1) | |
|
| |||
| AUTOLOGOUS HSCT | |||
|
| |||
| Number of patients | 19 | 57 | |
| Gender, male | 10 (53) | 28 (49) | 0.791 |
| Age, median (range), years | 58 (4 – 71) | 45 (2 – 68) | 0.060 |
| Race | 0.601 | ||
| Caucasian | 18 (95) | 50 (88) | |
| African-American | 0 | 3 (5) | |
| Asian | 0 | 1 (2) | |
| Hispanic | 1 (5) | 1 (2) | |
| Other | 0 | 2 (4) | |
| Co-existing Autoimmune disease | 0.04 | ||
| No | 17 (89) | 56 (98) | |
| Yes | 2 (11) | 0 | |
| Missing | 0 | 1 (2) | |
For those with supplemental information
Figure 3. Relative Risk (RR) of PJP control/case after allogeneic (allo) HSCT.
The relative risk of controls to PJP cases for risk factors after allo HSCT shows that non-Caucasian ethnicity, peripheral blood stem cell source, T cell depletion (TCD) in vivo and donor mismatch were high in PJP cases vs. controls were significantly associated with PJP infection compared to controls after allo HSCT.
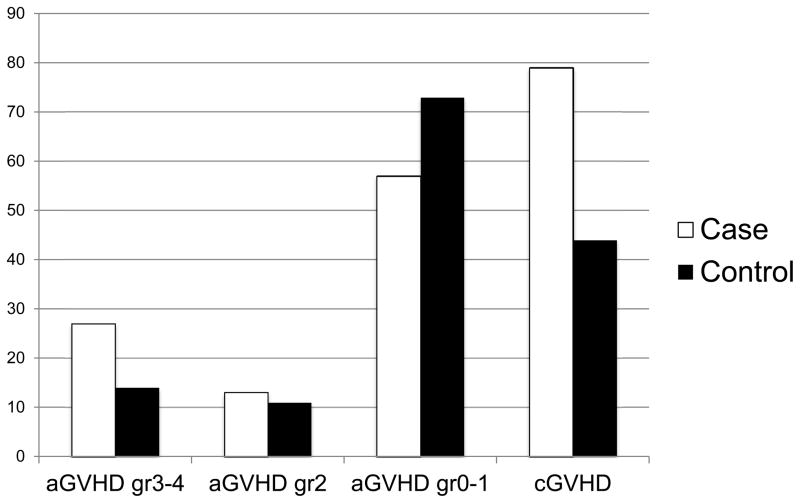
Association of GVHD with PJP infection after HSCT
For allogeneic recipients, acute and chronic GVHD was higher in PJP cases than controls. Of PJP cases, 27% had grade 3–4 acute GVHD compared to 14% of controls, with 73% of controls experiencing none or grade 1 GVHD vs. 57% of cases (Figure 4). Fifty-five percent of cases lacked chronic GVHD vs. 61% of controls. However, for patients developing PJP beyond day 270, 79% of cases experienced cGVHD vs. 44% of controls. While the transplant time period precluded analysis of severity of chronic GVHD, 33% of cases who developed PJP greater than 270 days after HSCT had been exposed to steroids prior to development of PJP, compared to 0% of controls, which could be a reflection of more significant GVHD.
Figure 4. Acute and Chronic Graft vs. Host Disease (GVHD) in PJP Cases versus Controls.
Prevalence of acute and chronic GVHD after allo HSCT in PJP cases was higher than in controls.
