Abstract
Abdominal aortic aneurysm is a multifactorial disease that is a leading cause of death in developed countries. Matrix-metalloproteases (MMPs) are part of the disease process, however, assessing their role in disease initiation and progression has been difficult and animal models have become essential. Combining Förster resonance energy transfer (FRET) proteolytic beacons activated in the presence of MMPs with 2-photon microscopy allows for a novel method of evaluating MMP activity within the extracellular matrix (ECM). Single and 2-photon spectra for proteolytic beacons were determined in vitro. Ex vivo experiments using the apolipoprotein E knockout angiotensin II-infused mouse model of aneurysm imaged ECM architecture simultaneously with the MMP-activated FRET beacons. 2-photon spectra of the two-color proteolytic beacons showed peaks for the individual fluorophores that enable imaging of MMP activity through proteolytic cleavage. Ex vivo imaging of the beacons within the ECM revealed both microstructure and MMP activity. 2-photon imaging of the beacons in aneurysmal tissue showed an increase in proteolytic cleavage within the ECM (p < 0.001), thus indicating an increase in MMP activity. Our data suggest that FRET-based proteolytic beacons show promise in assessing MMP activity within the ECM and will therefore allow future studies to identify the heterogeneous distribution of simultaneous ECM remodeling and protease activity in aneurysmal disease.
Keywords: AAA, MMP, ApoE, FRET, multiphoton
Introduction
Abdominal Aortic Aneurysm (AAA) has become an increasingly prevalent disease in patients over 65 years of age in the United States, with over 12% of men and 5% of women affected by the disease (Go et al., 2013). Although rates of AAA have declined in some European and other nations (Lederle, 2011), rates have stayed constant in the United States at about 45,000 cases a year, with 10,000 deaths annually (Dua et al., 2014). Furthermore, though mortality rates have fallen in recent years, AAAs still have a mortality rate that can be up to 85% and even over 50% when rupture occurs in a medical setting. In addition, AAAs have been an extenuating factor in over 35,000 deaths in the past 2 years and are responsible for nearly 150,000 hospitalizations, each costing on average nearly $60,000, in the same time period (Weintraub 2009; Kent et al., 2010; Roger et al., 2012; Go et al., 2013; Dua et al., 2014).
Although a definitive cause for AAA remains unknown, it is generally accepted that the pathogenesis of AAA is combinatorial in nature involving several factors including atherosclerosis, inflammation, hemodynamics, smoking status, gender, age, and genetic predisposition (Vorp & Geest, 2005; Vorp, 2007). One of the reasons that so little is known about the initiation and progression of AAA disease is that data on the mechanobiology of the disease are limited to that collected at one time point, typically from a sample acquired during an open surgical repair of an already mature (large) AAA.
In order to overcome the challenges of studying AAA initiation and progression in humans, an apolipoprotein E knockout (ApoE−/−) angiotensin II (AngII)-infused mouse model has been developed that produces an aortic aneurysm from a focal breakage point in the medial elastin separating the adventitial and medial layers (Goergen et al., 2011). This animal model has become prominent for the study of AAA as it displays many of the same histological and biological characteristics as native human AAA, including atherosclerotic plaque formation, medial degeneration, thrombus formation, and an increase in matrix-metalloprotease (MMP) activity (Daugherty et al., 2000; Daugherty et al., 2006). This model has allowed recent work to focus on understanding the underlying mechanisms of disease initiation and development, specifically the role of increased MMP production in aneurysmal tissue that appears to be associated with extensive remodeling of the extracellular matrix (ECM) (Freestone et al., 1995; Thompson et al., 1995; Curci et al., 1998a; Davis et al., 1998; Longo et al., 2002).
Although MMPs are known to be up-regulated in both animal and human AAA (Thompson et al., 1995; Curci et al., 1998a, 1998b; Thompson & Baxter, 1999; Baxter et al., 2002; Thompson et al., 2002; Daugherty & Cassis, 2004a, 2004b; Vorp & Geest, 2005; Bartoli et al., 2006; Thompson et al., 2006; Vorp, 2007; Baxter et al., 2008), the spatial distribution of their activity and how this correlates with AAA initiation and progression has yet to be quantified. In order to address this issue, recent work has used molecular imaging agents to study the spatial distribution of biological events in mouse AAA (Frangioni, 2003; Miyama et al., 2012; Ramaswamy et al., 2013). One novel imaging technique that has the potential to provide spatial in vivo imaging of MMP activity, developed for imaging tumors in a mouse model, involves the use of Förster resonance energy transfer (FRET)-based proteolytic beacons (PBs) (McIntyre & Matrisian, 2003; McIntyre et al., 2004; McIntyre & Matrisian, 2009; McIntyre et al., 2010). However, this modality has yet to be used in a model of AAA, nor has it been combined with 2-photon (2P) imaging that would also provide imaging of the ECM [e.g., using a nonlinear optical technique known as second harmonic generation (SHG) and 2P excited fluorescence (2PEF)]. 2P imaging also has the benefits of superior image quality, deep optical sectioning, and reduced photo damage to tissue. Combining PBs with 2P imaging offers the ability to relate local MMP activity to local ECM remodeling.
The spatial distribution of MMP activity in the ApoE−/− AngII-infused mouse model of AAA has yet to be coupled to the resulting functional tissue damage, and as such the link between the local protease activity, load-dependent ECM remodeling, mechanical wall stress, and aortic dilation remains unclear (Manning et al., 2003; Xiong et al., 2008). In order to generate a basis for further studies into AAA disease progression, the objective of this work is to determine the feasibility of using novel PBs combined with 2P imaging techniques to assess spatial alterations in MMP activity with simultaneous collagen and elastin imaging in the ApoE−/− AngII-infused mouse model of aneurysm. The results reported here show significantly enhanced activation of PB in aneurysmal aortic tissue as compared with non-aneurysmal and wildtype controls, demonstrating the feasibility of using PBs in combination with 2P imaging to assess the spatial distribution of MMP activity within developing aortic aneurysms.
Materials and Methods
PB Synthesis and Characterization
PBs employed for this study with MMP cleavable specific peptide linkers were synthesized at the Vanderbilt University Institute of Imaging Science based on previous protocols (McIntyre & Matrisian, 2003; McIntyre et al., 2004; Scherer et al., 2008b; McIntyre & Matrisian, 2009; McIntyre et al., 2010). These PBs used for in vitro and ex vivo optical imaging of MMP activity had in particular a fluorescein (FL)-labeled peptide sequence (BR1 or BR2) cleavable by MMP2 and MMP9 that show selectivity either for gelatin or collagen attached to a generation 4 polyamidoamine (G4-PAMAM) dendrimer scaffold that previously had the addition of polyethylene glycol (PEG) to increase solubility. The highly branched (FL-BR2)-PAMAM-PEG dendrimer was then labeled with tetramethylrhodamine (TMR) to generate the completed PB using methodology established by the McIntyre laboratory (McIntyre et al., 2004; McIntyre & Matrisian, 2009; McIntyre et al., 2010). The advantages of using dendrimers are controlled assembly, size, biocompatibility, long circulation time due to decreased vehicle uptake, and the multivalency of the dendrimer allowing for the addition of multiple sensor and reference fluorophores. In addition, PAMAM dendrimer backbones with either FL-peptide or TMR attached were used for initial imaging calibrations (Fig. 1). As the FL fluorophore donates energy to the TMR fluorophore when FRET occurs, they are referred to as the sensor and reference, respectively.
