Abstract
Purpose of review
Endothelial cells line the surface of the cardiovascular system and display a large degree of heterogeneity due to developmental origin and location. Despite this heterogeneity, all endothelial cells are exposed to wall shear stress (WSS) imparted by the frictional force of flowing blood, which plays an important role in determining the endothelial cell phenotype. Although the effects of WSS have been greatly studied in vascular endothelial cells, less is known about the role of WSS in regulating cardiac function and cardiac endothelial cells.
Recent findings
Recent advances in genetic and imaging technologies have enabled a more thorough investigation of cardiac hemodynamics. Using developmental models, shear stress sensing by endocardial endothelial cells has been shown to play an integral role in proper cardiac development including morphogenesis and formation of the conduction system. In the adult, less is known about hemodynamics and endocardial endothelial cells, but a clear role for WSS in the development of coronary and valvular disease is increasingly appreciated.
Summary
Future research will further elucidate a role for WSS in the developing and adult heart, and understanding this dynamic relationship may represent a potential therapeutic target for the treatment of cardiomyopathies.
Keywords: development, endocardium, heart, mechanotransduction, shear stress
INTRODUCTION
Endothelial cells line the inner surface of the heart and all blood vessels. They are present in all tissues and originate early in embryonic development. Despite a tremendous heterogeneity of endothelial cells due to location and developmental origin, a common and critical feature of these cells is their exposure to fluid wall shear stress (WSS), which initiates as soon as the heart begins beating. Research over the past several decades has revealed an important role for the mechanical force of blood flow in regulating development of the cardiovascular system, as well as in the adult. Here, we will review the role of hemodynamics and endothelial cell mechanotransduction in cardiovascular development, with a focus on the embryonic and adult heart.
ENDOTHELIAL CELLS AND MECHANOTRANSDUCTION
Endothelial cells were first recognized in the mid-1800s, and following these early descriptions it took almost 150 years for their complex and diverse biology to be appreciated [1]. In the mid-1960s, Lord Florey was among the first to describe the heterogeneity of the vascular network and to introduce the first descriptions of specialization to individual organs [2]. A large degree of endothelial cell heterogeneity can be attributed to the microenvironment to which the cells are exposed during development. Gradients of signaling molecules, diverse cell-to-cell interactions, as well as exposure to mechanical forces drive specialization and further affect endothelial cell function.
Despite increased appreciation for their heterogeneous nature, one universal factor affecting endothelial cells is their continuous exposure to WSS, the frictional force of blood flow. Shear stress varies by location and caliber of blood vessel, and spatiotemporal heterogeneities have important consequences on endothelial cell biology [3]. In regions of undisturbed, laminar blood flow, such as the descending thoracic aorta, endothelial cells express a repertoire of genes and regulate a number of pathways that maintain normal physiology [4]. In contrast, in regions of disturbed blood flow, which are characterized by flow reversals and typically occur at arterial segments with high curvatures, branch points, and bifurcations, endothelial cells are primed for the formation of disease, with reduced expression of protective genes and activation of proinflammatory pathways. These susceptible regions are typically the sites of atherosclerosis formation [5].
Given that WSS plays a key role in regulating endothelial cell form and function, a significant amount of work has focused on understanding the ways in which endothelial cells sense and respond to flow. Using knockout cell lines and animals, a number of mechanosensors have been identified (reviewed in [6]). These include, but are not limited to, a mechanosensory complex of platelet endothelial cell adhesion molecule-1 (PECAM-1), vascular endothelial-cadherin, and vascular endothelial growth factor receptor-2, ion channels, and integrins among others [7-9]. Furthermore, the downstream signaling from these mechanosensors has been studied, and a canonical set of ‘shear-responsive’ genes and signaling events has been identified in vascular endothelial cells [10].
MECHANICAL FORCES AND MECHANOTRANSDUCTION IN CARDIOVASCULAR DEVELOPMENT
The cardiovascular system is the first system to develop in vertebrate embryos, and disruptions in this process have significant effects on viability. Genetic manipulation of Drosophila, zebrafish, and mouse embryos has identified a complex milieu of signaling molecules (morphogens) and their cognate receptors that are required for proper cardiovascular development. These include Notch [11,12], sonic hedgehog (Shh) [13,14], and bone morphogenic protein (BMP) signaling (reviewed in [15]) among many others. Increasingly studied, but less well understood, are the effects of mechanical forces on cells as they divide, migrate, and differentiate [16].