Association of PJP prophylaxes and risk of PJP infection
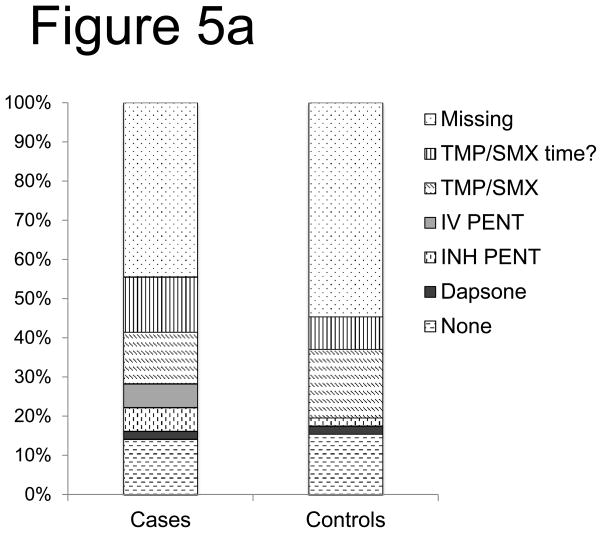
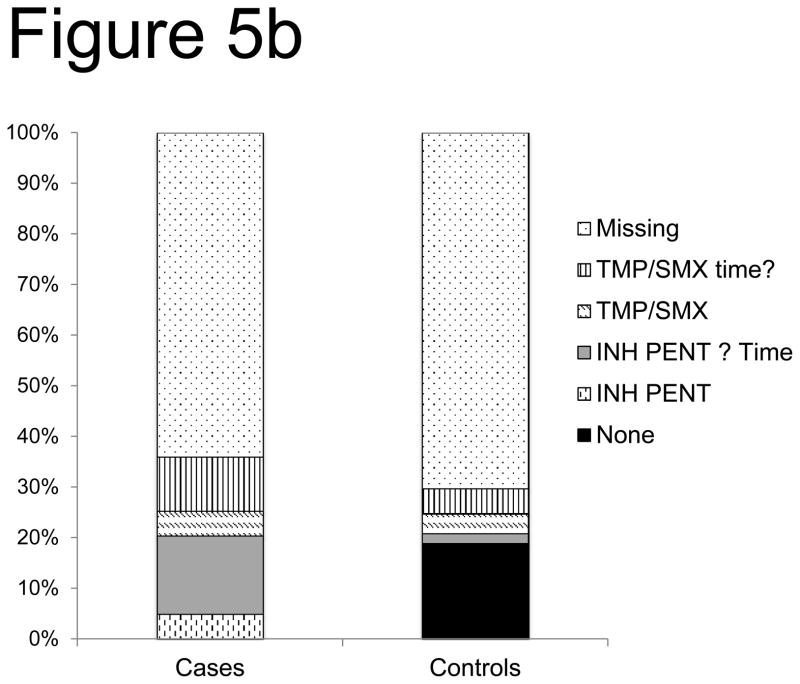
Determination of the breakthrough risk of PJP as a function of PJP prophylaxis agents was limited due to both missing and incomplete secondary forms. This large quantity of missing or incomplete data precluded statistical analyses of the association between PJP prophylaxis regimens and the risk of PJP infection. For description, ‘time unknown’ is combined with the known prophylaxis. Despite these limitations, more cases received only inhaled pentamidine for both autologous (10% of cases vs. 2% of controls) and allogeneic (20% of cases vs. 2% of controls) recipients developing PJP (Figure 5a,b). Additionally, in allogeneic recipients there was a case of IV pentamidine alone and none in controls. Allogeneic cases were more likely to be on prophylaxis greater than 60 days after HSCT-likely due to recognition of impaired immune reconstitution, though less likely to receive TMP/SMX prophylaxis. Overall, 14% of cases and 15% of controls were on no prophylaxis in the 30 days prior to onset of PJP infection.
Figure 5. PJP Prophylaxis agent association with breakthrough risk after allo-HSCT (a) and auto- HSCT (b).
Using secondary forms, the use of PJP prophylaxis agents was interrogated in PJP cases and controls after allo HSCT (a) and auto HSCT (b). Notably, missing data was included as a variable (TMP/SMX of time? = trimethoprim/sulfamethoxazole of uncertain timing in relation to PJP disease or control time point, IV PENT= intravenous pentamidine, INH = inhaled), none= the center confirmed the absence of PJP prophylaxis).
Mortality after PJP infection
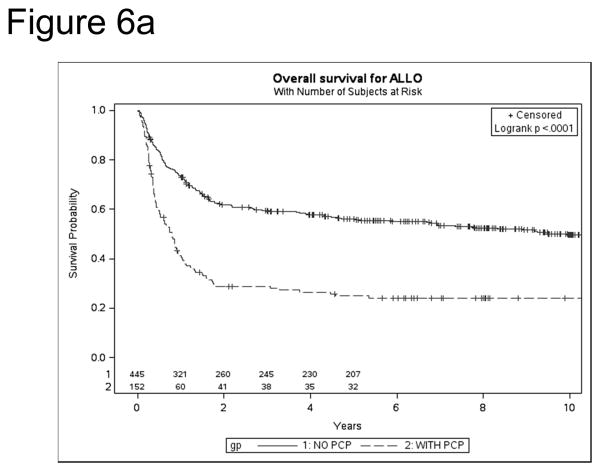
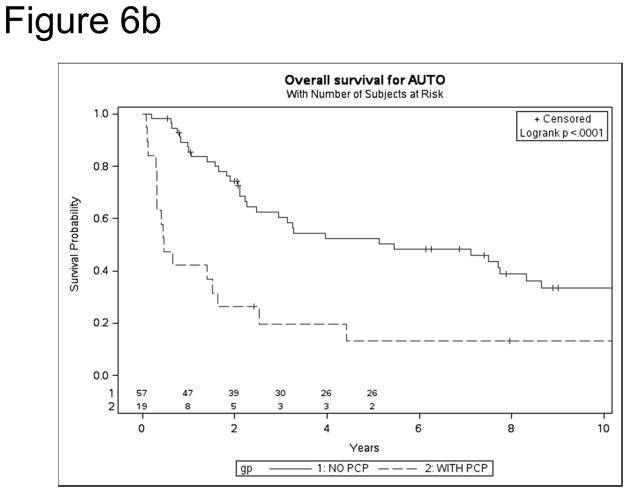
After allo HSCT, overall survival (OS) was significantly poorer among PJP cases vs. controls [1 year: 41% vs. 73% (p<0.0001); 5 year: 25% vs. 56% (p<0.0001)] (Figure 6a). Similarly, following auto HSCT, OS was lower in cases vs. controls [1year: 42% vs. 86% (p=0.0004); 5 year: 13% vs. 52% (p=0.0003)] (Figure 6b). Mortality was directly attributable to PJP in nearly a quarter of HSCT recipients [auto, 24%, allo 23%]. For allo-HSCT cases, other infections were the largest second contributor to mortality (21%). In auto HSCT cases, recurrent disease accounted for 43% of deaths in PJP cases and 69% of controls. After controlling for significant variables (age, mismatch, race, GVHD prophylaxis, year of HSCT, aGVHD), PJP cases had 6.87-fold increase in mortality (p<0.0001, 95% CI 4.84–9.75, p<0.0001).
