Abstract
Importance
Safe, efficacious second-line pharmacological treatment options exist for the large portion of older adults with major depressive disorder that does not respond to first-line pharmacotherapy. However, limited evidence exists to aid clinical decision-making regarding which patients will benefit from which second-line treatments.
Objective
To test the moderating role of pretreatment executive function, anxiety severity, and medical comorbidity on remission to aripiprazole augmentation for treatment-resistant late-life depression.
Design
We conducted a National Institute of Mental Health sponsored 12-week, multi-site, randomized, placebo-controlled, double-blind trial of aripiprazole augmentation for first-line resistant late-life major depressive disorder. Using logistic regression, we evaluated the main effects of the following potential moderators and their interactions with treatment: baseline assessments of executive function (set shifting measured by a Trail Making test) and response inhibition control (measured by a color-word interference task), anxiety symptoms, and medical comorbidity.
Setting
Specialty care.
Participants
We included 181 participants aged 60 and older whose major depression had failed to remit with venlafaxine monotherapy.
Intervention
Aripiprazole or placebo tablets were started at 2 mg daily and titrated as tolerated, to a maximal dose of 15 mg daily.
Main outcome measure
The outcome was remission defined as a MADRS score ≤10 at both of the last two consecutive visits.
Results
Baseline set-shifting moderated aripiprazole efficacy (p for interaction with treatment = 0.03): among participants with Trail-Making test Scaled Scores ≥7, the odds of remitting were significantly higher with aripiprazole than with placebo (53% versus 28%; NNT = 4; OR = 4.11, 95% CI: 1.83-9.20); among participants with Trail-Making test Scaled Scores <7, aripiprazole and placebo were equally efficacious (aripiprazole vs. placebo remission OR=0.64, 95% CI: 0.15-2.80). Greater severity of anxiety at baseline predicted a lower remission rate but did not moderate aripiprazole efficacy: each standard deviation greater anxiety severity was associated with 50% reduced odds of remission in both aripiprazole and placebo arms. Medical comorbidity and Color-Word interference test performance were neither general predictors nor treatment moderating factors.
Conclusion and relevance
Set-shifting performance indicates which older adults with treatment-resistant depression may respond favorably to augmentation with aripiprazole and thus may help to personalize treatment.
Over half of older adults with major depressive disorder (MDD) fail to respond adequately to first-line pharmacotherapy with serotonin reuptake inhibitors (SSRI) or serotonin-norepinephrine reuptake inhibitors (SNRI)1. Persistent depression in this population heightens the risk for disability, non-adherence to treatment for other medical disorders, cognitive impairment leading to dementia, low quality of life, caregiver burden, suicidality and early mortality2-6. Data from controlled trials to guide second-line treatment in older adults are sparse7.
Recently, we reported the results of a randomized controlled trial testing the efficacy and safety of aripiprazole for late-life depression that is resistant to first-line treatment8. Aripiprazole is an atypical antipsychotic approved by the FDA for such second-line augmentation treatment of MDD. Its pharmacodynamic actions involve dopamine D2 and D3 receptor partial agonism and serotonin 5HT1a and 5HT2a receptor antagonism9,10. Aripiprazole is not an anticholinergic drug, and aripiprazole's partial dopamine receptor agonism may have favorable effects in late-life depression wherein the mesolimbic dopamine system may be disrupted11,12. Indeed, the addition of aripiprazole to venlafaxine was well tolerated and effective in inducing and maintaining remission for up to 12 weeks: 44% of participants treated with aripiprazole remitted, as compared with 29% of placebo-randomized participants.
While our initial report8 focused on the safety and efficacy of aripiprazole for treatment resistant late-life depression (the first two aims of our study), the current report focuses on our third aim, which was to examine the roles of pre-specified variables as moderators of response to aripiprazole. The importance of identifying treatment moderators has been highlighted previously13. Briefly, it is important to determine if specific clinical characteristics are general prognostic variables (i.e., that predict the course of depression regardless of treatment) or treatment moderating factors (i.e., that predict the effect size of active treatment vs. placebo). Identifying moderators of aripiprazole response could help clinicians tailor treatment to individual patients, thereby minimizing exposure to inefficacious trial-and-error pharmacotherapy.