Figure 1.
Schematic illustrations of reference compounds and proteolytic beacon (PB) showing (left to right) the polyamidoamine (PAMAM) dendrimer backbone with reference tetramethylrhodamine (TMR) fluorophores, PAMAM dendrimer backbone with peptide linker and sensor fluorescein (FL) fluorophores, and the Förster resonance energy transfer (FRET)-based PB with both reference TMR and peptide-attached sensor FL fluorophores.
Quantitative Fluorescence Imaging of PBs Using Single-Photon and 2P Microscopy
Initial single-photon calibration of the PBs was done on a Synergy H1 plate reader from BioTek® (Winooski, VT, USA), with excitation for the individual reference containing only one each of the individual fluorophores (FL-PAMAM and TMR-PAMAM dendrimers) measured in both sensor and reference channels between 480–490 nm and 520–530 nm, respectively, in order to determine the efficiency of color discrimination of the two channels based on previous studies (McIntyre & Matrisian, 2009). Characterization of the newly synthesized PBs was done by first confirming the desired proteolytic cleavage using known activator trypsin (25200-056, Life Technologies™, Carlsbad, CA, USA), and confirmation of selective cleavage in the presence of specific MMPs was demonstrated in vitro using active MMP-2 and -9 (Enzo® Life Sciences BLM-SE360 & BLM-SE237) in a reaction buffer containing 0.05 M Tris-HCL, 0.15 M NaCl, 5mM CaCl2, 0.2 mM sodium azide pH 7.6 (E12055, Life Technologies™, USA) through setting up a number of assays that varied from 0 to 1U/ml MMP concentration and up to 120min of incubation. The PB concentrations are expressed based on molar concentrations of the PB, i.e., the PAMAM core rather than the peptide substrate. For this PB, the TMR fluorescence shows the distribution of the PB, whereas the FL/TMR ratio is an index of the extent of cleavage (McIntyre & Matrisian, 2009).
Sensor (FL) and reference (TMR) fluorescence of the PBs were then measured using 2P excitation conducted on the advanced intravital microscope (AIM), an National Institute of Health (NIH)-sponsored shared device (Bal et al., 2013; Keyes et al., 2013; Utzinger et al., 2015). The AIM uses a pulsed Titanium–Sapphire laser (680–1060 nm) for simultaneous 2PEF excitation and SHG. For this study, imaging was done using an Olympus XLUMPLFL 20 × (Edmund Optics Inc., Barrington, NJ, USA) water immersion objective with a numerical aperture of 0.9. Multiple channels were used with band-pass filters set to capture the sensor (FL—bandpass filter 525/50 nm) and reference (TMR—bandpass filter 607/70 nm) emissions. Modifications to the AIM imaging system were done by calibrating both in vitro and ex vivo testing with appropriate fluorescence standards and phantoms (McIntyre et al., 2010) in preparation for quantitative fluorescence imaging studies. In 2P imaging, proteolytic cleavage of the PBs was evident by an increase in the sensor/reference fluorescence ratio of the PB as compared with the uncleaved control using known protease concentrations used for quantitative assessment (McIntyre et al., 2010).
Ex vivo Imaging of MMP Activity in the AngII-Infused ApoE−/− Mouse Model of Aneurysm
The AngII-infused ApoE−/− mouse model of aneurysm has been used in our lab previously (Haskett et al., 2013), and all experimental procedures for mouse testing were performed according to the approved protocol (No. 06-045) of the University of Arizona Institutional Animal Care and Use Committee and Animal Welfare Assurance Number (A3248-01). ApoE−/− mutant mice were obtained from Jackson Lab (Bar Harbor, ME, USA) (Stock No. 002052) (Piedrahita et al., 1992). ApoE−/− mice on C57BL/6J background were maintained as a colony of heterozygous animals in order to produce age-matched wildtype and ApoE−/− individuals, fed a standard diet, and provided water ad libitum (Haskett et al., 2013). Briefly, 4- to 6-month-old adult ApoE−/− mice were administered AngII (1,000 ng/min/kg, A9525 Sigma-Aldrich, St. Louis, MO, USA) through a subcutaneously implanted Azlet® mini-osmotic pump (model 2002, Durect Corp) and placed on a high fat diet for 14 days as described in the literature (Cassis et al., 2009; Haskett et al., 2012b).
AngII-infused ApoE−/− mice exhibiting aneurysm (defined as a >50% increase in aortic diameter or the onset of dissection), AngII-infused ApoE−/− mice not exhibiting aneurysm, and wildtype control (ApoE+/+) mice had their aortas isolated and surgically excised from the ascending aorta to the iliac bifurcation and cleaned of perivascular tissue as described previously (Haskett et al., 2012b; Haskett et al., 2013). The aorta was then trimmed isolating the suprarenal segment of the aorta, immersed in a PB solution (0.144 nmol/ml), and incubated at 37°C overnight to allow for diffusion through the tissue. The aortas were then cannulated on custom-pulled micropipette capillary tubes and secured using cyanoacrylate adhesive gel and placed in the microbiaxial optomechanical device (MOD) (Keyes et al., 2011). The abdominal aortas were then imaged on the AIM (configured for MOD imaging) configured for PBs (Quantitative Fluorescence Imaging of PBs Using Single-Photon and 2P Microscopy section) at an excitation wavelength of 780 nm with a laser power of 40 mW (25 mW power on the sample). A third collection channel was added to collect SHG [for imaging collagen fibers of the adventitia (bandpass filter—377/50 nm)]. En face image stacks were taken using a 500 × 500 µm field of view at 4 µm steps from 100 to 200 µm in depth based on thickness of the sample and signal loss. The aorta was then immersed in 5% low melting temp agarose and cross-sectional sections 200 µm in thickness were cut using a vibratome (model 5100mz-Plus, Lafayette Instrument Ltd.). The cross-sectional slices were then imaged as described above and analyzed based on full image stack maximum intensity projections and for individual regions of the media, thrombus, and adventitia. Additional cross-sectional image stacks were also taken of suprarenal wildtype mice without being immersed in PBs. The FL/TMR ratio was then determined as an index of the extent of cleavage, where an increase in the sensor/reference ratio indicates an increase in MMP activity. Thus, when the channels are overlaid the image can appear anywhere from blue (low cleavage and MMP activity) to yellow (high cleavage and MMP activity).