Figure 6.
Kaplan Meier survival for PJP cases and controls after allo (a) and auto (b) HSCT
Discussion
We have evaluated the incidence, risk factors, timing and mortality for PJP in HSCT recipients using a large HSCT registry. The CIBMTR database included over 50,000 allogeneic recipients at the time of the data query, overcoming common limitations of prior studies, including those of small patient numbers and single institution data. We show that the incidence of PJP following allogeneic and autologous HSCT is much less common than prior publications have suggested. This lower rate is likely due to freedom from publication bias; centers were more likely to publish their findings after an outbreak of PJP, enriching for this rare disease. This suggestion is supported by the fact that large multi-year studies including all infections affecting HSCT recipients often report lower rates of PJP infections as compared to smaller PJP-focused studies (1% vs. 2.5–6%)4, 7, 9–13. This is further supported by the fact that although PJP infections were initially thought to represent an opportunistic event related to chronic colonization, genotyping of P. jiroveci in clusters has confirmed that de novo outbreaks do occur in immunosuppressed patients, and could prompt these publications.1, 15–17, 41 Interestingly, despite the increased sensitivity of disease detection including the use of beta-D-glucan testing,42–48 and improving survival of severely immunosuppressed patients, the incidence of PJP after HSCT has not increased over time, consistent with the hypothesis that prophylaxes are protective in HSCT patients8, 20, 26, 49–53. Notably, our data are unable to assess for a true incidence of PJP in patients on any prophylaxis (i.e. breakthrough PJP) due to the nested case cohort study design. However, since similar numbers of cases and controls (14 – 15%) within the subset of patients with supplemental data were not on prophylaxis, our estimated overall incidence of PJP following allogeneic and autologous HSCT are a reasonable estimate of the true incidence of PJP after HSCT.
Our data show that PJP infection occurred both early and late after HSCT. Prior reports have often highlighted the period between day 80–270 after HSCT as the time of highest risk, with some including up to 1 year18, 36. In contrast, our data reveal 26% of cases occur before day 60, with the earliest event just 5 days after HSCT.18, 36 Current practice often involves PJP prophylaxis before or during the preparative regimen, to ‘diminish PJP burden’, and re-institution of prophylaxis after counts have recovered due to medication intolerance (e.g. blood count suppression with TMP/SMX, intolerance for inhaled pentamidine) or challenges with oral absorption (atovaquone).31, 54 In addition, while 50% of cases did occur in the period that has been most reported most often previously as the period of highest risk (day 80–270), 24% occurred after day 270, with a median of nearly a year, suggesting that the recommendation of a year of prophylaxis may be insufficient. These data suggest that PJP prophylaxis should be started early and continued late (beyond a year)—at least for patients at highest risk for this disease, with the caveat that these suggestions are limited by the retrospective nature of this study.
This study identifies risk factors for PJP disease, including some of those reported in prior studies such as: corticosteroid exposure, in vivo or in vitro T cell depletion, lymphopenia, immunosuppression, and GVHD.4, 11, 32–35, 39, 55 After auto HSCT, factors associated with ongoing immunosuppression, such as co-existing autoimmune disease and lymphopenia, were higher in PJP patients, although, a previously identified risk factor—steroids did not achieve statistical significance as a risk factor for PJP disease. This likely reflects the small sample size, as steroids were more common in the PJP diseased cohort. After allo HSCT, factors associated with impaired immune reconstitution (steroid exposure, lymphopenia, neutropenia) and/or GVHD, non-Caucasian decent, peripheral blood stem cell graft, receipt of either GVHD prophylaxis other than tacrolimus/methotrexate and/or alemtuzumab/ATG, and greater HLA mismatch were more common in PJP patients as compared to controls. This is not surprising as PJP eradication relies upon functional CD4 immunity, which is impaired in the setting of GVHD and steroid exposure. As anticipated from these data, lymphopenia as well as both acute and chronic GVHD were greater in recipients developing PJP. Both the aberrant lymphocyte response to infection in patients with GVHD and the effect of immune suppressive therapy, likely contribute to the heightened risk for opportunistic infections. In addition, impaired immune reconstitution can occur in the absence of these overt risk factors, due to absence of thymus reconstitution and/or the use of HLA-mismatched grafts, which may demonstrate absent CD4 function with or without normal peripheral counts.56 Together, these data suggest that those HSCT patients with impaired immunity (from GVHD, steroids, T cell antibody therapy, or other factors predictive of T cell dysfunction), should start PJP prophylaxis early and continue on such prophylaxis until functional immunity is restored—evidenced both by CD4 reconstitution and functional immunity via vaccine response or evidence of thymus reconstitution.