Evidence regarding moderators of response to first-line treatment for late-life depression has advanced to the level of meta-analysis14. This literature has found that cognitive impairment15, anxiety16, and medical comorbidity17 may moderate response to first-line late-life depression treatments. However, to our knowledge, no such data exist regarding the moderators of response to second-line treatments like aripiprazole. Since more than half of older adults do not adequately respond to first-line pharmacological therapy for major depressive disorder1, it is important to identify for whom such second-line pharmacotherapy treatments are likely to be efficacious.
We therefore evaluated the potential moderating roles of three factors, specified a priori in the clinicaltrials.gov registration (executive dysfunction, anxiety severity, and medical burden), on remission to 12-weeks of aripiprazole augmentation therapy. We selected these factors based on prior literature regarding their role as moderators of first line treatments15-17, as well as the fact that these factors are highly but variably prevalent among older adults with depression. We hypothesized that pretreatment executive function, severity of anxiety, and severity of medical comorbidity would moderate the efficacy of aripiprazole augmentation.
Methods
Data from the NIMH-sponsored study of “Incomplete Response in Late-Life Depression: Getting to Remission” (“IRL-GRey”) were used for these analyses. IRL-GRey was a multi-site, placebo-controlled, randomized clinical trial conducted to test the efficacy, safety, and tolerability of aripiprazole augmentation in older adults whose depression had not remitted with venlafaxine8. The methods of IRL-GRey have been described in detail previously8 and are summarized below.
Participants
Participants were aged ≥60 with a current major depressive episode (diagnosed by SCID/DSM-IV criteria) and a Montgomery Asberg Depression Rating Scale (MADRS)8 score of ≥15. Individuals with a diagnosis of bipolar disorder, dementia, schizophrenia, current psychotic symptoms, and alcohol or substance abuse during the last 6 months were excluded from the study. All participants provided written, informed consent. The conduct of the study was overseen by a Data Safety and Monitoring Board.
Interventions
After open treatment with venlafaxine XR (up to 300 mg/day) for 12 weeks to establish treatment resistance, the 181 participants who did not achieve remission (defined by a MADRS score ≤ 10 for two sequential assessments) were randomly assigned, using permuted block randomization, to the addition of aripiprazole or placebo for 12 weeks, while maintaining the dose of venlafaxine achieved during initial monotherapy. This randomized augmentation phase was conducted under double-blind conditions with outcomes assessed by independent evaluators. In terms of allocation concealment, no member of the research team other than the pharmacist was aware of treatment assignment. Aripiprazole or placebo tablets were started at 2 mg daily and titrated as tolerated, to a maximal dose of 15 mg daily.
Assessments
Outcome
The primary outcome of the treatment trial and current analysis was final remission, defined as a MADRS score ≤10, at the last two consecutive visits (week 10 and 12 of the augmentation treatment phase). Depression symptoms were measured over time with the total MADRS score assessed at each weekly or biweekly visit. Assessments were administered by an independent evaluator and regular inter-site sessions were conducted to maintain inter-rater reliability (intraclass correlation coefficient [ICC] = 0.997).
Baseline Clinical Factors
We administered a neuropsychological test battery (supervised by senior neuropsychologist, MAB) before starting venlafaxine XR open treatment. Executive function was evaluated using two tests from the Delis-Kaplan Executive Function Scale (D-KEFS)—the Color-Word Interference task (measuring response inhibition) and two Trail-Making tasks (measuring set shifting)18. Color-Word condition 3, called “inhibition,” assess ability to inhibit an automatic response (i.e., reading words); instead, participants must produce a response that requires more effort (i.e., naming the colors of words). The Trail Making Test condition 4 (also known as the Number-Letter Switching condition) requires that examinees switch back and forth between connecting numbers and letters (i.e., 1, A, 2, B, etc., to 16, P). Condition 5 is a motor speed condition in which examinees trace over a dotted line connecting circles on the page as quickly as possible, in order to gauge their motor drawing speed. Comparing performance on Condition 4 (which assesses cognitive flexibility) with performance on Condition 5 (which assesses motor speed) removes the motor speed element from the test score to ascertain cognitive flexibility19; we used the D-KEFS normed scaled score (with a mean of 10 and standard deviation of 3) based on the difference in speeds on condition 4 and 5, representing set-shifting performance20.
The Brief Symptom Inventory (BSI), a self-report scale with strong construct validity, internal consistency, and test-retest reliability21 was used as a continuous score to assess anxiety symptoms. The Cumulative Illness Rating Scale for Geriatrics (CIRS-G)22 expressed as a total score, was used to assess medical comorbidity. The BSI and CIRS-G were administered at the start of aripiprazole/placebo augmentation.