As mentioned above, WSS is a key epigenetic factor in endothelial cell biology. Shear stress occurs simultaneously with the onset of a heartbeat, which occurs early in embryogenesis (Table 1). The initiation of WSS plays an important role in the development of all components of the cardiovascular system, including remodeling of blood vessels [21,22■], hematopoiesis [18,23], angiogenesis [24,25], as well as lymphangiogenesis [26,27]. In the following section, we focus on the specific requirements of shear stress in aspects of heart development.
Table 1.
Onset of heartbeat in organisms
| Species | Heart tube formation | Onset heartbeat | Reference |
|---|---|---|---|
| Human | Day 19 | Day 27 | [17] |
| Mouse | E8–8.5 | E8.5 | [18] |
| Chick | HH-stage 9–10 | HH-stage 10–11 | [19] |
| Zebrafish | 24 hpf | 24 hpf | [20] |
E, embryonic day; HH, Hamburger–Hamilton; hpf, hours postfertilization.
MECHANICAL FORCES IN HEART DEVELOPMENT
Heart development is a complex and tightly regulated process and disruption is linked to a number of congenital birth defects, including hypoplastic left heart syndrome, tetralogy of Fallot, and atrioventricular septal defects. The process of cardiogenesis has been well studied and revealed important spatiotemporal information regarding activation and expression of key signaling molecules (e.g. Notch, Tbx2, Nkx2–5) [28,29]. In addition to signaling molecules and transcription factors, the role for hemodynamic force in heart development is also becoming increasingly appreciated. The availability of models that allow surgical or genetic manipulation of contraction and visualization of the cardiac flow environment has greatly advanced the field [30-35].
Like blood vessels, the embryonic heart chambers are lined by a monolayer of endothelial cells. These ‘endocardial’ endothelial cells (EECs) are formed prior to the initiation of contraction and contribute significantly to heart development [36]. In-vivo developmental studies suggest that EECs are shear stress responsive, but the mechanisms by which they sense shear stress are still under investigation. A role for the primary cilium has been described [37-39], but additional mechanosensors have yet to be identified. Many of the studies that implicate a role for mechanotransduction in heart development rely on detection of endothelial shear-responsive transcription factors and signaling molecules [e.g. BMP, neuregulin-1 (NRG1), Krüppel-like factor 2 (KLF2), endothelin-1 (ET-1), endothelial nitric oxide synthase (eNOS)] [40]. Recent evidence suggests that mechanosensitive ion channels are important as well [41■■]. It is highly likely that endothelial cell mechanosensors previously identified in blood vessel mechanotransduction also contribute to heart development, but a role for many of these proteins has not yet been explored in this context. Additionally, it is entirely possible that EECs utilize other mechanisms of mechanotransduction.
One key aspect in studying hemodynamics in heart development is the ability to visualize and characterize the forces. With the advent of new imaging modalities, as well as the use of models that allow in-vivo assessment of mechanical forces, is it possible to link the two. These techniques range from in-vivo confocal microscopy [32,42], micro-computed tomography [43], high-frequency ultrasound, and optical coherence tomography [44,45]. Detailed analysis of cardiac flow has revealed that despite the existence of unidirectional flow through the developing blood vessels, flow in the embryonic heart is bidirectional, which exposes the heart to unique spatial shear stress [46].
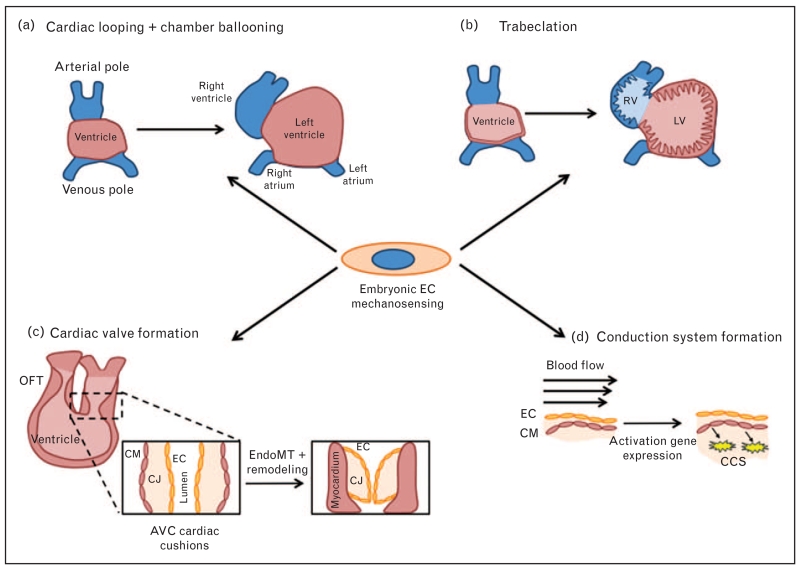
Hemodynamics plays an important role in all aspects of heart development, including looping, trabeculation, cardiac cushion/valve formation, and maturation of the conduction system (Fig. 1). The role WSS plays in each will be described in detail below.