While missing data preclude full analysis of the relative efficacy of PJP prophylaxis agents, our data do provide some possible insights into this important question. Most surprisingly, approximately 15% of cases and controls were on no agent for PJP prophylaxis following allogeneic HSCT. This could suggest either patients did not adhere to the recommended regimen or because physician practice did not include PJP prophylaxis, which would be particularly important in high risk patients at high risk time frames after HSCT. Not surprisingly, after auto HSCT, more controls were on no prophylaxis, likely reflecting remission, and/or absence of immune suppression. In both auto and allo patients, there were more cases with PJP vs. controls receiving pentamidine, which has been previously suggested in one of the largest investigations of PJP disease in allo HSCT recipients.23 Data from non-HSCT diseases have shown higher breakthrough rates for pentamidine as well, leading to recommendations of greater than the third line choice for pentamidine for PJP prophylaxis.31, 34 Although one single center study suggests that intravenous pentamidine may provide efficacy, this was published during an outbreak of cases (with cases occurring despite known adherence to the pentamidine regimen), with unclear generalizability or efficacy in light of the rarity we report here. Further, data show that the intravenous route does not result in high intra-alveolar concentrations of drug, providing poor protection for high risk patients.57, 58 In our study, there were similar cases and controls on dapsone (though small numbers), which may reflect the high rate of intolerance of this agent (greater than 40%) and concern regarding toxicities such as aplastic anemia and agranulocytosis, precluding interpretation of efficacy. 21, 22, 27, 59 There were no cases or controls on atovaquone, though this may simply reflect the era of evaluation, given that atovaquone has only recently been incorporated in recommendations and requires fatty foods and oral absorption, limiting its use in HSCT patients.31 Breakthroughs were also observed with the best prophylaxis agent, TMP/SMX, which may reflect decreased adherence, frequency of dosing, or resistance of the organism, which is difficult to prove and often unknown.60, 61 Emerging, albeit anecdotal, data, have recently reported the activity of caspofungin against PJP62, though after the time frame of the current analysis. In summary, while these data are limited due to missing or incomplete secondary forms, our data would support that of prior publications that TMP/SMX be strongly recommended and careful consideration regarding cessation as no other alternative agent has been shown to be equally effective. Before stopping this medication for count suppression or rashes, perhaps, we recommend that TMP/SMX prophylaxis should be reconsidered in high risk patients with growth factor support or desensitization utilized whenever possible, as has been recommended by the recent international guidelines for PJP prophylaxis.19, 31
Mortality was high in patients with PJP disease, with nearly 7-fold increased risk of death compared to matched controls. The absolute overall survival matches that previously published for early time points, approximately 40% at 2 years4, 5 and decreased with time to < 25% at 5 years after allogeneic HSCT, and even lower for autologous recipients at 5 years. While this is compelling data that PJP portends poor survival, it is possible that PJP is a surrogate marker for other high-risk predictors of mortality due to impaired immunity or relapse as well.
There were several notable limitations of our study. The analyses addressing mortality, timing, and associated risk factors including therapeutic prevention, were limited by missing data due to secondary forms that were incomplete or not returned. Furthermore, limitations to our data include those of all registry studies, where data rely upon accurate capture of events into the database and that only a small proportion are audited for accuracy, thus possibly resulting in underestimation of the true incidence especially as time from HSCT increases and visits to the primary HSCT center decline. This could lead to intentional or accidental underreporting, either from a desire to report positive results or due to missed data that was not captured, though one would postulate that both the anonymity of these data and the onus to responsibly report would drive accurate reporting and minimize this risk. Because the transplant center is more likely to be contacted for sick patients than healthy controls, there may be increasing selection bias with longer time from transplantation as well.
In summary, our data demonstrate that the incidence of PJP disease is very low in autologous and allogeneic HSCT recipients, and most common among recipients with poor immune reconstitution, including those with mismatched grafts and GVHD. Our data demonstrate that PJP infection may occur at any time after HSCT and confers a high risk of mortality. In addition, these data suggest that TMP/SMX remains the most effective prophylactic drug. We provide guidance about duration of prophylaxis during periods of immune compromise, including very early and very late after HSCT.