Statistical analysis
All continuous variables, including executive function measures, were standardized before analysis. In our primary analyses, the hypothesized moderators were expressed as continuous predictors using separate logistic regression models of remission status. We defined general prognostic factors as main effects significantly (p<0.05) associated with the odds of remission that did not demonstrate interaction with treatment assignment. Moderating factors were defined as those baseline variables that interacted with treatment in predicting remission. To illustrate moderating effects, we present the odds of remission associated with each hypothesized moderator stratified by treatment assignment. All models were adjusted for age, gender, study site, and treatment assignment; models including executive function variables were further adjusted for educational attainment.
To test whether the associations detected in separate models were independent of each other, we constructed a final multivariable model including the significant main effects and interactions identified. (Main effects of variables composing the selected interactions were also included, as required for properly interpreting the regression analysis). To increase interpretability and clinical relevance, when interactions were detected between treatment and continuously expressed executive function performance, we also examined the effect of impairment in the executive function domain. We defined executive impairment as scores more than 1 standard deviation below age-normed performance, and present the odds of remission associated with aripiprazole (vs. placebo) among patients with and without impaired executive function. Clinical effect sizes are expressed in numbers needed to treat (NNT).
Results
Demographic and clinical characteristics are presented in Table 1, broken down by treatment arm and remission status. Examining the potential moderators separately (Table 2) indicated that better baseline performance on the Trail-Making task (condition 4 vs. 5; set-shifting) was associated with higher odds of remission among patients treated with aripiprazole but not placebo (p for interaction with treatment=0.03); this interaction was significant with (Table 2) and without (p=0.03) adjustments for age and sex. Performance on the Color-Word Interference task (response inhibition) was not a significant predictor of remission (i.e. confidence intervals overlapped with 1, see Table 2), and there was no moderating effect (p for interaction with treatment=0.38).
Table 1. Descriptive information by treatment arm and remission status.
| Aripiprazole | Placebo | |||
|---|---|---|---|---|
|
|
||||
| Remitters (n=40) | Non-remitters (n=51) | Remitters (n=26) | Non-remitters (n=64) | |
|
|
||||
| Age | 68.08 (4.53) | 67.34 (7.42) | 66.59 (4.90) | 67.25 (5.87) |
| Gender female, % (n) | 55 (22) | 58.82 (30) | 38.46 (10) | 64.06 (41) |
| White, % (n) | 95.00 (38) | 82.35 (42) | 84.62 (22) | 89.06 (57) |
| Educational attainment (years) | 15.03 (2.75) | 13.51 (2.96) | 14.19 (2.88) | 14.14 (2.62) |
| Depression (MADRS) | 21.45 (6.42) | 25.09 (5.99) | 19.78 (5.36) | 24.34 (6.60) |
| Anxiety (BSI) | 0.81 (0.57) | 1.27 (0.81) | 0.51 (0.60) | 0.99 (0.81) |
| Physical health (CIR) | 10.48 (9.59) | 9.59 (4.39) | 9.42 (3.43) | 9.30 (4.49) |
| Color-Word Interference (condition 3)* | 10.98 (2.57) | 9.90 (3.53) | 10.24 (2.67) | 10.08 (3.14) |
| Trail Making Test (condition 4 vs. 5, scaled score) | 9.18 (3.18) | 7.55 (4.04) | 8.50 (3.44) | 8.98 (3.58) |
used with permission from Pearson©; Abbreviations: BSI, Brief Symptom Inventory; CIR, Cumulative illness rating scale;
Table 2. Odds ratios and 95% confidence intervals for remission associated with potential moderators within aripiprazole and placebo arms.