FIGURE 1.
The major stages of cardiac development in which WSS is involved. (a) Cardiac looping coincides with the onset of the heartbeat and therefore flow. After looping, the heart chambers begin to take shape. (b) Trabeculation occurs following looping and increased the surface area of the ventricular chambers. (c) Concomitant with trabeculation is cardiac valve formation, which involves a process of EndoMT and remodeling of the tissue. (d) Conduction system formation results from hemodynamically-driven differentiation of CMs. AVC, atrioventricular canal; CCS, cardiac conduction system; CJ, cardiac jelly; CM, cardiomyocyte; EC, endothelial cell; EndoMT, endothelial to mesenchymal transition; LV, left ventricle; OFT, outflow tract; RV, right ventricle; WSS, wall shear stress.
Looping
One of the earliest and most fundamental processes in heart development is cardiac looping. The primitive heart begins as a straight tube with an atrial and ventricular pole, and is composed of three layers: myocardium and endocardium, separated by cardiac jelly. At this stage, flow through the heart is based on its function as a suction pump [47]. Early in development, the heart tube begins to elongate and then transform into a c-shaped heart loop. In mice, this occurs around E8.5, which is approximately the time at which the heart starts beating. Following looping, a primitive atria, ventricle, and outflow tract form. Using an elegant model of flow disruption in zebrafish in which a bead was placed in the heart to obstruct flow, Hove et al. [32] demonstrated that a 10-fold decrease in WSS impaired cardiac looping. Additionally, after cardiac looping, activation of shear-responsive transcription factors is required for ballooning of the cardiac chambers [48■■], which is important for defining the ventricular chamber.
Trabeculation
Following looping, the heart becomes highly trabeculated. Trabeculae are specialized sheets of developing myocardium that form endocardial cell-lined protrusions into the ventricular lumen. Functionally, they are proposed to increase surface area for oxygen–nutrient exchange in the developing myocardium [49]. A zebrafish model of altered flow through the left ventricle demonstrated that blood flow is required for this process to occur [34]. In these studies, a mutation in the atrial myosin heavy chain (amhc) gene leads to a noncontractile atrium, causing a reduction in left ventricular flow without altering left ventricular function. Under these conditions, the authors observe reduced invaginations in the lumen. Interestingly, the authors also demonstrate a requirement for endothelial NRG-1 in trabeculation and speculate that EEC-cardiomyocyte signaling may be required for this process to proceed.
Cardiac cushion and valve formation
Concomitant with trabeculation, constrictions develop at the outflow tract and atrioventricular canal, which specify where the heart valves will develop. EECs in these regions undergo a process of endothelial to mesenchymal transition and proliferate to initiate endocardial cushion formation, which remodel into the four major valves: mitral, tricuspid, pulmonary, and aortic. There is a great deal known about the signaling pathways involved, which include Notch, BMP/TGFβ, Wnt/B-catenin, and vascular endothelial growth factor among others (reviewed in [50]).
