Abstract
Zebrafish intraspinal serotonergic neuron (ISN) morphology and distribution have been examined in detail at different ages; however, some aspects of the development of these cells remain unclear. Although antibodies to serotonin (5-HT) have detected ISNs in the ventral spinal cord of embryos, larvae, and adults, the only tryptophan hydroxylase (tph) transcript that has been described in the spinal cord is tph1a. Paradoxically, spinal tph1a is expressed transiently in embryos, which brings the source of 5-HT in the ISNs of larvae and adults into question. Because the pet1 and tph2 promoters drive transgene expression in the spinal cord, we hypothesized that tph2 is expressed in spinal cords of zebrafish larvae. We confirmed this hypothesis through in situ hybridization. Next, we used 5-HT antibody labeling and transgenic markers of tph2-expressing neurons to identify a transient population of ISNs in embryos that was distinct from ISNs that appeared later in development. The existence of separate ISN populations may not have been recognized previously due to their shared location in the ventral spinal cord. Finally, we used transgenic markers and immunohistochemical labeling to identify the transient ISN population as GABAergic Kolmer-Agduhr double-prime (KA″) neurons. Altogether, this study revealed a novel developmental paradigm in which KA″ neurons are transiently serotonergic before the appearance of a stable population of tph2-expressing ISNs.
Keywords: Kolmer-Agduhr, pet1, serotonin, spinal cord, tryptophan hydroxylase
Introduction
Spinal locomotor networks contain all of the necessary components to produce coordinated motor activity autonomously from the brain (Grillner and Zangger, 1979; Delcomyn, 1980). It is well documented that serotonin (5-HT) is a powerful neuromodulator of these networks (Jacobs and Fornal, 1997; Jordan et al., 2008). Unfortunately, the complexity of the 5-HT system makes understanding the mechanisms by which it shapes locomotor output difficult. First, numerous 5-HT receptor subtypes exist in vertebrates and can function presynaptically or postsynaptically (Barnes and Sharp, 1999; Hoyer et al., 2002). Further, 5-HT signaling can occur through classical synaptic transmission or 5-HT can be released extrasynaptically to produce paracrine effects through volume transmission (De-Miguel and Trueta, 2005; Fuxe et al., 2007). Finally, the cellular sources of spinal 5-HT vary between species. Projections descending into the spinal cord from the raphe nuclei are well-conserved in vertebrates; however, both the number and location of intraspinal serotonergic neurons (ISNs) are highly variable, in that mammals possess relatively few of these cells when compared to other vertebrates (Schmidt and Jordan, 2000).
The inter-species variability of ISN cell number and location suggests that they perform different functional roles between species. In fish, the proximity of ISNs and their processes to spinal motor neurons indicates that they may play a role in modulating motor output (Van Dongen et al., 1985; Van Raamsdonk et al., 1996; Brustein et al., 2003a; McLean and Fetcho, 2004b) and are therefore an interesting candidate cell class for the study of serotonergic modulation of spinal locomotor networks. The transparency and amenability of larval zebrafish to labeling techniques, such as transgenesis and whole-mount immunohistochemistry, make them an attractive model for the study of ISNs and their function. Antibodies to 5-HT and stochastic fluorescent protein expression have been used to describe zebrafish ISNs as a homogenous cell population that is distributed along the rostrocaudal axis of the ventral spinal cord (McLean and Fetcho, 2004a). These cells possess oval-shaped somas and a single process that projects dorsally and laterally and bifurcates to form a dendritic arbor and a single descending axon (Brustein et al., 2003a; McLean and Fetcho, 2004a).