Table 2.
Known PJP prophylaxis therapies and development of PJP as a function of time after allo HSCT
| Time after HSCT | ||||||
|---|---|---|---|---|---|---|
| <60 days | 60–270 days | >270 days | ||||
| Proportion of PJP cases | 26% | 51% | 24% | |||
| Cases | Controls | Cases | Controls | Cases | Controls | |
| Bactrim | 36% | 35% | 14% | 43% | 11% | 21% |
| Prophylaxis use | ||||||
| No prophylaxis | 7% | 35% | 34% | 20% | 26% | 50% |
| Dapsone | 0% | 0% | 0% | 0% | 0% | 1% |
| Pentamidine as part of regimen | 14% | 8% | 17% | 4% | 0% | 0% |
| Missing data | 21% | 13% | 11% | 6% | 5% | 9% |
Above is a table showing the proportion of cases and controls who received these known prophylaxis regimens in relation to the timing of the case onset of PJP. Notably, the pentamidine data could include inhaled or intravenous medication. Time unknown data was excluded from this table (though included in figures 4a and b) and missing data shows the forms returned without this prophylaxis section completed.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Conflict of Interest Statement: There are no relevant conflicts of interest to disclose.
References
- 1.Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Seminars in Respiratory and Critical Care Medicine. 2011;32(6):775–782. doi: 10.1055/s-0031-1295725. [DOI] [PubMed] [Google Scholar]
- 2.Wakefield AE, Peters SE, Banerji S, Bridge PD, Hall GS, Hawksworth DL, et al. Pneumocystis carinii shows DNA homology with the ustomycetous red yeast fungi. Molecular Microbiology. 1992;6(14):1903–1911. doi: 10.1111/j.1365-2958.1992.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 3.Gluck T, Geerdes-Fenge HF, Straub RH, Raffenberg M, Lang B, Lode H, et al. Pneumocystis carinii pneumonia as a complication of immunosuppressive therapy. Infection. 2000;28(4):227–230. doi: 10.1007/s150100070041. [DOI] [PubMed] [Google Scholar]
- 4.Tuan IZ, Dennison D, Weisdorf DJ. Pneumocystis carinii pneumonitis following bone marrow transplantation. Bone Marrow Transplantation. 1992;10(3):267–272. [PubMed] [Google Scholar]
- 5.Wazir JF, Ansari NA. Pneumocystis carinii infection. Update and review Archives of Pathology & Laboratory Medicine. 2004;128(9):1023–1027. doi: 10.5858/2004-128-1023-PCI. [DOI] [PubMed] [Google Scholar]
- 6.Torres HA, Chemaly RF, Storey R, Aguilera EA, Noqueras GM, Safdar A, et al. Influence of type of cancer and hematopoietic stem cell transplantation on clinical presentation of Pneumocystis jiroveci pneumonia in cancer patients. European Journal of Clinical Microbiology & Infectious Diseases : Official Publication of the European Society of Clinical Microbiology. 2006;25(6):382–388. doi: 10.1007/s10096-006-0149-4. [DOI] [PubMed] [Google Scholar]
- 7.Yoo JH, Lee DG, Choi SM, Choi JH, Park YH, Kim YJ, et al. Infectious complications and outcomes after allogeneic hematopoietic stem cell transplantation in Korea. Bone Marrow Transplantation. 2004;34(6):497–504. doi: 10.1038/sj.bmt.1704636. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clinical Microbiology Reviews. 2004;17(4):770–782. doi: 10.1128/CMR.17.4.770-782.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. Journal of Infection and Chemotherapy : Official Journal of the Japan Society of Chemotherapy. 2012;18(6):793–806. doi: 10.1007/s10156-012-0453-0. [DOI] [PubMed] [Google Scholar]
- 10.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. The Cochrane Database of Systematic Reviews. 2007;(3):CD005590. doi: 10.1002/14651858.CD005590.pub2. [DOI] [PubMed] [Google Scholar]
- 11.De Castro N, Neuville S, Sarfati C, Ribaud P, Derouin F, Gluckman E, et al. Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplantation. 2005;36(10):879–883. doi: 10.1038/sj.bmt.1705149. [DOI] [PubMed] [Google Scholar]
- 12.Mikaelsson L, Jacobsson G, Andersson R. Pneumocystis pneumonia--a retrospective study 1991–2001 in Gothenburg, Sweden. The Journal of infection. 2006;53(4):260–265. doi: 10.1016/j.jinf.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Kruger W, Russmann B, Kroger N, Salomon C, Ekopf N, Elsner HA, et al. Early infections in patients undergoing bone marrow or blood stem cell transplantation--a 7 year single centre investigation of 409 cases. Bone Marrow Transplantation. 1999;23(6):589–597. doi: 10.1038/sj.bmt.1701614. [DOI] [PubMed] [Google Scholar]