In addition to activation of signaling pathways, WSS has been demonstrated to greatly influence cardiac cushion formation and valvulogenesis [30,51]. During early looping, flow-mediated expression of the microRNA, miR-21, is required for constriction of the region that will eventually become the AV valve [52]. In E9.5-12.5 hearts, mice lacking endocardial cilia (Kif3a−/− embryos) exhibit abnormal cardiac cushion development [39]. This implicates flow sensing as an important factor in cushion development. Furthermore, it has been shown that in the atrioventricular canal, EECs experience higher WSS than other regions of the developing heart [46,53]. An additional component of WSS, flow reversal, is also important for proper valvulogenesis, as it induces elevated expression of shear-related genes KLF2a, Notch1b, and BMP4, which are required for valve formation [46]. In the absence of these forces, there is reduced gene expression and inhibition of valvulogenesis [32,46,54■]. These observations were further validated by Heckel et al. [41■■], who demonstrated that oscillatory flow amplitude mediates KLF2 activation, as well as an endocardial calcium response, via activation of the mechanosensitive ion channels Trpv2 and Trpp2. Interestingly, these ion channels have also been identified as mechanosensors in adult blood vessels [55].
Conduction system
Also critical for proper cardiogenesis is development of the cardiac conduction system (CCS), which is required for coordinated contraction of the heart. Improper formation can lead to the presence of ectopic pacemakers and arrhythmias. The CCS is derived from cardiomyocytes that express markers of the CCS early after looping [56,57]. In addition to the importance of cardiomyocytes, endothelial cells are, primarily through paracrine signaling, critical for development of this system [58]. Importantly, shear stress in the developing embryo has been shown to influence expression of endothelin-converting enzyme (ECE), which is converts ‘big endothelin’ to active endothelin (ET-1) [59,60]. More recently identified is the role of WSS in the expression and patterning of genes necessary for the proper development of the atrial conduction system [61].
HEMODYNAMICS AND MECHANOTRANSDUCTION IN THE ADULT HEART
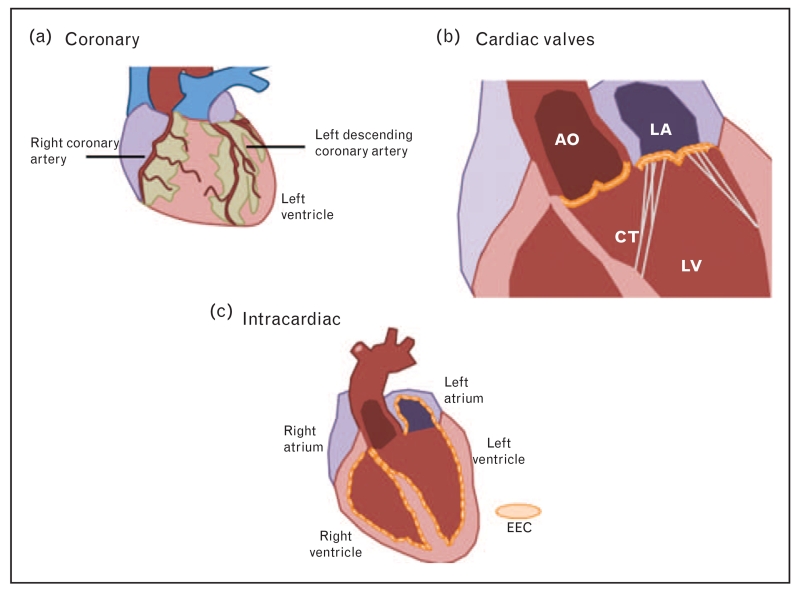
Although the role of hemodynamics in heart development has been established, significantly less is known about WSS in the adult heart. Most current knowledge relates to the coronary arteries and valves because most endothelial cell-associated cardiac abnormalities occur at these sites. The relevance of hemodynamics to the different endothelial cell populations of the heart will be discussed in the following sections (Fig. 2).
FIGURE 2.
Different populations of endothelial cells in the heart. (a) Coronary vessels supply blood to the myocardium and are found on the surface of the heart. (b) Valvular ECs line the surface of the major heart valves: mitral, tricuspid, aortic, and pulmonary. Shown are the mitral and aortic. (c) Endocardial ECs line the entire surface area of the four heart chambers. Shown are cross-sections of the left atria and ventricle. AO, aorta; CT, chordae tendinae; EC, endothelial cell; EEC, endocardial endothelium; LA, left atrium; LV, left ventricle.