Statistics
For full image analysis, a one-way analysis of variance (ANOVA) test followed by a Tukey pairwise analysis was used to determine differences in MMP activity between aneurysmal ApoE−/− AngII-infused, non-aneurysmal ApoE−/− AngII-infused, and wildtype control aortas in ex vivo 2P imaging using PBs based on means from image stack maximum intensity projections. A two-way ANOVA was used when analyzing differences in medial, thrombus, and adventitial sensor to reference ratio between groups. All statistical analyses were performed in SigmaPlot (v. 13.0, SPSS, Chicago, IL, USA).
Results
PB Characterization Through Single-Photon and 2P Spectra with Proteolytic Cleavage
Initial single-photon emission sweeps (485 nm excitation) found the sensor (FL-PAMAM) gave a strong peak at 520 nm and the reference (TMR-PAMAM) showed almost no excitation with a small emission peak around 580 nm(Fig. 2, left). However, uncleaved PBs containing both sensor and reference molecules give a strong emission peak at 580 nm when excited between 480 and 490 nm, signifying FRET activity. Emission spectra were also gathered from in vitro assays using trypsin, which is also capable of cleaving the peptide linkers, demonstrating the increase in sensor/reference fluorescence ratio of the PBs and distinguishing increases in enzymatic levels (Fig. 2, right). Note that the large increase in sensor fluorescence is also accompanied by a slight increase in reference fluorescence. This is due to the energy loss that can occur with FRET. When the fluorophores are separated through cleavage of the peptide bond, both the sensor (FL) and reference (TMR) are able to be excited and both will emit. However, this increase in sensor fluorescence is much greater than the increase in reference fluorescence so the sensor/reference ratio will increase dramatically upon cleavage.
Figure 2.
(Left) Spectra from fluorescein polyamidoamine (FL-PAMAM) dendrimer excited by 485 nm light and emitting a peak around 520 nm, the tetramethylrhodamine (TMR)-PAMAM dendrimer shows greatly reduced fluorescence at its peak emission (590 nm), whereas the proteolytic beacon (PB) shows minimal FL fluorescence but strong fluorescence corresponding with TMR indicating efficient Förster resonance energy transfer (FRET) in the uncleaved PB. (Right) Emission spectra (ex 490 nm) of PB after 2-h incubation with varying concentrations of trypsin (as indicated) showing enhanced FL sensor fluorescence with increasing trypsin indicating cleavage of PBs in vitro.
Single-photon in vitro assays demonstrated that PBs containing BR1 and BR2 peptide sequence were cleavable by both MMP 9 and MMP 2 with a sensor to reference ratio determined at varying time and concentrations (Fig. 3). Sensitivity down to 0.03 U/ml MMP was found after 120 min of incubation with higher concentrations requiring shorter incubation times. Longer incubation times (~16 h) for the PBs were found to have higher sensitivity and were used in the ex vivo mouse aorta tests as described below.
Figure 3.
(Left) Sensor/reference ratios for single-photon measurements of kinetics of the BR2 peptide proteolytic beacons (PBs) at multiple concentrations (U/ml) incubated in ~72 pMoles PB/ml in reaction buffer for up to 3 h. (Right) The sensor/reference ratio at 120 min plotted against matrix-metalloprotease (MMP) concentration representing the reaction progress enzyme kinetics.
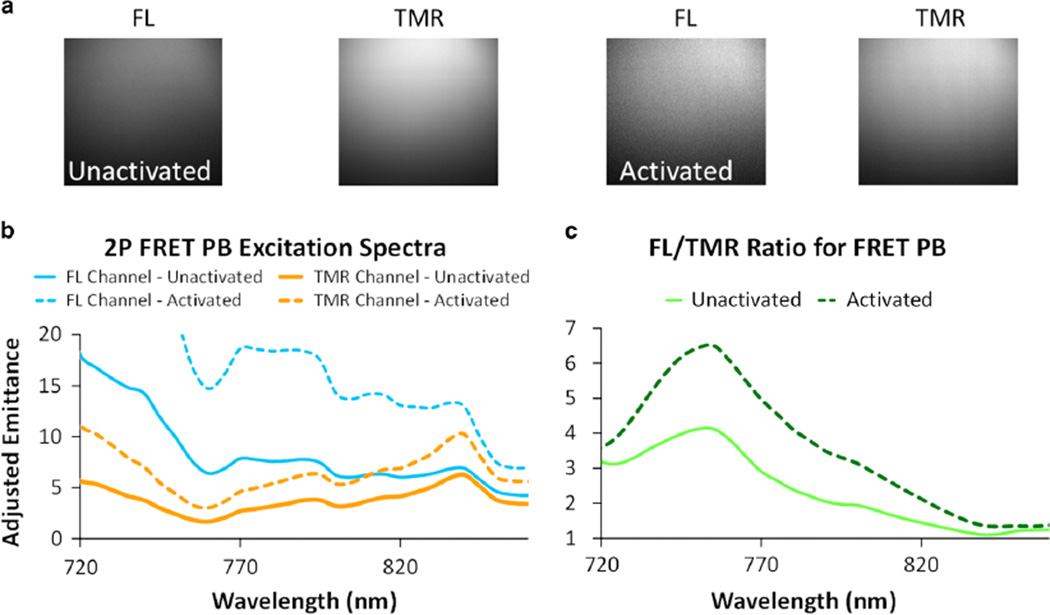
Initial 2P studies, with PBs diluted to ~72pMoles PB/ml in a slide well suspended and illuminated, showed unactivated PBs imaged in both the sensor (FL) and reference (TMR) channels from 720 to 980 nm for excitation and were then activated using trypsin, incubated for 120 min, and imaged again (Fig. 4a). Mean intensities of the 2P spectra showed a large difference in the emission between unactivated and activated PBs (Fig. 4b). Analysis of the sensor/reference ratio through the 2P spectra revealed a peak in sensor reference ratio around 760 nm and the largest increase in sensor reference ratio with proteolytic activation also occurred at 760 nm (Fig. 5c). However, an excitation wavelength of 780 nm was chosen for all following 2P imaging as it provides a sufficient increase in sensor/reference ratio, is near the peak power output of the titanium–sapphire laser enhancing fluorescence signal, and allows for the collection of SHG in ex vivo imaging distinct from the FL and TMR channels.
Figure 4.
a: 2-photon (2P) images of the fluorescence of proteolytic beacons (PBs) in suspension in the fluorescein (FL) and tetramethylrhodamine (TMR) channels for both unactivated and activated PBs. Activation is evidenced by the increase in the mean intensity in the FL channel for the activated PBs and the enhanced FL/TMR ratio. b: 2P spectral excitation sweeps for the PB before (solid lines) and after (dotted lines) protease activation for both the FL (blue) and TMR (orange) emission channels. c: Changes in the FL/TMR (sensor/reference) 2P fluorescence ratio illustrates a peak around 760 nm excitation that increases significantly with protease activation. FRET, Förster resonance energy transfer.
Figure 5.