The time-course of morphological ISN development is consistent with the hypothesis that they play a role in shaping zebrafish locomotor output. These cells and the descending serotonergic raphe fiber tracts, which modulate sensory responsiveness during arousal (Yokogawa et al., 2012), appear to establish a mature pattern of spinal innervation around 4 days post fertilization (dpf; Brustein et al., 2003a; McLean and Fetcho, 2004a) that is retained in the adult zebrafish spinal cord (Van Raamsdonk et al., 1996). Four dpf coincides with development of the mature beat-and-glide swimming pattern that persists through the life of the fish (Fuiman and Webb, 1988; Muller et al., 2000; Drapeau et al., 2002; Brustein et al., 2003b). Further, functional studies demonstrated that acute and chronic perturbations of 5-HT signaling affect properties of locomotor output in 4 dpf and older larvae, but not in younger larvae or embryos (Brustein et al., 2003a; Airhart et al., 2007). Thus, the spinal population of serotonergic neurons ostensibly reaches morphological and functional maturity as early as 4 dpf.
A number of studies have examined ISN development and innervation patterns. Antibodies to 5-HT label ISNs in the ventral spinal cord as early as 24 hours post fertilization (hpf; Sallinen et al., 2009), although others initially detected them slightly later in development, between 32 and 48 hpf (Brustein et al., 2003a; McLean and Fetcho, 2004a). Despite extensive labeling of ventrally-located cell bodies throughout the spinal cord at 2 dpf, the characteristic descending axons are not discernible until approximately 3 dpf and the cells do not acquire their final innervation pattern until 4 dpf (Brustein et al., 2003a; McLean and Fetcho, 2004a). Because the ventral soma location and projection pattern of ISNs persist at 10 dpf (McLean and Fetcho, 2004a) and in adults (Van Raamsdonk et al., 1996), it has been assumed that the ISNs that are initially detected in 1 and 2 dpf embryos are the same population that is observed in larvae, juveniles, and adults. If, in fact, ISNs do play a role in modulating locomotor networks as proposed (Van Raamsdonk et al., 1996; Brustein et al., 2003a; McLean and Fetcho, 2004b), it is somewhat surprising that 5-HT does not noticeably influence motor output until 4 dpf, given their initial appearance between 1 and 2 dpf.
The delay between detection of spinal 5-HT at 1–2 dpf and 5-HT’s influence on locomotor output at 4 dpf may be due to later development of post-synaptic cells and receptor expression; however, evidence suggests that ISNs undergo further gene expression changes and/or differentiation between these ages. Zebrafish possess three known genes that encode the rate-limiting enzyme in 5-HT synthesis, tryptophan hydroxylase (Tph): tph1a, tph1b, and tph2 (Bellipanni et al., 2002; Teraoka et al., 2004). It was recently shown that tyrosine hydroxylase 2 (Th2) also has Tph activity in diencephalic 5-HT neurons (Ren et al., 2013). While there is no evidence of tph1b or th2 expression in the raphe or spinal cord, tph1a transcripts are present in the ventral spinal cord at 24 hpf (Bellipanni et al., 2002), which is consistent with the onset of neuronal 5-HT antibody labeling in the same location around 32 hpf (McLean and Fetcho, 2004a). Surprisingly, spinal tph1a expression is only transient (Bellipanni et al., 2002), even as 5-HT antibody labeling of ventral spinal neurons persists in older larvae and adults (Van Raamsdonk et al., 1996; Brustein et al., 2003a; McLean and Fetcho, 2004a). Finally, tph2 is expressed in the raphe, which projects to the spinal cord (Teraoka et al., 2004; Norton et al., 2005; Lillesaar et al., 2007), although there is no direct evidence that tph2 is expressed in the cord itself (Rauch et al., 2003). Spinal transgene expression was noted in two separate transgenic lines using promoters (tph2 and pet1 promoters) that drive expression in tph2-expressing cells (Lillesaar et al., 2009; Yokogawa et al., 2012). Although the timing and location of spinal transgene expression was not described in detail, this does suggest that tph2 is expressed in spinal neurons. Expression of tph2 represents an intriguing potential mechanism to account for continued spinal 5-HT synthesis even after the loss of tph1a expression.