Coronary
Due to the difficulty in imaging the coronary arteries, unlike other regions of the vasculature, the hemodynamic environment and measurements of WSS in these vessels are still under investigation [62]. Despite detailed WSS measurements, it is clear that coronary endothelial cells are mechanoresponsive and that this characteristic plays an important role in the focal development and progression of coronary artery disease [63-66]. Endothelial cells in regions of the coronary arteries exposed to low, disturbed flow up-regulate common atherosusceptible pathways, including endoplasmic reticulum stress and reactive oxygen species [66,67]. Interestingly, coronary endothelial cells express these pathways at higher levels than their noncoronary counterparts, which may indicate why these vessels are more susceptible to atherosclerotic plaque formation and disease progression [67]. Furthermore, low WSS sites correlate with increased lipid insudation and modification [68]. It is generally accepted that coronary endothelial cells utilize the same mechanosensors identified in vascular endothelial cells, but this has not been thoroughly investigated [6].
Cardiac valves
Valvular endothelial cells are exposed to hemodynamics throughout the cardiac cycle. Most often studied are the effects of WSS on the mitral and aortic valves as these are closely associated with the development of human disease such as mitral stenosis and calcific aortic valve disease. These hemodynamic studies have demonstrated that the aortic side of the aortic valve is most susceptible to disease. Laser Doppler velocimetry has demonstrated that the two sides of the aortic valve are exposed to different flow patterns [69,70]. Using healthy pig tissue, Simmons et al. [71] investigated gene expression on the aortic and ventricular side of the aortic valve and showed that there was differential gene expression, with up-regulation of BMP4 on the aortic side of the valve among others.
Intracardiac
Similarly to vascular endothelial cells, EECs lining the adult heart chambers are constantly exposed to WSS. Unlike vascular endothelial cells, the effect of WSS on EECs remains understudied and poorly defined. In-vitro studies using primary EECs suggest that these cells are shear stress responsive as they up-regulate prostacyclin production in response to flow [72]. Although informative, these studies lack detailed information regarding the hemodynamic environment and rely on WSS values derived from the vasculature. Studies in vivo support this hypothesis, demonstrating that manipulating the hemodynamic environment influences EEC phenotype [73-75]. Again, these studies lack information about the hemodynamic environment, which presumably is altered through manipulation of heart rate and pressure. The use of four-dimensional (4D) flow MRI has allowed the more thorough description of cardiovascular hemodynamics, in particular intracardiac blood flow [76,77]. Our recent data suggest that like both vascular endothelial cells and embryonic EECs, adult EECs are sensitive to their hemodynamic environment (manuscript in review). Using 4D flow MRI, we have recently measured WSS in adult humans and pigs, and isolated EECs from these regions. Using next-generation RNA sequencing, we show that EECs from the different regions have dramatically different phenotypes, suggesting that in a normal heart, WSS has an important effect.
CONTRIBUTION OF ENDOTHELIAL CELL MECHANOTRANSDUCTION IN CELL-TO-CELL COMMUNICATION
Studies using isolated preparations of myocardium have demonstrated the requirement for EECs in regulating cardiac function [78-81]. Specifically, early studies of endocardial surface damage resulted in altered myocardial contraction, suggesting that the endothelial layer functions to regulate peak contraction and relaxation of the myocardial layer [78]. Other studies identified a role of ET-1, nitric oxide, prostacyclin, and NRG-1 (all factors released from endothelial cells) in regulation of cardiomyocyte contractility. Brutsaert provides an excellent review of this in [82]. Importantly, not only are these paracrine factors similar to those identified in endothelial cell-vascular smooth muscle cell cross-talk, but several are known to be regulated by shear stress in vascular endothelial cells. Recent work from our laboratory has identified a role for the mechanosensor PECAM-1 in the regulation of cardiac contractility and function. We showed that aberrant release of NRG-1 in PECAM-1 knockout hearts and endothelial cell results in increased ErbB2 phosphorylation and signaling [83]. Although this might seem at odds with the described cardioprotective effects of NRG-1 [84], it does underscore the importance of precise spatiotemporal regulation of NRG-1/ErbB2 signaling and the significance of endothelial cell mechanotransduction in EEC-cardiomyocyte cross-talk [83]. The present study is focused on identifying the molecular mechanisms by which PECAM-1 regulates NRG-1 release, and the role of hemodynamics in this pathway.
CONCLUSION
Here, we discuss the importance of shear stress and mechanotransduction in numerous aspects of cardiac biology from development, to the adult and in disease. A deeper understanding of hemodynamic forces in the heart may represent a new pharmacologically tractable area for treating cardiac disease.
KEY POINTS.