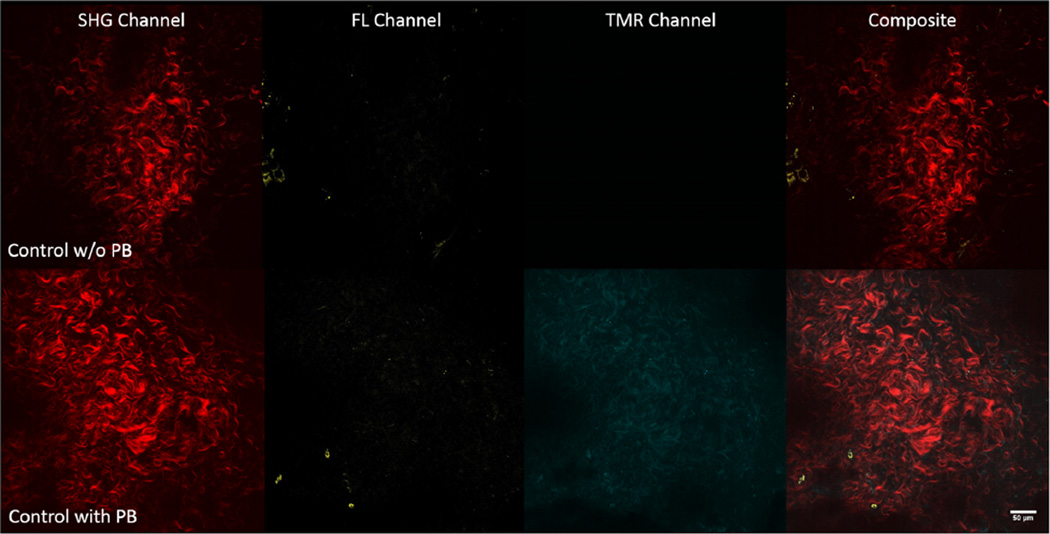
(Top row) Representative 2-photon (2P) single-slice images of the adventitia for second harmonic generation (SHG), fluorescein (FL), tetramethylrhodamine (TMR), and overlaid channels (from left to right) before incubation with proteolytic beacons (PBs) for a wildtype control mouse depicting collagen signal (red), whereas signal in the FL (yellow) and TMR (cyan) channels is virtually absent. (Bottom row) Representative 2P single-slice images of the adventitia for SHG, FL, TMR, and overlaid (from left to right) channels after 16 h of incubation with PBs for a wildtype control mouse depicting collagen signal (red), and signal in the FL (yellow) and TMR (cyan) channels. The dynamic range of the FL channel and TMR channel of the top and bottom rows are 0–64 and 0–255, respectively, in order to illustrate the difference in signal in the two channels within the adventitia before and after the addition of PBs.
Ex vivo Imaging of MMP Activity in the AngII-Infused ApoE−/− Mouse Model of Aneurysm
Wildtype (ApoE+/+) control (n = 4) and ApoE−/− mice infused with AngII for 14 days (n = 5) were sacrificed and their abdominal aortas removed and cleaned for imaging whether the ApoE−/− AngII aortas were fully aneurysmal or not. From the five ApoE−/− 14-day AngII-infused aortas, three presented with remodeling and aneurysm, whereas two did not. Multiple en face 2P image stacks were taken for both wildtype and ApoE−/− mice infused with AngII for 14 days before PB treatment and after 16 h of incubation on the MOD at 0 mmHg. Within the adventitia where SHG denoted the presence of collagen, without PBs there was little to no signal in either the FL (sensor) or TMR (reference) channel (Fig. 5, top row). After immersion in a ~72 pmol/ml PB solution for up to 16 h, there was a distinct increase in signal found in both FL (sensor) and TMR (reference) channels (Fig. 5, bottom row) where the PBs are present but not undergoing a large amount of proteolytic cleavage. Thus, the prevailing fluorescence is from the TMR channel showing the distribution of the probe. After immersion in PBs for 16 h, ApoE−/− 14-day AngII-infused mouse aortas that had undergone remodeling displayed increased FL signal relative to wildtype control aortas that had also been immersed in PBs (Fig. 6).
Figure 6.
(Top row) Representative 2-photon (2P) single-slice images of the adventitia for second harmonic generation (SHG), fluorescein (FL), tetramethylrhodamine (TMR), and overlaid (from left to right) channels after being incubated with proteolytic beacons (PBs) for a wildtype control mouse aorta depicting collagen signal (red), with signal in the FL (yellow) and TMR (cyan) channels indicating MMP activity. (Bottom row) Representative 2P single-slice images of the adventitia for SHG, FL, TMR, and overlaid channels after 16 h of incubation with PBs for an apolipoprotein E knockout 14-day angiotensin II-infused remodeled mouse aorta depicting collagen signal (red), with increased signal in the FL (yellow) and TMR (cyan) channels. Notice that where SHG is present, autofluorescence is absent, so in the corresponding images, the fluorescence is being generated primarily by the PBs.
Cross-Sectional 2P Imaging of the AngII-Infused ApoE−/− Mouse Model of Aneurysm
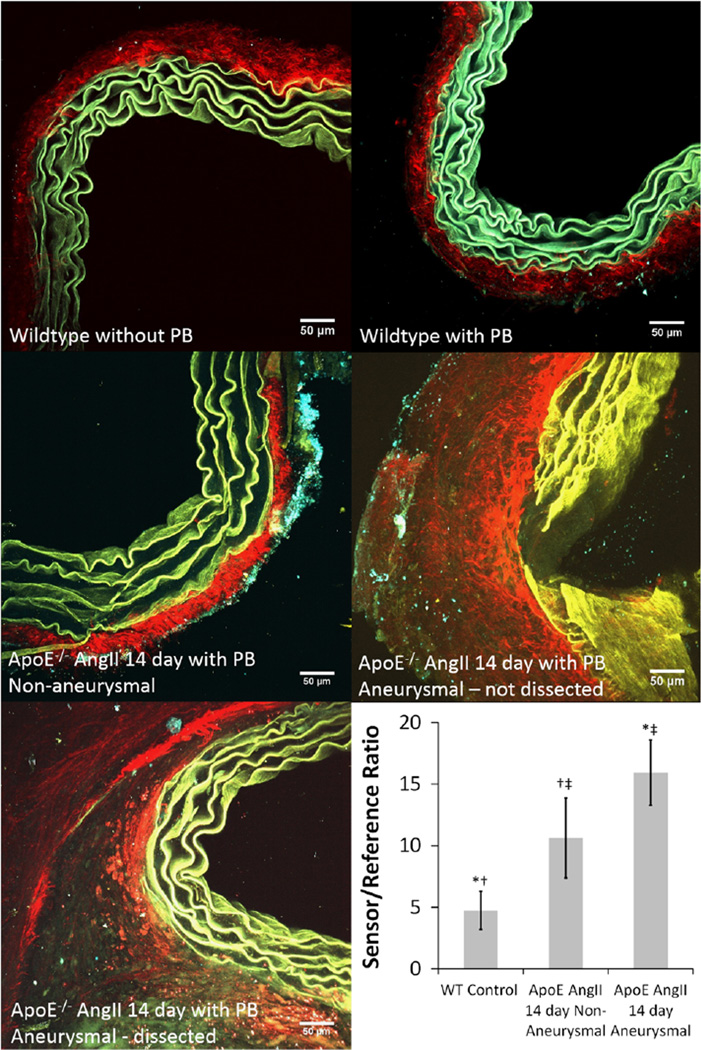
Multiple cross-sectional image stacks taken after en face imaging displayed differences in signal between wildtype control and ApoE−/− AngII-infused aortas. Although autofluorescence of the elastin within the medial layers contributed to signal in both the FL and TMR channels, wildtype mouse aorta sections without PBs showed an almost even amount of autofluorescence signal in both channels. This confirmed that differences in fluorescence between the FL and TMR channels due to the PBs can be attributed to the sensor/reference fluorophores and any proteolytic activity they undergo and not autofluorescence. Aortas were then placed in groups of wildtype control (n = 4), ApoE−/− AngII-infused non-aneurysmal (n = 2), and ApoE−/− AngII-infused aneurysmal (n = 3). Although the wildtype aortas with PBs had relatively greater signal in the TMR channel, both ApoE−/− AngII-infused groups had an increase of signal in the FL channel and thus an increase in the sensor/reference ratio indicating increased MMP activity. The medial layer of wildtype mouse aortas without PBs appeared green, whereas wildtype mouse aortas with PBs appeared blue and the ApoE−/− 14-day AngII-infused remodeled aortas appeared yellow indicating differences in the amount of signal from each channel and increased activity in the remodeled aneurysmal aortas. The mean intensity taken from Z-stack maximum intensity projections from cross-sectional images (n = 7 for each group) showed a significant increase in sensor/reference ratio between wildtype controls and both ApoE−/− AngII-infused non-aneurysmal and aneurysmal aortas (each p < 0.001). It was also found that there was a significant increase in sensor/reference ratio between ApoE−/− AngII-infused non-aneurysmal and aneurysmal aortas (p = 0.019) (Fig. 7).