| Odds Ratio (95% confidence interval) | Interaction p-value (Treatment* Moderator) | |
|---|---|---|
|
|
|
|
| Anxiety (BSI) among patients treated with: | 0.64 | |
| Aripiprazole (n=91) | 0.54 (0.32-0.93) | |
| Placebo (n=90) | 0.44 (0.22-0.87) | |
| Physical health (CIR total) among patients treated with: | 0.57 | |
| Aripiprazole (n=91) | 1.13 (0.74-1.70) | |
| Placebo (n=90) | 0.93 (0.56-1.56) | |
| Color-Word Interference (condition 3) among patients treated with: | 0.38 | |
| Aripiprazole (n=89) | 1.43 (0.90-2.26) | |
| Placebo (n=86) | 1.05 (0.63-1.74) | |
| Trail Making Test (condition 4 vs. 5, scaled score) among patients treated with: | 0.03 | |
| Aripiprazole (n=89) | 1.66 (1.05-2.62) | |
| Placebo (n=84) | 0.75 (0.44-1.27) |
Moderators are separately modeled (per standard deviation) adjusted for age, gender, and study site; Abbreviations: BSI, Brief Symptom Inventory; CIR, Cumulative illness rating scale;
For each standard deviation (overall BSI SD=0.77) higher pre-treatment anxiety severity, the odds of remission were reduced by 54% (Table 2). Note that this association was consistent within both aripiprazole and placebo arms, indicating pre-treatment anxiety was a general prognostic but not moderating factor.
Medical comorbidity was not a significant predictor of remission, and we detected no interaction between medical comorbidity and treatment arm (Table 2).
A final multivariable model including the above-detected crude associations indicated that both the general prognostic role of anxiety and the moderating effect of trail-making test performance were independent (Table 3). We did not detect significant interactions of age or gender with treatment (p=0.33 and 0.11, respectively), and further adjusting the multivariable model (Table 3) for these interaction terms did not alter the associations of anxiety with remission or the interaction of trail-making test performance with treatment.
Table 3. Parameter estimates from the final multivariable model* predicting remission (n=173).
| β (SE) | p-value | |
|---|---|---|
|
|
|
|
| Aripiprazole (main effect vs. placebo) | 0.50 (0.18) | 0.006 |
| Anxiety (BSI main effect per SD) | -0.70 (0.22) | 0.002 |
| Trails Making Test 4 vs. 5 (main effect per SD) | 0.11 (0.20) | 0.59 |
| Treatment*Trails Making Test 4 vs. 5 (interaction) | 0.44 (0.19) | 0.02 |
Adjusted for all factors above, plus age, gender, study site, and education; Abbreviations: BSI, Brief Symptom Inventory;
Table 4 further highlights the treatment moderating effect of Trail-Making task performance. Among participants with a Trail-Making baseline score equal to or greater than 7 (within one standard deviation of age-normed performance or better19), the odds of remitting were more than four times higher with aripiprazole treatment than with placebo (OR = 4.11, 95% CI: 1.83-9.20). However, among patients with Trail-Making task (condition 4 vs. 5) scores less than 7, there was no statistically significant difference between the treatment arms (Table 4).
Table 4. Adjusted odds ratios (OR) and 95% confidence intervals (CI) for remission illustrating the interaction between trail-making test impairment and treatment (aripiprazole vs. placebo).
| OR (95% CI) | |
|---|---|
|
|
|
| Effect of aripiprazole (vs. placebo) among patients with: | |
| Trail Making Test condition 4 vs. 5 scaled scores ≥ -1 SD | 4.11 (1.83-9.20) |
| Trail Making Test condition 4 vs. 5 scaled scores < -1 SD | 0.64 (0.15-2.80) |
Adjusted for age, gender, study site, education, anxiety, and the main effects of treatment and trails condition 4 vs. 5 comparing patients with scaled scores <-1 SD below the norm vs. the rest; Abbreviations: SD, standard deviation;
Remission rates also illustrate this moderating effect (Figure 1, top): among patients with Trail-Making task (condition 4 vs. 5) scores ≥7, aripiprazole was associated with higher remission rates when compared with placebo (aripiprazole: 53.3%, placebo: 28.1%). Thus, among participants with in-tact set-shifting performance, the number needed to treat with aripiprazole was 4. However, among patients with Trail-Making task (condition 4 vs. 5) scores less than 7, remission rates were low in both treatment arms (aripiprazole: 21.7%, placebo: 30.0%).
Figure 1. Remission rates in aripiprazole vs. placebo stratified by the presence of high anxiety (top) and set-shifting impairment (bottom).