Endothelial cells are continuously exposed to WSS, the frictional force imparted by blood flow, and are able to adapt to changing shear stress conditions.
The response of vascular endothelial cells in both development and in the adult has been studied in detail, but much less is known about EECs, which line the chambers of the heart.
Recent studies have shown that shear stress and EEC mechanotransduction play a critical role in all aspects of heart development, including chamber morphogenesis and valve development.
Significantly less is known about adult EECs and whether and how they respond to shear stress.
Acknowledgements
None.
Financial support and sponsorship
Supported by NIH NRSA Training Grant T32 HL07954 (M.E.M.) and Wellcome Trust Senior Fellowship and NIH HL117256 (E.T.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Laubichler MD, Aird WC, Maienschein J. The endothelium in history. In: Aird WC, editor. Endothelial biomedicine. Cambridge University Press; Cambridge: 2007. pp. 5–22. [Google Scholar]
- 2.Florey HW. The endothelial cell. Br Med J. 1966;2:487–490. doi: 10.1136/bmj.2.5512.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46:9–15. [PubMed] [Google Scholar]
- 4.Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117:1082–1089. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- 5.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll of Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 7.Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 8.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 9.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 10.Dekker RJ, van Soest S, Fontijn RD, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 11.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 12.Krebs LT, Shutter JR, Tanigaki K, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- 14.Izraeli S, Lowe LA, Bertness VL, et al. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature. 1999;399:691–694. doi: 10.1038/21429. [DOI] [PubMed] [Google Scholar]
- 15.Yuasa S, Fukuda K. Multiple roles for BMP signaling in cardiac development. Drug Discov Today Ther Strateg. 2008;5:209–214. [Google Scholar]
- 16.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdulla R, Blew GA, Holterman MJ. Cardiovascular embryology. Pediatr Cardiol. 2004;25:191–200. doi: 10.1007/s00246-003-0585-1. [DOI] [PubMed] [Google Scholar]
- 18.Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinsen BJ. Reference guide to the stages of chick heart embryology. Dev Dyn. 2005;233:1217–1237. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- 20.Staudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Ann Rev Genet. 2012;46:397–418. doi: 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucitti JL, Jones EA, Huang C, et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boselli F, Freund JB, Vermot J. Blood flow mechanics in cardiovascular development. Cell Mol Life Sci. 2015;72:2545–2559. doi: 10.1007/s00018-015-1885-3. ■ A comprehensive review of the role for signaling and blood flow in heart development.
- 23.North TE, Goessling W, Peeters M, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galie PA, Nguyen DH, Choi CK, et al. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci USA. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaffari S, Leask RL, Jones EA. Flow dynamics control the location of sprouting and direct elongation during developmental angiogenesis. Development. 2015;142:4151–4157. doi: 10.1242/dev.128058. [DOI] [PubMed] [Google Scholar]
- 26.Sabine A, Agalarov Y, Maby-El Hajjami H, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Sweet DT, Jimenez JM, Chang J, et al. Lymph flow regulates collecting lymphatic maturation in vivo. J Clin Invest. 2015;125:2995–3007. doi: 10.1172/JCI79386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Bartman T, Walsh EC, Wen KK, et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2:E129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harh JY, Paul MH, Gallen WJ, et al. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am J Cardiol. 1973;31:51–56. doi: 10.1016/0002-9149(73)90810-2. [DOI] [PubMed] [Google Scholar]
- 32.Hove JR, Köster RW, Forouhar AS, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 33.Nishii K, Morimoto S, Minakami R, et al. Targeted disruption of the cardiac troponin T gene causes sarcomere disassembly and defects in heartbeat within the early mouse embryo. Dev Biol. 2008;322:65–73. doi: 10.1016/j.ydbio.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Peshkovsky C, Totong R, Yelon D. Dependence of cardiac trabeculation on neuregulin signaling and blood flow in zebrafish. Dev Dyn. 2011;240:446–456. doi: 10.1002/dvdy.22526. [DOI] [PubMed] [Google Scholar]
- 35.Sedmera D, Pexieder T, Rychterova V, et al. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 36.Harris IS, Black BL. Development of the endocardium. Pediatr Cardiol. 2010;31:391–399. doi: 10.1007/s00246-010-9642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Heiden K, Groenendijk BC, Hierck BP, et al. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- 38.Egorova AD, Khedoe PP, Goumans MJ, et al. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res. 2011;108:1093–1101. doi: 10.1161/CIRCRESAHA.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn. 2008;237:2304–2314. doi: 10.1002/dvdy.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groenendijk BC, Hierck BP, Gittenberger-De Groot AC, Poelmann RE. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn. 2004;230:57–68. doi: 10.1002/dvdy.20029. [DOI] [PubMed] [Google Scholar]
- 41.Heckel E, Boselli F, Roth S, et al. Oscillatory flow modulates mechanosensitive klf2a expression through trpv4 and trpp2 during heart valve development. Curr Biol. 2015;25:1354–1361. doi: 10.1016/j.cub.2015.03.038. ■ ■ In this study, the authors identify two ion channels found in endocardial endothelial cells that are required for mechanotransduction and valve development in zebrafish.