Figure 7.
2-photon maximum intensity projections for cross-sectional image stacks of suprarenal aortas from wildtype (WT) mouse without proteolytic beacons (PBs) (top left), WT mouse after 16-h immersion in PBs (top right), apolipoprotein E knockout (ApoE−/−) 14-day angiotensin II (AngII)-infused nonaneurysmal (middle left), ApoE−/− 14-day AngII-infused non-dissected aneurysmal (middle right), and ApoE−/− 14-day AngII-infused dissected aneurysmal (bottom left) aorta using 4 µm steps up to 100 µm. Images are an overlay of three channels: second harmonic generation (red) primarily depicting collagen, fluorescein (FL) channel (yellow) signifying autofluorescence of the elastin in the medial layer of the aorta and the cleaved FL fluorophore, and tetramethylrhodamine (TMR) channel (cyan) signifying autofluorescence of the elastin in the medial layer of the aorta and the remaining uncleaved PBs still undergoing Förster resonance energy transfer. The mean intensity in the FL and TMR channels for each maximum intensity projection for every slice from each specimen was used to give the sensor/reference ratio (bottom right) and indicates an increase in matrix-metalloprotease (MMP) activity for both ApoE−/− AngII-infused non-aneurysmal and aneurysmal aortas (*, †p < 0.001). It was also found that there was a significant increase in MMP activity between ApoE−/− AngII-infused non-aneurysmal and aneurysmal aortas (‡p = 0.019).
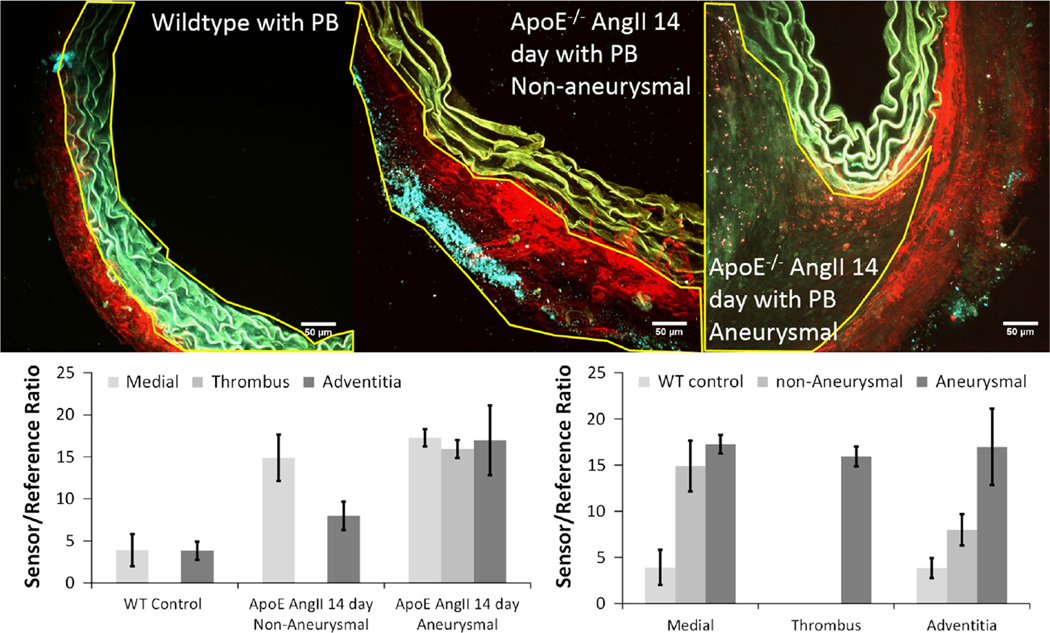
Further analysis of the cross-sectional images, differentiating between areas of the media, thrombus, and adventitia (Fig. 8, top) found that the sensor/reference ratio was not different between the medial and adventitial regions in wildtype control mouse aortas and that aneurysmal ApoE−/− AngII-infused aortas also had no significant differences between regions. Yet all regions of the ApoE−/− AngII-infused aortas were significantly higher in sensor/reference ratio compared with wildtype control regions (p < 0.001); however, in non-aneurysmal ApoE−/− AngII-infused aortas, the medial region had a significantly elevated sensor/reference ratio compared with the adventitial region (p = 0.001) (Fig. 8, bottom). In addition, though both the medial and adventitial regions had increased sensor/reference ratios compared with wildtype control mice, the medial sensor/reference ratio in non-aneurysmal ApoE−/− AngII-infused aortas was comparable with the levels found in aneurysmal ApoE−/− AngII-infused aortas, whereas the adventitial layer was not comparable.
Figure 8.