- 14.Tomonari A, Takahashi S, Ooi J, Tsukada N, Konuma T, Kato S, et al. No occurrence of Pneumocystis jiroveci (carinii) pneumonia in 120 adults undergoing myeloablative unrelated cord blood transplantation. Transplant Infectious Disease : an Official Journal of the Transplantation Society. 2008;10(5):303–307. doi: 10.1111/j.1399-3062.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 15.Chapman JR, Marriott DJ, Chen SC, MacDonald PS. Post-transplant Pneumocystis jirovecii pneumonia--a re-emerged public health problem? Kidney International. 2013;84(2):240–243. doi: 10.1038/ki.2013.212. [DOI] [PubMed] [Google Scholar]
- 16.Nankivell BJ, Firacative C, Kable K, Chen SC, Meyer W. Molecular epidemiology linking multihospital clusters of opportunistic Pneumocystis jirovecii pneumonia. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2013;57(7):1058–1059. doi: 10.1093/cid/cit413. [DOI] [PubMed] [Google Scholar]
- 17.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clinical Microbiology Reviews. 2012;25(2):297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dykewicz CA. Preventing opportunistic infections in bone marrow transplant recipients. Transplant Infectious Disease : an Official Journal of the Transplantation Society. 1999;1(1):40–49. doi: 10.1034/j.1399-3062.1999.10105.x. [DOI] [PubMed] [Google Scholar]
- 19.Fontanet ACY, Roosnek E, Mohty B, Passweg JR. Cotrimoxazole myelotoxicity in hematopoietic SCT recipients: time for reappraisal. Bone Marrow Transplantation. 2011;46(9):1272–1273. doi: 10.1038/bmt.2010.285. [DOI] [PubMed] [Google Scholar]
- 20.Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T. A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplantation. 1999;24(8):897–902. doi: 10.1038/sj.bmt.1702004. [DOI] [PubMed] [Google Scholar]
- 21.Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2001;33(8):1397–1405. doi: 10.1086/323129. [DOI] [PubMed] [Google Scholar]
- 22.Sangiolo D, Storer B, Nash R, Corey L, Davis C, Flowers M, et al. Toxicity and efficacy of daily dapsone as Pneumocystis jiroveci prophylaxis after hematopoietic stem cell transplantation: a case-control study. Biology of Blood and Marrow Transplantation : Journal of the American Society for Blood and Marrow Transplantation. 2005;11(7):521–529. doi: 10.1016/j.bbmt.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Marras TK, Sanders K, Lipton JH, Messner HA, Conly J, Chan CK. Aerosolized pentamidine prophylaxis for Pneumocystis carinii pneumonia after allogeneic marrow transplantation. Transplant Infectious Disease : an Official Journal of the Transplantation Society. 2002;4(2):66–74. doi: 10.1034/j.1399-3062.2002.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh M, Chandrasekar PH. Effective alternates to trimethoprim-sulfamethoxazole as antimicrobial prophylaxis in stem cell recipients: are there any? Pediatric Transplantation. 2008;12(8):823–826. doi: 10.1111/j.1399-3046.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 25.Utili R, Durante-Mangoni E, Basilico C, Mattei A, Ragone E, Grossi P. Efficacy of caspofungin addition to trimethoprim-sulfamethoxazole treatment for severe pneumocystis pneumonia in solid organ transplant recipients. Transplantation. 2007;84(6):685–688. doi: 10.1097/01.tp.0000280546.91617.6c. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez M, Sifri CD, Fishman JA. Failure of low-dose atovaquone prophylaxis against Pneumocystis jiroveci infection in transplant recipients. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2004;38(8):e76–8. doi: 10.1086/383150. [DOI] [PubMed] [Google Scholar]
- 27.Maltezou HC, Petropoulos D, Choroszy M, Gardner M, Mantzouranis EC, Rolston KV, et al. Dapsone for Pneumocystis carinii prophylaxis in children undergoing bone marrow transplantation. Bone Marrow Transplantation. 1997;20(10):879–881. doi: 10.1038/sj.bmt.1700978. [DOI] [PubMed] [Google Scholar]
- 28.Vasconcelles MJ, Bernardo MV, King C, Weller EA, Antin JH. Aerosolized pentamidine as pneumocystis prophylaxis after bone marrow transplantation is inferior to other regimens and is associated with decreased survival and an increased risk of other infections. Biology of Blood and Marrow Transplantation : Journal of the American Society for Blood and Marrow Transplantation. 2000;6(1):35–43. doi: 10.1016/s1083-8791(00)70050-4. [DOI] [PubMed] [Google Scholar]
- 29.Souza JP, Boeckh M, Gooley TA, Flowers ME, Crawford SW. High rates of Pneumocystis carinii pneumonia in allogeneic blood and marrow transplant recipients receiving dapsone prophylaxis. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 1999;29(6):1467–1471. doi: 10.1086/313509. [DOI] [PubMed] [Google Scholar]