- 42.Kowalski WJ, Teslovich NC, Menon PG, et al. Left atrial ligation alters intracardiac flow patterns and the biomechanical landscape in the chick embryo. Dev Dyn. 2014;243:652–662. doi: 10.1002/dvdy.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degenhardt K, Wright AC, Horng D, et al. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ Cardiovasc Imaging. 2010;3:314–322. doi: 10.1161/CIRCIMAGING.109.918482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midgett M, Chivukula VK, Dorn C, et al. Blood flow through the embryonic heart outflow tract during cardiac looping in HH13-HH18 chicken embryos. J R Soc Interface. 2015;12:20150652. doi: 10.1098/rsif.2015.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garita B, Jenkins MW, Han M, et al. Blood flow dynamics of one cardiac cycle and relationship to mechanotransduction and trabeculation during heart looping. Am J Physiol Heart Circ Physiol. 2011;300:H879–H891. doi: 10.1152/ajpheart.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermot J, Forouhar AS, Liebling M, et al. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 2009;7:e1000246. doi: 10.1371/journal.pbio.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forouhar AS, Liebling M, Hickerson A, et al. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 2006;312:751–753. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- 48.Dietrich AC, Lombardo VA, Veerkamp J, et al. Blood flow and Bmp signaling control endocardial chamber morphogenesis. Dev Cell. 2014;30:367–377. doi: 10.1016/j.devcel.2014.06.020. ■■ A very interesting study using zebrafish to study chamber ballooning morphogenesis and demonstrating that the hemodynamic-responisive transcription factor Klf2a plays a key role in regulating chamber growth.
- 49.Sedmera D, Pexieder T, Vuillemin M, et al. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 50.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Phil Trans R Soc London Biol. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butcher JT, McQuinn TC, Sedmera D, et al. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100:1503–1511. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- 52.Banjo T, Grajcarek J, Yoshino D, et al. Haemodynamically dependent valvulogenesis of zebrafish heart is mediated by flow-dependent expression of miR-21. Nat Commun. 2013;4:1978. doi: 10.1038/ncomms2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yalcin HC, Shekhar A, McQuinn TC, Butcher JT. Hemodynamic patterning of the avian atrioventricular valve. Dev Dyn. 2011;240:23–35. doi: 10.1002/dvdy.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalogirou S, Malissovas N, Moro E, et al. Intracardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardiovasc Res. 2014;104:49–60. doi: 10.1093/cvr/cvu186. ■ This study uses genetic manipulation in zebrafish to separate blood flow and myocardial contractility to demonstrate that blood flow regulates valvulogenesis independent of contracility.