2-Photon maximum intensity projections for cross-sectional image stacks of suprarenal aortas from wildtype (WT) mouse (top left), apolipoprotein E knockout (ApoE−/−) 14-day angiotensin II (AngII)-infused non-aneurysmal (top middle), and ApoE−/− 14-day AngII-infused dissected aneurysmal aortas (top right) with regions of interest (ROIs) outlined indicating spatial distinctions between the media (top left), adventitia (top middle), and thrombus (top right). Images are an overlay of three channels: second harmonic generation (red) primarily depicting collagen, fluorescein (FL) channel (yellow) signifying autofluorescence of the elastin in the medial layer of the aorta and the cleaved FL fluorophore, and tetramethylrhodamine (TMR) channel (cyan) signifying the autofluorescence of the elastin in the medial layer of the aorta and the remaining uncleaved PBs still undergoing Förster resonance energy transfer. Sensor/reference ratios (bottom row) for the ROIs based on region and groups. Ratios for each region are comparable within the WT control and AopE−/− AngII-infused aneurysmal groups; however, there was a significant difference between regions within the AopE−/− AngII-infused non-aneurysmal group (p = 0.001).
Discussion
Summary of Results
With the single-photon FRET characteristics demonstrated, the 2P excitation spectra for FL and TMR were obtained and used to characterize the proteolytic cleavage of PBs. Proteolytic cleavage of the PBs was observed in the 2P spectra and an excitation wavelength of 780 nm was chosen for imaging studies due to the significant increase in sensor/reference ratio upon cleavage at that wavelength. This also allowed for collection of SHG at ~390 nm, distinct from the FL and TMR fluorescence channels.
From the five ApoE−/− mice infused with AngII, three presented with remodeling and aneurysm, whereas two did not, which is as expected based on previous work with this model (Cassis et al., 2009; Xie et al., 2012). However, even with these small numbers, significantly altered MMP activity was still observed when combining PBs and 2P imaging ex vivo. In cross-sectional image stacks, tests showed a significant threefold increase in sensor/reference ratio between wildtype control and ApoE−/− AngII-infused aneurysmal aortas indicating an increase in MMP activity. Unexpectedly, ApoE−/− AngII-infused aortas that did not show overt signs of remodeling or aneurysm formation still were found to have significantly increased sensor/reference ratios, thus indicating increased MMP activity compared with wildtype controls, yet these non-aneurysmal aortas have ratios significantly lower than aneurysmal aortas. In addition, spatial analysis found that in the non-aneurysmal AngII-infused ApoE−/− mouse aortas there was a significant increase in MMP activity in the medial layer over the adventitial layer that was not present in either wildtype control or aneurysmal AngII-infused ApoE−/− mouse aortas. This could be the basis for medial degradation of the elastic lamina leading to the onset of dissection.
Relation to Previous Work
2P imaging has recently been used to characterize endogenous fiber structure within the AngII-infused ApoE−/− mouse model of aneurysm (Haskett et al., 2013; Phillips et al., 2015) and activatable fluorescent imaging tools have recently also been used to show increased MMP activity localized within the aneurysmal region in the AngII-infused ApoE−/− mouse model at 28 days (Goergen et al., 2011). However, the combination of the two techniques in a mouse model has not been previously reported. Activatable fluorescent imaging tools have become very important in imaging biological systems, especially during the disease process both in vitro and ex vivo, and increasingly in vivo. Much of this prior work has been done using quenched agents that then generate high levels of fluorescence when the fluorophore is released by an enzyme-mediated reaction. Agents such as these have been put to commercial use such as EnzChek® Gelatinase/Collagenase FRET based Assay Kit from (Molecular Probes®, Invitrogen, Carlsbad, CA, USA) or MMPSense®, MMPSense FAST®, and ProSense® (PerkinElmer, Inc., Waltham, MA, USA) in studies where MMPs play a central role in diseases such as cancer (invasion, progression, and metastasis), rheumatism, pulmonary disease, and cardiovascular disease (Leahy et al., 2015; Lin et al., 2015; Pivoraite et al., 2015). Each of these can have both advantages and disadvantages, such as EnzChek® is specifically for in vitro assays. For ex vivo and in vivo assays, MMPSense® will often have issues with vehicle uptake or reduced circulation time (Scherer et al., 2008a, 2008b), and specifically in the AngII-infused ApoE−/− mouse model, both activation of MMPSense® and ProSense® were observed in the liver and kidneys under standard conditions (Goergen et al., 2011), creating a significant amount of nonspecific signal and limiting possible in vivo usage. Unlike these commercially available products, use of PBs comes with certain advantages, especially in the use of the dendrimer backbone, which allows for a much more customizable product.
Other studies (Eagleton et al., 2006) have looked into temporal MMP activity in the ApoE−/− 14-day AngII-infused mouse model and showed significantly increased MMP expression after 21 days in the ApoE−/− AngII-infused aneurysmal model through gene expression and zymography. In our work, use of PBs was able to show elevated MMP activity levels in aneurysmal aortas compared with wildtype control aortas as early as 14 days. However, as not all ApoE−/− 14-day AngII-infused mice presented with fully formed suprarenal aneurysms, they cannot be directly compared with previous results, mechanical or otherwise (Rateri et al., 2011; Phillips et al., 2015), and those that did undergo remodeling but did not present with full aneurysm formation expressed a phenotype similar to wildtype mice infused with AngII (Haskett et al., 2012b). In addition, to the best of our knowledge, this is the only study to look into the 2P spectra of such PBs and their fluorophores as well as FRET activity under 2P conditions. We believe that the current work is also the first to collect SHG simultaneously with FRET PBs in untreated tissue, a methodology that may be extremely useful in future mechanobiological studies.
Limitations
The ApoE−/− AngII-infused mouse model of aneurysm has become a standard in genetic animal models for AAA. Although previous work in our lab has found no difference between wildtype mice with and without vehicle infusion via pump implantation (Haskett et al., 2012b; Haskett et al., 2013), any further studies would need to include such groups to provide evidence that pump implantation does not alter MMP expression. In addition, for the ex vivo studies, it is unknown when the aneurysms formed in the ApoE−/− 14-day AngII-infused mice that presented with suprarenal aneurysms, and there is the possibility that the aneurysms being compared could have developed in days or over a week apart and could be at varying stages of development (Rateri et al., 2011; Phillips et al., 2015). Ex vivo results presented here also do not contain comparison with gelatin zymography, which has been used previously as a standard measurement for detecting MMP expression (Leber & Balkwill, 1997; Mannello & Sebastiani, 2003; Troeberg & Nagase, 2003). However, previous works with these specific peptide sequences have shown them to be selectively cleaved by MMPs (Welch et al., 1995; Kraft et al., 2001; Chen et al., 2003; McIntyre et al., 2010). Limitations involving imaging with the AIM and MOD have been described previously (Haskett et al., 2012a, 2012b, 2013). One problem that was encountered when imaging ex vivo aortas was the difficulty in matching locations of before image stacks and images stacks taken after being immersed in PBs. Moreover, there is the assumption that the PBs are completely diffuse in the aorta when image stacks were taken.