- 30.Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Seminars in Respiratory and Critical Care Medicine. 2006;27(3):297–309. doi: 10.1055/s-2006-945530. [DOI] [PubMed] [Google Scholar]
- 31.Gea-Banacloche J, Masur H, Arns da Cunha C, Chiller T, Kirchhoff LV, Shaw P, et al. Regionally limited or rare infections: prevention after hematopoietic cell transplantation. Bone Marrow Transplantation. 2009;44(8):489–494. doi: 10.1038/bmt.2009.260. [DOI] [PubMed] [Google Scholar]
- 32.Sepkowitz KA, Brown AE, Telzak EE, Gottlieb S, Armstrong D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA : the Journal of the American Medical Association. 1992;267(6):832–837. [PubMed] [Google Scholar]
- 33.Russian DA, Levine SJ. Pneumocystis carinii pneumonia in patients without HIV infection. The American Journal of the Medical Sciences. 2001;321(1):56–65. doi: 10.1097/00000441-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR, et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Medecine et Maladies Infectieuses. 2014 doi: 10.1016/j.medmal.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Worth LJ, Dooley MJ, Seymour JF, Mileshkin L, Slavin MA, Thursky KA. An analysis of the utilisation of chemoprophylaxis against Pneumocystis jirovecii pneumonia in patients with malignancy receiving corticosteroid therapy at a cancer hospital. British Journal of Cancer. 2005;92(5):867–872. doi: 10.1038/sj.bjc.6602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahuja J, Kanne JP. Thoracic infections in immunocompromised patients. Radiologic Clinics of North America. 2014;52(1):121–136. doi: 10.1016/j.rcl.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Seo S, Kanda Y, Shoji N, Ogasawara T, Murakami J, et al. Early onset Pneumocystis carinii pneumonia after allogeneic peripheral blood stem cell transplantation. American Journal of Hematology. 2001;67(3):206–209. doi: 10.1002/ajh.1109. [DOI] [PubMed] [Google Scholar]
- 38.Bjorklund A, Aschan J, Labopin M, Remberger M, Ringden O, Winiarski J, et al. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplantation. 2007;40(11):1055–1062. doi: 10.1038/sj.bmt.1705856. [DOI] [PubMed] [Google Scholar]
- 39.Roblot F, Le Moal G, Kauffmann-Lacroix C, Bastides F, Boutoille D, Verdon R, et al. Pneumocystis jirovecii pneumonia in HIV-negative patients: a prospective study with focus on immunosuppressive drugs and markers of immune impairment. Scandinavian Journal of Infectious Diseases. 2014;46(3):210–214. doi: 10.3109/00365548.2013.865142. [DOI] [PubMed] [Google Scholar]
- 40.Lee EW, Wei LJ, Amato DA. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. In: Goel JPKaPK., editor. Survival Analysis: State of the Art. Kluwer Academic Publishers; Dordrecht, Netherlands: 1992. pp. 237–247. [Google Scholar]
- 41.Olsson M, Eriksson BM, Elvin K, Strandberg M, Wahlgren M. Genotypes of clustered cases of Pneumocystis carinii pneumonia. Scandinavian Journal of Infectious Diseases. 2001;33(4):285–289. doi: 10.1080/003655401300077324. [DOI] [PubMed] [Google Scholar]
- 42.Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-beta-D-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. Journal of Clinical Microbiology. 2013;51(10):3380–3388. doi: 10.1128/JCM.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteves F, Lee CH, de Sousa B, Badura R, Seringa M, Fernandes C, et al. (1-3)-Beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. European Journal of Clinical Microbiology & Infectious Diseases : Official Publication of the European Society of Clinical Microbiology. 2014;33(7):1173–1180. doi: 10.1007/s10096-014-2054-6. [DOI] [PubMed] [Google Scholar]
- 44.Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PloS One. 2013;8(9):e73099. doi: 10.1371/journal.pone.0073099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maillet M, Maubon D, Brion JP, Francois P, Molina L, Stahl JP, et al. Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients. European Journal of Clinical Microbiology & Infectious Diseases : Official Publication of the European Society of Clinical Microbiology. 2014;33(3):331–336. doi: 10.1007/s10096-013-1960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumura Y, Ito Y, Yamamoto M, Matsushima A, Nagao M, Takakura S, et al. Pneumocystis polymerase chain reaction and blood (1-->3)-beta-D-glucan assays to predict survival with suspected Pneumocystis jirovecii pneumonia. Journal of Infection and Chemotherapy : Official Journal of the Japan Society of Chemotherapy. 2014;20(2):109–114. doi: 10.1016/j.jiac.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Olsson M, Stralin K, Holmberg H. Clinical significance of nested polymerase chain reaction and immunofluorescence for detection of Pneumocystis carinii pneumonia. Clinical Microbiology and Infection : the Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2001;7(9):492–497. doi: 10.1046/j.1469-0691.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 48.Tasaka S, Tokuda H. Recent advances in the diagnosis of Pneumocystis jirovecii pneumonia in HIV-infected adults. Expert Opinion on Medical Diagnostics. 2013;7(1):85–97. doi: 10.1517/17530059.2012.722080. [DOI] [PubMed] [Google Scholar]