- 55.Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- 56.Cheng G, Litchenberg WH, Cole GJ, et al. Development of the cardiac conduction system involves recruitment within multipotent cardiomyogenic lineage. Development. 1999;126:5041–5049. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- 57.Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generated Purkinje fibers of the cardiac conduction system. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- 58.Rentschler S, Zander J, Meyers K, et al. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci USA. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall CE, Hurtado R, Hewett KW, et al. Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting Purkinje fibers in the embryonic heart. Development. 2004;131:581–592. doi: 10.1242/dev.00947. [DOI] [PubMed] [Google Scholar]
- 60.Reckova M, Rosengarten C, deAlmeida A, et al. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93:77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 61.Bressan MC, Louie JD, Mikawa T. Hemodynamic forces regulate developmental patterning of atrial conduction. PloS One. 2014;9:e115207. doi: 10.1371/journal.pone.0115207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Gutierrez-Chico JL, Holm NR, et al. Impact of side branch modeling on computation of endothelial shear stress in coronary artery disease: coronary tree reconstruction by fusion of 3D angiography and OCT. J Am Coll Cardiol. 2015;66:125–135. doi: 10.1016/j.jacc.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254. [DOI] [PubMed] [Google Scholar]
- 64.Pedrigi RM, Poulsen CB, Mehta VV, et al. Inducing persistent flow disturbances accelerates atherogenesis and promotes thin cap fibroatheroma development in D374Y-PCSK9 hypercholesterolemic minipigs. Circulation. 2015;132:1003–1012. doi: 10.1161/CIRCULATIONAHA.115.016270. [DOI] [PubMed] [Google Scholar]
- 65.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 66.Stone PH, Coskun AU, Kinlay S, et al. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J. 2007;28:705–710. doi: 10.1093/eurheartj/ehl575. [DOI] [PubMed] [Google Scholar]
- 67.Civelek M, Manduchi E, Riley RJ, et al. Coronary artery endothelial transcriptome in vivo: identification of endoplasmic reticulum stress and enhanced reactive oxygen species by gene connectivity network analysis. Circ Cardiovasc Genet. 2011;4:243–252. doi: 10.1161/CIRCGENETICS.110.958926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 69.Yap CH, Saikrishnan N, Tamilselvan G, Yoganathan AP. Experimental measurement of dynamic fluid shear stress on the aortic surface of the aortic valve leaflet. Biomech Model Mechanobiol. 2012;11:171–182. doi: 10.1007/s10237-011-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yap CH, Saikrishnan N, Yoganathan AP. Experimental measurement of dynamic fluid shear stress on the ventricular surface of the aortic valve leaflet. Biomech Model Mechanobiol. 2012;11:231–244. doi: 10.1007/s10237-011-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanada T, Hashimoto M, Nosaka S, et al. Shear stress enhances prostacyclin release from endocardial endothelial cells. Life Sci. 2000;66:215–220. doi: 10.1016/s0024-3205(99)00583-4. [DOI] [PubMed] [Google Scholar]
- 73.Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 74.Kapur NK, Deming CB, Kapur S, et al. Hemodynamic modulation of endocardial thromboresistance. Circulation. 2007;115:67–75. doi: 10.1161/CIRCULATIONAHA.106.640698. [DOI] [PubMed] [Google Scholar]
- 75.Yamashita T, Sekiguchi A, Iwasaki YK, et al. Thrombomodulin and tissue factor pathway inhibitor in endocardium of rapidly paced rat atria. Circulation. 2003;108:2450–2452. doi: 10.1161/01.CIR.0000102969.09658.F2. [DOI] [PubMed] [Google Scholar]
- 76.Markl M, Frydrychowicz A, Kozerke S, et al. 4D flow MRI. J Magn Reson Imaging. 2012;36:1015–1036. doi: 10.1002/jmri.23632. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez Muñoz D, Markl M, Moya Mur JL, et al. Intracardiac flow visualization: current status and future directions. Eur Heart J Cardiovasc Imaging. 2013;14:1029–1038. doi: 10.1093/ehjci/jet086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mebazaa A, Mayoux E, Maeda K, et al. Paracrine effects of endocardial endothelial cells on myocyte contraction mediated via endothelin. Am J Physiol Heart Circ Physiol. 1993;34:H1841–H1846. doi: 10.1152/ajpheart.1993.265.5.H1841. [DOI] [PubMed] [Google Scholar]
- 79.Shen X, Tan Z, Zhong X, et al. Endocardial endothelium is a key determinant of force-frequency relationship in rat ventricular myocardium. J Appl Physiol. 2013;115:383–393. doi: 10.1152/japplphysiol.01415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith JA, Shah AM, Lewis MJ. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol. 1991;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brutsaert DL, Meulemans AL, Sipido KR, Sys SU. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circ Res. 1988;62:358–366. doi: 10.1161/01.res.62.2.358. [DOI] [PubMed] [Google Scholar]
- 82.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 83.McCormick ME, Collins C, Makarewich CA, et al. Platelet endothelial cell adhesion molecule-1 mediates endothelial-cardiomyocyte communication and regulates cardiac function. J Am Heart Assoc. 2015;4:e001210–e11210. doi: 10.1161/JAHA.114.001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X, Gu X, Li Z, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]