In this study, it was found that autofluorescence given off by elastin in the medial layer bled over into both the FL and TMR channels. However, measurements taken in the adventitial layer did not contain any autofluorescence as evidenced by the lack of any signal in the FL and TMR channels in before image stacks, whereas the signal in the SHG channel was well defined. We are certain that any signal in the FL and TMR channels in the adventitial layer was due to fluorescence given off by the PBs in this situation. If the autofluorescence of elastin needed to be avoided, then studies could be reserved to just the adventitia. However, aortic cross-sections without PBs displayed an autofluorescence signal that was even across both channels and comparison between cross-sectional images of the wildtype control, non-aneurysmal ApoE−/− AngII-infused aortas, and aneurysmal ApoE−/− AngII-infused aortas immersed in PBs showed a measurable difference in the signal/reference ratio of the PBs that was significant and demonstrated increased MMP activity within the medial layer despite the influence of autofluorescence.
Future Studies
Continued use of PBs in the study of the ApoE−/− AngII-infused mouse model would allow for investigation into a more detailed assessment of the temporal and spatial distributions of protease activity in the initiation, progression, and rupture of aneurysms. By quantifying changes in protease activity, load-dependent ECM microstructure, and biomechanical response at early time points post AngII infusion, a more complete picture of aneurysmal disease can be formed. Further use of PBs in the ApoE−/− AngII model of AAA will also allow for temporal studies and investigations into the time course of disease initiation.
Future studies in this line of research also have the possibility of moving toward FRET imaging in vivo as has been done previously in cancer research (McIntyre & Matrisian, 2003; McIntyre et al., 2004; McIntyre & Matrisian, 2009; McIntyre et al., 2010). This ability to image the tumor microenvironment has potential applications in imaging vascular disease, especially endoscopically, distinguishing specific functions of MMPs in disease progression and in different models.
In addition, the ability to create more specific amino acid sequences for the chain linkers of the PBs would allow for the activity of specific MMPs to be observed. The FRET pair of the PBs is also modifiable and can be adapted for imaging through the visual spectrum and into the infrared for deeper tissue imaging that would allow for imaging of the deep vasculature when combined with emerging endovascular techniques. Further modifications to the dendrimer backbone could also allow for other applications, such as changing the hydrophobicity and altering the efficiency of delivery.
PB imaging of MMP activity also provides a means to assess possible therapies for treating vascular disease; that is, being able to image biomarkers in vascular disease and their alterations with treatment. Imaging in this way could offer an early indication of disease progression and any responsiveness toward treatment. Although there remain important advances that need to be made in order to apply this technology directly toward treating of human disease, this study represents a first step toward simultaneously imaging MMP activity and ECM microstructure, something that current instrumentation and methodologies lack.
Conclusions
In summary, our study measured the 2P spectra of FRET-based PBs and used 2P imaging ex vivo to determine changes in MMP activity within the ECM of the ApoE−/− AngII-infused mouse model of aneurysm. We found that alongside the PBs, it was also possible to simultaneously image collagen microstructure through SHG. Although some autofluorescence from elastin found within the medial layer did extend into both the FL and TMR channels, there was a clear increase in sensor/reference ratio seen from non-aneurysmal to aneurysmal ApoE−/− AngII-infused aortas and both had higher sensor/reference ratios than wildtype controls indicating increasing MMP activity. Moreover, PBs were able to show spatial variance in sensor/reference ratio indicating differences in MMP activity within ApoE−/− AngII-infused aortas. As a result, PBs can prove to be a useful tool for future studies looking into and investigating a more detailed time course and spatial distribution of the initiation, progression, and rupture of aneurysm in the ApoE−/− AngII-infused mouse model and other models of AAA.
Acknowledgments
The authors would like to acknowledge sources of support: Cardiovascular Training Grant to Dr. Jennifer Barton (NIH 232HL007955), NIH research grant (1RO1EY020890-02A1 to Dr. J.P. Vande Geest), partial support came from the NIH (NHLBI-1R21HL111990-01A1 to Dr. J.P. Vande Geest), the Vascular Training Grant to Dr. Edith Tzeng (NIH 2T32HL098036-06), and partial support to Dr. J.O.McIntyre from NIH (P50CA128323, RO1CA048360) and Susan G. Komen for the Cure. Imaging was done on an NIH-sponsored shared device (NIH/NCRR S10RR023737).
References
- Bal U, Andresen V, Baggett B, Utzinger U. Intravital confocal and two-photon imaging of dual-color cells and extracellular matrix mimics. Microsc Microanal. 2013;19:201–212. doi: 10.1017/S1431927612014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, Buckley C, Ennis TL, Thompson RW. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20:228–236. doi: 10.1007/s10016-006-9017-z. [DOI] [PubMed] [Google Scholar]
- Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: Report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EI, Li W, Godzik A, Howard EW, Smith JW. A residue in the S2 subsite controls substrate selectivity of matrix metalloproteinase-2 and matrix metalloproteinase-9. J Biol Chem. 2003;278:17158–17163. doi: 10.1074/jbc.M210324200. [DOI] [PubMed] [Google Scholar]
- Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998a;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curci JA, Petrinec D, Liao S, Golub LM, Thompson RW. Pharmacologic suppression of experimental abdominal aortic aneurysms: A comparison of doxycycline and four chemically modified tetracyclines. J Vasc Surg. 1998b;28:1082–1093. doi: 10.1016/s0741-5214(98)70035-7. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Cassis L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc Med. 2004a;14:117–120. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004b;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A, Rateri DL, Cassis LA. Role of the renin-angiotensin system in the development of abdominal aortic aneurysms in animals and humans. Ann NY Acad Sci. 2006;1085:82–91. doi: 10.1196/annals.1383.035. [DOI] [PubMed] [Google Scholar]
- Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- Dua A, Kuy S, Lee CJ, Upchurch GR, Jr, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59:1512–1517. doi: 10.1016/j.jvs.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Eagleton MJ, Ballard N, Lynch E, Srivastava SD, Upchurch GR, Jr, Stanley JC. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during Angiotensin II induced aneurysm formation. J Surg Res. 2006;135:345–351. doi: 10.1016/j.jss.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB C. American Heart Association Statistics & S. Stroke Statistics. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goergen CJ, Azuma J, Barr KN, Magdefessel L, Kallop DY, Gogineni A, Grewall A, Weimer RM, Connolly AJ, Dalman RL, Taylor CA, Tsao PS, Greve JM. Influences of aortic motion and curvature on vessel expansion in murine experimental aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:270–279. doi: 10.1161/ATVBAHA.110.216481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskett D, Azhar M, Utzinger U, Vande Geest JP. Progressive alterations in microstructural organization and biomechanical response in the ApoE mouse model of aneurysm. Biomatter. 2013;3:e24648. doi: 10.4161/biom.24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskett D, Doyle JJ, Gard C, Chen H, Ball C, Estabrook MA, Encinas AC, Dietz HC, Utzinger U, Vande Geest JP, Azhar M. Altered tissue behavior of a non-aneurysmal descending thoracic aorta in the mouse model of Marfan syndrome. Cell Tissue Res. 2012b;347:267–277. doi: 10.1007/s00441-011-1270-y. [DOI] [PubMed] [Google Scholar]