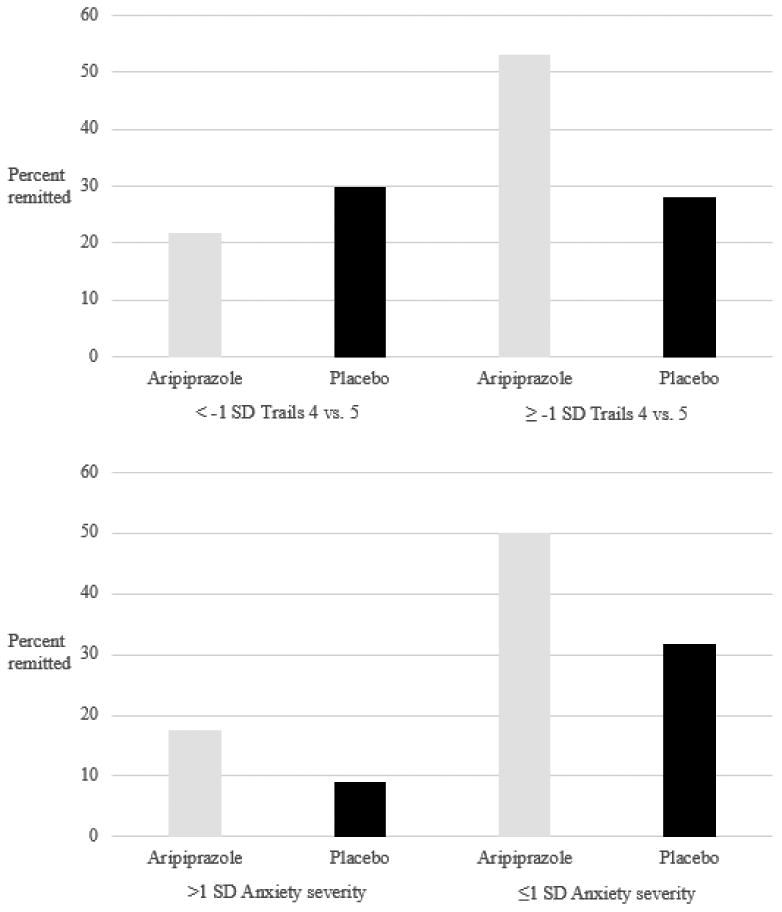
High anxiety is associated with lower remission rates, but no difference in aripiprazole-placebo separation (anxiety is a general prognostic factor that does not moderate aripiprazole efficacy). In contrast, set-shifting impairment is a treatment moderator: in the absence of set-shifting impairment, aripiprazole is clearly superior to placebo; however, in the presence of set-shifting impairment, there is no difference between treatment arms (see odds ratios in Table 4).
To illustrate the general prognostic role of baseline anxiety symptoms, we present remission rates stratified by treatment arm and the presence BSI-measured anxiety symptoms >1 standard deviation of the sample's mean (Figure 1, bottom): remission rates are reduced in the groups with higher baseline anxiety severity, but the difference in remission rates between aripiprazole and placebo arms is similar across groups with lower and higher anxiety severity. We also performed a post-hoc descriptive analysis of basic patient characteristics in groups with and without set-shifting impairment (Supplemental Table). Patient with and without set-shifting performances did not differ on the clinical characteristics examined, including the rate of treatment-emergent akathisia (the most common adverse effect we previously identified8). Patients with set-shifting impairment were somewhat older and less often white.
Discussion
Given the high rate of treatment resistance in late-life depression, clinicians, patients, and family caregivers need data from controlled clinical trials to inform treatment decisions. The IRL-GRey study previously demonstrated that aripiprazole is efficacious and well tolerated for inducing and maintaining remission in older adults 8. However this prior work did not address which patients might benefit from aripiprazole. Based on pre-specified moderator analyses, the current work now adds that pre-treatment performance on the Trail-Making task condition 4 vs. 5 (set-shifting) moderated the efficacy of aripiprazole response. Aripiprazole was associated with higher odds of remission (compared with placebo) only among participants without set-shifting impairment.
The observed moderating effect of set shifting performance is consistent with previous studies showing that cognitive dysfunction in general and executive impairment in particular correlate with (predict) poor treatment outcomes in late-life depression23,24. The current work provides, to our knowledge, the first test of these moderators (including two measures of executive function) in a large randomized trial of second-line treatment. Our findings making clear the distinction between the general prognostic and moderating effects in treatment resistant late-life depression. We found that pre-treatment performance on the Color-Word Interference task (response inhibition) showed neither a prognostic nor moderating effect over the trial. The two sub-domains of executive function examined (set-shifting and response inhibition) may therefore dissociable in their ability to moderate the efficacy of aripiprazole for venlafaxine-resistant late-life depression. Specificity of executive function deficits may indicate differences in the neurobiological basis of resistance to particular treatments (e.g. first and second line moderators).