- 49.Coyle PV, McCaughey C, Nager A, McKenna J, O’Neill H, Feeney SA, et al. Rising incidence of Pneumocystis jirovecii pneumonia suggests iatrogenic exposure of immune-compromised patients may be becoming a significant problem. Journal of Medical Microbiology. 2012;61(Pt 7):1009–1015. doi: 10.1099/jmm.0.043984-0. [DOI] [PubMed] [Google Scholar]
- 50.Maini R, Henderson KL, Sheridan EA, Lamagni T, Nichols G, Delpech V, et al. Increasing Pneumocystis pneumonia, England, UK, 2000–2010. Emerging Infectious Diseases. 2013;19(3):386–392. doi: 10.3201/eid1903.121151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madden RM, Pui CH, Hughes WT, Flynn PM, Leung W. Prophylaxis of Pneumocystis carinii pneumonia with atovaquone in children with leukemia. Cancer. 2007;109(8):1654–1658. doi: 10.1002/cncr.22562. [DOI] [PubMed] [Google Scholar]
- 52.Meyers B, Borrego F, Papanicolaou G. Pneumocystis carinii pneumonia prophylaxis with atovaquone in trimethoprim-sulfamethoxazole-intolerant orthotopic liver transplant patients: a preliminary study. Liver Transplantation : Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001;7(8):750–751. doi: 10.1053/jlts.2001.26433. [DOI] [PubMed] [Google Scholar]
- 53.Shankar SM, Nania JJ. Management of Pneumocystis jiroveci pneumonia in children receiving chemotherapy. Paediatric Drugs. 2007;9(5):301–309. doi: 10.2165/00148581-200709050-00003. [DOI] [PubMed] [Google Scholar]
- 54.Neumann S, Krause SW, Maschmeyer G, Schiel X, von Lilienfeld-Toal M. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematological malignancies and solid tumors : guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Annals of Hematology. 2013;92(4):433–442. doi: 10.1007/s00277-013-1698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overgaard UM, Helweg-Larsen J. Pneumocystis jiroveci pneumonia (PCP) in HIV-1-negative patients: a retrospective study 2002–2004. Scandinavian Journal of Infectious Diseases. 2007;39(6–7):589–595. doi: 10.1080/00365540601150497. [DOI] [PubMed] [Google Scholar]
- 56.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Seminars in Immunology. 2007;19(5):318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SY, Dabb AA, Glenn DJ, Snyder KM, Chuk MK, Loeb DM. Intravenous pentamidine is effective as second line Pneumocystis pneumonia prophylaxis in pediatric oncology patients. Pediatric Blood & Cancer. 2008;50(4):779–783. doi: 10.1002/pbc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debs RJ, Straubinger RM, Brunette EN, Lin JM, Lin EJ, Montgomery AB, et al. Selective enhancement of pentamidine uptake in the lung by aerosolization and delivery in liposomes. The American Review of Respiratory Disease. 1987;135(3):731–737. doi: 10.1164/arrd.1987.135.3.731. [DOI] [PubMed] [Google Scholar]
- 59.Williams S, MacDonald P, Hoyer JD, Barr RD, Athale UH. Methemoglobinemia in children with acute lymphoblastic leukemia (ALL) receiving dapsone for pneumocystis carinii pneumonia (PCP) prophylaxis: a correlation with cytochrome b5 reductase (Cb5R) enzyme levels. Pediatric Blood & Cancer. 2005;44(1):55–62. doi: 10.1002/pbc.20164. [DOI] [PubMed] [Google Scholar]
- 60.Queener SF, Cody V, Pace J, Torkelson P, Gangjee A. Trimethoprim resistance of dihydrofolate reductase variants from clinical isolates of Pneumocystis jirovecii. Antimicrobial Agents and Chemotherapy. 2013;57(10):4990–4998. doi: 10.1128/AAC.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caselli D, Petris MG, Rondelli R, Carraro F, Colombini A, Muggeo P, et al. Single-day trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis pneumonia in children with cancer. The Journal of Pediatrics. 2014;164(2):389–392 e1. doi: 10.1016/j.jpeds.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Link H, Vohringer HF, Wingen F, Bragas B, Schwardt A, Ehninger G. Pentamidine aerosol for prophylaxis of Pneumocystis carinii pneumonia after BMT. Bone Marrow Transplantation. 1993;11(5):403–406. [PubMed] [Google Scholar]