- Haskett D, Speicher E, Fouts M, Larson D, Azhar M, Utzinger U, Vande Geest J. The effects of angiotensin II on the coupled microstructural and biomechanical response of C57BL/6 mouse aorta. J Biomech. 2012a;45:772–779. doi: 10.1016/j.jbiomech.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- Keyes JT, Haskett DG, Utzinger U, Azhar M, Vande Geest JP. Adaptation of a planar microbiaxial optomechanical device for the tubular biaxial microstructural and macroscopic characterization of small vascular tissues. J Biomech Eng. 2011;133:075001. doi: 10.1115/1.4004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes JT, Lockwood DR, Utzinger U, Montilla LG, Witte RS, Vande Geest JP. Comparisons of planar and tubular biaxial tensile testing protocols of the same porcine coronary arteries. Ann Biomed Eng. 2013;41:1579–1591. doi: 10.1007/s10439-012-0679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft PJ, Haynes-Johnson DE, Patel L, Lenhart JA, Zivin RA, Palmer SS. Fluorescence polarization assay and SDS-PAGE confirms matrilysin degrades fibronectin and collagen IV whereas gelatinase A degrades collagen IV but not fibronectin. Connect Tissue Res. 2001;42:149–163. doi: 10.3109/03008200109014256. [DOI] [PubMed] [Google Scholar]
- Leahy AA, Esfahani SA, Foote AT, Hui CK, Rainbow RS, Nakamura DS, Tracey BH, Mahmood U, Zeng L. Analysis of the trajectory of osteoarthritis development in a mouse model by serial near-infrared fluorescence imaging of matrix metalloproteinase activities. Arthritis Rheumatol. 2015;67:442–453. doi: 10.1002/art.38957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber TM, Balkwill FR. Zymography: A single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem. 1997;249:24–28. doi: 10.1006/abio.1997.2170. [DOI] [PubMed] [Google Scholar]
- Lederle FA. The rise and fall of abdominal aortic aneurysm. Circulation. 2011;124:1097–1099. doi: 10.1161/CIRCULATIONAHA.111.052365. [DOI] [PubMed] [Google Scholar]
- Lin SA, Suresch DL, Connolly B, Mesfin G, Gonzalez RJ, Patel MR, Shevell D, Johnson T, Bednar B. Optical imaging biomarkers of drug-induced vascular injury. Mol Imaging. 2015;14 doi: 10.2310/7290.2014.00054. [DOI] [PubMed] [Google Scholar]
- Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannello F, Sebastiani M. Zymographic analyses and measurement of matrix metalloproteinase-2 and-9 in nipple aspirate fluids. Clin Chem. 2003;49:1546–1550. doi: 10.1373/49.9.1546. [DOI] [PubMed] [Google Scholar]
- Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J. 2004;377:617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JO, Matrisian LM. Molecular imaging of proteolytic activity in cancer. J Cell Biochem. 2003;90:1087–1097. doi: 10.1002/jcb.10713. [DOI] [PubMed] [Google Scholar]
- McIntyre JO, Matrisian LM. Optical proteolytic beacons for in vivo detection of matrix metalloproteinase activity. Methods Mol Biol. 2009;539:155–174. doi: 10.1007/978-1-60327-003-8_9. [DOI] [PubMed] [Google Scholar]
- McIntyre JO, Scherer RL, Matrisian LM. Near-infrared optical proteolytic beacons for in vivo imaging of matrix metalloproteinase activity. Methods Mol Biol. 2010;622:279–304. doi: 10.1007/978-1-60327-299-5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyama N, Dua MM, Schultz GM, Kosuge H, Terashima M, Pisani LJ, Dalman RL, McConnell MV. Bioluminescence and magnetic resonance imaging of macrophage homing to experimental abdominal aortic aneurysms. Mol Imaging. 2012;11:126–134. [PubMed] [Google Scholar]
- Phillips EH, Yrineo AA, Schroeder HD, Wilson KE, Cheng JX, Goergen CJ. Morphological and biomechanical differences in the elastase and AngII apoE (−/−) rodent models of abdominal aortic aneurysms. Biomed Res Int. 2015;2015:413189. doi: 10.1155/2015/413189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivoraite U, Jarmalaviciute A, Tunaitis V, Ramanauskaite G, Vaitkuviene A, Kaseta V, Biziuleviciene G, Venalis A, Pivoriunas A. Exosomes from human dental pulp stem cells suppress carrageenan-induced acute inflammation in mice. Inflammation. 2015;38:1933–1941. doi: 10.1007/s10753-015-0173-6. [DOI] [PubMed] [Google Scholar]
- Ramaswamy AK, Hamilton M, 2nd, Joshi RV, Kline BP, Li R, Wang P, Goergen CJ. Molecular imaging of experimental abdominal aortic aneurysms. Scientific World Journal. 2013;2013:973150. doi: 10.1155/2013/973150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin II in apoE(−/−) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB C. American Heart Association Statistics & S. Stroke Statistics. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer RL, McIntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008a;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol Imaging. 2008b;7:118–131. [PMC free article] [PubMed] [Google Scholar]
- Thompson RW, Baxter BT. MMP inhibition in abdominal aortic aneurysms. Rationale for a prospective randomized clinical trial. Ann N Y Acad Sci. 1999;878:159–178. doi: 10.1111/j.1749-6632.1999.tb07682.x. [DOI] [PubMed] [Google Scholar]
- Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: Insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: Basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeberg L, Nagase H. Measurement of matrix metalloproteinase activities in the medium of cultured synoviocytes using zymography. Methods Mol Biol. 2003;225:77–87. doi: 10.1385/1-59259-374-7:77. [DOI] [PubMed] [Google Scholar]
- Utzinger U, Baggett B, Weiss JA, Hoying JB, Edgar LT. Large-scale time series microscopy of neovessel growth during angiogenesis. Angiogenesis. 2015;136:021001. doi: 10.1007/s10456-015-9461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorp DA. Biomechanics of abdominal aortic aneurysm. J Biomech. 2007;40:1887–1902. doi: 10.1016/j.jbiomech.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorp DA, Geest JPV. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol. 2005;25:1558–1566. doi: 10.1161/01.ATV.0000174129.77391.55. [DOI] [PubMed] [Google Scholar]
- Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–1116. doi: 10.1056/NEJMcibr0905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AR, Holman CM, Browner MF, Gehring MR, Kan CC, Van Wart HE. Purification of human matrilysin produced in Escherichia coli and characterization using a new optimized fluorogenic peptide substrate. Arch Biochem Biophys. 1995;324:59–64. doi: 10.1006/abbi.1995.9929. [DOI] [PubMed] [Google Scholar]
- Xie X, Lu H, Moorleghen JJ, Howatt DA, Rateri DL, Cassis LA, Daugherty A. Doxycycline does not influence established abdominal aortic aneurysms in angiotensin II-infused mice. PLoS One. 2012;7:e46411. doi: 10.1371/journal.pone.0046411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47:166–172. doi: 10.1016/j.jvs.2007.09.016. discussion 172. [DOI] [PMC free article] [PubMed] [Google Scholar]