We also found that greater severity of anxiety was associated with lower odds of remission, but did not influence the strength of (moderate) aripiprazole efficacy. The absence of treatment moderation associated with anxiety severity suggests that aripiprazole is efficacious regardless of pre-treatment anxiety levels. Nevertheless, the observed evidence for a general prognostic role of pre-treatment anxiety is useful information for determining which patients are likely to have a more challenging treatment course. Medical comorbidity as assessed with the CIRS-G was neither a moderating nor general prognostic factor. We previously reported that higher medical burden undermines the stability of remission over two years, placing patients at higher risk for recurrence of major depressive episodes17. In light of these findings, it will be important for future studies to determine whether pre-treatment medical comorbidity has a differential effect on clinical outcomes over short-term and long-term aripiprazole treatment.
Strengths of our study include the placebo-controlled design following open-label venlafaxine treatment that prospectively established treatment resistance. Identifying set-shifting performance as a moderator of aripiprazole efficacy could be an important step towards personalizing intervention strategies for older adults with MDD. Our initial report from the IRLGRey RCT found aripiprazole was associated with a NNT of 6.625, the current report now adds that, among patients with normal set-shifting functioning, aripiprazole is associated with a NNT of 4.0. Equally important, our current findings suggest aripiprazole may not be efficacious for treatment resistant late-life depression occurring in the presence of set-shifting impairment. Since set-shifting tests, including a version of the Trail Making Test, are fairly easily administered and available in the public domain19 clinicians may find such tests useful in objectively evaluating who is likely or not likely to respond to aripiprazole augmentation.
Executive dysfunction, including impaired set-shifting, is also present in teenagers and young adults with major depression26, even during the first major depressive episode27. Given the relatively “young” older adults included in this study (mean age of 68), we can hypothesize that executive dysfunction might also be a moderator of treatment response in younger age patients as well. Future research is needed to investigate whether executive dysfunction is a moderator of antidepressant treatment response in other age groups.
Although our study focused on clinical rather than biological factors, the observed moderating effect of set-shifting impairment suggests a possible neurobiological basis of aripiprazole resistance among older adults with treatment-resistant depression. Set shifting is plausibly related to aripiprazole's known mechanisms of action, as set-shifting involves co-operative interaction between D1 and D2 receptors in the prefrontal cortex (PFC)28. A recent, small study of treatment-resistant depression found that response to aripiprazole treatment is associated with enhanced dopaminergic activity in the striatum10. Set-shifting impairment and aripirazole non-response may therefore share a common substrate in dopamine receptor imbalance, and/or loss of structural integrity of the frontostriatal connections (potentially due to cerebrovascular, neurodegenerative or other pathological processes). These changes may lead to reduced aripiprazole target engagement and therapeutic success by preventing effective activation of the relevant dopaminergic circuits. Although plausible, the biological basis of aripiprazole's antidepressant effect and the moderating role of set-shifting impairment must be confirmed in future research.
Other limitations of our study should also be noted. Most participants in the IRL-GRey study could be characterized as being “young-old”, with a mean age of 68. As a result of this somewhat truncated distribution of participant ages, our results may not be generalizable to the “older-old” patient population. Future research is needed to generalize our findings beyond groups of predominately older white patients, because we did not have sufficient representation of participants self-identifying as non-white ethnic and racial groups; future research is needed to test the potential moderating effect of race and ethnicity on achieving remission with aripiprazole vs. placebo. In addition, although a significant interaction between treatment and set-shifting performance was detected, the confidence intervals of the association between set-shifting and remission within aripiprazole and placebo treatment arms did somewhat overlap (Table 2); this may be a result of the relatively restricted sample size, large variability in these estimates, and/or a relatively small moderator effect size.
Future research is needed to characterize more thoroughly patients who benefit from aripiprazole augmentation. The current work focused on only three a priori specified potential response predictors. The observed moderating effect of Trail-Making performance suggests that set-shifting abilities are relevant to the capacity for aripiprazole response; nevertheless, it remains possible that other aspects of neuropsychological function (not specified for moderator analyses a priori) may also moderate aripiprazole efficacy. Our finding that set-shifting impairment marks a sub-group of patients who are not likely remit following aripiprazole treatment may help avoid prescribing aripiprazole to patients who are unlikely to benefit. However, even though aripiprazole was efficacious (compared with placebo) in the absence of set-shifting impairments, remission rates in this group remained modest (53.37%). Identifying additional moderators of aripiprazole's effect could lead to greater precision in determining which patients will remit following aripiprazole treatment. Future exploratory analyses utilizing a wider range of clinical data to create combined moderators29 may be necessary to accomplish this goal.
In conclusion, our study extends published observations of executive impairment, anxiety, and medical burden as correlates or predictors of poorer outcomes in late-life depression. Our findings support set-shifting performance as a moderator of aripiprazole short-term remission (that is, influencing the efficacy of aripiprazole) and distinguish anxiety as a general short-term prognostic variable (predictor). Further examining a wider range of pre-treatment factors including other aspects of cognition, as well as the neurobiological basis of these observed effects, will continue to improve our understanding of how treatments work and for whom they do or do not work.
Supplementary Material
Acknowledgments
This study was supported primarily by the National Institute of Mental Health (R01 MH083660, P30 MH90333 and T32 MH019986 to University of Pittsburgh, R01 MH083648 to Washington University, and R01 MH083643 to University of Toronto). Additional funding was provided by the UPMC Endowment in Geriatric Psychiatry, the Taylor Family Institute for Innovative Psychiatric Research (at Washington University), the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS), and the Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health, Toronto. The funding sources had no role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the paper for publication. SFS and SJA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by R01 MH083660, R01 MH083648, R01 MH083643,P30 MH90333, T32 MH19986. Additional support from the Taylor Family Institute for Innovative Psychiatric Research and the Campbell Family Mental Health Research Institute
Biographies
Dr. Butters has received research support from the National Institutes of Health. She served as a consultant for Medtronic and Northstar Neuroscience from whom she received remuneration for neuropsychological evaluations performed within the context of clinical trials. She also served as a consultant for GlaxoSmithKline, from whom she received remuneration for participating in cognitive disorder diagnostic consensus conferences for research participants in a clinical trial. None of the above represents a conflict of interest with the manuscript under consideration. Dr. Lenze has received research support from the National Institute of Mental Health (NIMH), National Institute on Aging (NIA), National Center for Complementary and Integrative Health (NCCIH), Roche, Lundbeck, the Sidney R. Baer Foundation, the Taylor Family Institute for Innovative Psychiatric Research, the Barnes-Jewish Foundation, and the McKnight Brain Research Foundation. Dr. Mulsant currently receives research funding from Brain Canada, the CAMH Foundation, the Canadian Institutes of Health Research, and the US National Institute of Health (NIH). During the last five years, he also received research support from Bristol-Myers Squibb (medications for a NIH-funded clinical trial), Eli-Lilly (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial). He directly owns stocks of General Electric (less than $5,000).
Dr. Blumberger DMB receives research support from the Canadian Institutes of Health Research (CIHR), Brain Canada, National Institutes of Health (NIH), Temerty Family through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Family Research Institute. He receives non-salary operating funds and in-kind equipment support from Brainsway Ltd. for an investigator-initiated study. He is the site principal investigator for several sponsor-initiated clinical trials from Brainsway Ltd. He receives in-kind equipment support from Tonika/Magventure for an investigator-initiated study. Dr. Anderson reports receiving grant support from the NIH grants P30 MH090333, R01 MH090250, R01 MH084921. Dr. Dew receives grant support from the National Institutes of Health, and serves on the editorial boards of numerous clinical journals. None of these activities represent a conflict of interest with the manuscript under consideration. Drs. Lotrich, Aizenstein, Diniz and Smagula report no conflicts.
Dr. Reynolds reports receiving pharmaceutical support for NIH-sponsored research studies from Bristol-Myers Squibb, Forest, Pfizer, and Lilly; receiving grants from the National Institute of Mental Health, National Institute on Aging, National Center for Minority Health Disparities, National Heart Lung and Blood Institute, Center for Medicare and Medicaid Services (CMS), Patient Centered Outcomes Research Institute (PCORI),the Commonwealth of Pennsylvania, the John A Hartford Foundation, National Palliative Care Research Center (NPCRC), Clinical and Translational Science Institute (CTSI), and the American Foundation for Suicide Prevention; and serving on the American Association for Geriatric Psychiatry editorial review board. He has received an honorarium as a speaker from MedScape/WEB MD. He is the co-inventor (Licensed Intellectual Property) of Psychometric analysis of the Pittsburgh Sleep Quality Index (PSQI) PRO10050447 (PI: Buysse).
Footnotes
Contributors: EJL, JFK, BHM, MAD, MAB, SJA and CFR designed the study and wrote the protocol. EJL, BHM, DMB, JFK, and CFR recruited patients for the study and participated in coordination. EJL, MAB, JFK, SJA, and CFR had access to all the data and analyzed the data. EJL, JFK, BHM, SJA, and CFR were responsible for the decision to submit the report, and drafted it. All authors read, critically revised, and approved the report.
Declaration of Interests: Ms. Kaneriya and Mr. Welty have no conflicts of interest to disclose. Dr. Karp has received medication supplies for investigator initiated studies from Invidior and Pfizer.
References
- 1.Mulsant BH, Blumberger DM, Ismail Z, Rabheru K, Rapoport MJ. A systematic approach to pharmacotherapy for geriatric major depression. Clinics in geriatric medicine Aug. 2014;30(3):517–534. doi: 10.1016/j.cger.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. American Journal of Psychiatry. 2014;171(4):453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 3.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013 Nov 9;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 4.Callahan CM, Kroenke K, Counsell SR, et al. Treatment of depression improves physical functioning in older adults. Journal of the American Geriatrics Society. 2005;53:367–373. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- 5.Gallo JJ, Morales KH, Bogner HR, et al. Long term effect of depression care management on mortality in older adults: follow-up of cluster randomized clinical trial in primary care. BMJ (Clinical research ed) 2013;346:f2570–f2570. doi: 10.1136/bmj.f2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. 2004:0098–7484. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 7.Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: A critical review. American Journal of Geriatric Psychiatry. 2013;21:973–986. doi: 10.1097/JGP.0b013e31826576cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015 Sep 24; doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirose T, Uwahodo Y, Yamada S, et al. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. Journal of psychopharmacology (Oxford, England) 2004;18:375–383. doi: 10.1177/026988110401800308. [DOI] [PubMed] [Google Scholar]
- 10.Conway CR, Chibnall JT, Cumming P, et al. Antidepressant response to aripiprazole augmentation associated with enhanced FDOPA utilization in striatum: A preliminary PET study. Psychiatry Research - Neuroimaging. 2014;221:231–239. doi: 10.1016/j.pscychresns.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dombrovski AY, Szanto K, Clark L, et al. Corticostriatothalamic reward prediction error signals and executive control in late-life depression. Psychol Med May. 2015;45(7):1413–1424. doi: 10.1017/S0033291714002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Nag S, Boyle PA, et al. Brainstem aminergic nuclei and late-life depressive symptoms. JAMA psychiatry (Chicago, Ill) 2013 Dec;70(12):1320–1328. doi: 10.1001/jamapsychiatry.2013.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of general psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JC, Delucchi KL, Schneider LS. Moderators of outcome in late-life depression: a patient-level meta-analysis. The American journal of psychiatry Jun. 2013;170(6):651–659. doi: 10.1176/appi.ajp.2012.12070927. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy reduces disability more than supportive therapy in older adults with major depression and executive dysfunction. Archives of general psychiatry. 2011;68(3):77–77. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreescu C, Lenze EJ, Dew MA, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: Controlled study. British Journal of Psychiatry. 2007;190(APR):344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds CF, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. The New England journal of medicine. 2006;354:1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 18.Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. Journal of clinical and experimental neuropsychology. 2005;27:599–609. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- 19.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5th. 2012. Neuropsychological assessment (5th ed.) [Google Scholar]
- 20.Delis DC KE, Kramer JH. Dellis Kaplan Executive Function System Examiner's Manua. The Psychological Corporation; 2001. [Google Scholar]
- 21.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med Aug. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 22.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry research Mar. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of general psychiatry Mar. 2010;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenze E, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment- resistant depression in late life: a randomized placebo-controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baune BT, Czira ME, Smith AL, Mitchell D, Sinnamon G. Neuropsychological performance in a sample of 13-25 year olds with a history of non-psychotic major depressive disorder. Journal of affective disorders. 2012 Dec 10;141(2-3):441–448. doi: 10.1016/j.jad.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 27.Schmid M, Hammar A. Cognitive function in first episode major depressive disorder: poor inhibition and semantic fluency performance. Cognitive neuropsychiatry. 2013;18(6):515–530. doi: 10.1080/13546805.2012.754748. [DOI] [PubMed] [Google Scholar]
- 28.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in Neuroscience. 2013 doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer HC. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Statistics in medicine. 2013;32(11):1964–1973. doi: 10.1002/sim.5734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