We examined ISN development and gene expression between 1 and 4 dpf. First, we verified that tph2 was expressed in the spinal cord after tph1a expression was lost. We used immunohistochemical labeling of developing Tg(-3.2pet1:EGFP)ne0214 larvae to show that the ISNs observed at 2 dpf were a distinct population from those present at 4 dpf and beyond. The 4 dpf ISNs were positive for markers of tph2-expressing neurons, indicating that these cells synthesized 5-HT rather than taking it up from another source. Finally, we identified the population of transiently serotonergic cells as the most ventral subset of Kolmer-Agduhr (KA) neurons (designated KA″; Dale et al., 1987b; Bernhardt et al., 1992; Park et al., 2004; Yang et al., 2010; Huang et al., 2012). Altogether this study demonstrates that KA″ neurons are transiently 5-HT positive before giving way to a separate population of tph2-expressing ISNs in the ventral spinal cord.
Materials and Methods
Zebrafish maintenance and transgenic lines
Zebrafish lines were maintained at the University of Minnesota Zebrafish Core Facility or University of Würzburg fish facility using standard techniques (Westerfield, 2000). Embryos were collected from timed crosses and raised in egg water containing 60 μg/ml Instant Ocean (Blacksburg, VA) Sea Salt at 28.5°C with a 14 hour light:10 hour dark cycle. Larvae were screened for fluorescent transgene expression and staged prior to use (Kimmel et al., 1995). The Tg(-3.2pet1:EGFP)ne0214 (Lillesaar et al., 2009), Et(-1.5hsp70l:Gal4-VP16)s1003t, Tg(UAS-E1b:Kaede)s1999t (Scott et al., 2007), and Tg(tph2:nfsB-mCherry)y226 (Yokogawa et al., 2012) lines were described previously. All protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC) or performed in accordance with the animal welfare regulations of the District Government of Lower Franconia.
In situ hybridization and immunohistochemical double labeling
In situ hybridization was performed on Tg(-3.2pet1:EGFP)ne0214 embryos (20, 24, and 48 hpf) and larvae (68 and 96 hpf). Specimens were fixed in 4% PFA overnight at 4°C and then dehydrated in a methanol series and stored in 100% methanol at −20°C. Subsequently, the tissues were rehydrated in a reverse methanol series and processed for whole-mount in situ hybridization as described elsewhere (Thisse and Thisse, 2008). Transcripts for tph1a and tph2 were detected by using dig-labeled RNA probes (Bellipanni et al., 2002; Teraoka et al., 2004), followed by NBT/BCIP color precipitation. After the in situ hybridization, the specimens were immunostained for EGFP detection by incubation for three days at 4°C in rabbit anti-GFP (TP401, Torrey Pines Biolabs, Inc., Houston, TX) diluted 1:500 in PBS with 0.1% Tween 20, 2% normal sheep serum, and 2 mg/ml bovine serum albumin. After washing, the tissues were incubated for three days at 4°C in secondary goat anti-rabbit Alexa Fluor 488 antibodies (Thermo Fisher Scientific, Waltham, MA) diluted 1:1000 in the same buffer. Whole-mount preparations were imaged using a Nikon Eclipse Ti microscope (Tokyo, Japan) equipped with a 488 nm Sapphire laser.
Whole-mount immunohistochemistry
Embryos and larvae were anesthetized with a lethal dose of 0.2% Tricaine-S (Western Chemical, Ferndale, WA), fixed in 4% paraformaldehyde for three hours at room temperature or 4°C overnight, and washed with 0.5% Triton X-100 in PBS (PBS-Tx). Larvae older than 72 hpf were digested with Proteinase K (40 μg/ml) for 30 minutes to permeabilize the tissue, post-fixed with 4% paraformaldehyde for 20 minutes, and washed again with PBS-Tx. Specimens were incubated in blocking solution (0.2% bovine serum albumin and 10% normal goat serum in PBS-Tx) for one hour at room temperature. Primary antibodies were added to the blocking solution and specimens were incubated at 4°C overnight. Primary antibodies included rabbit polyclonal anti-5-HT (S5545, 1:400; Sigma-Aldrich, St. Louis, MO), rat monoclonal anti-5-HT (MAB352, 1:10; EMD Millipore, Billerica, MA), and rabbit polyclonal anti-GABA (A2052, 1:400, Sigma-Aldrich). Specimens were washed and incubated with secondary antibodies diluted in blocking solution containing 1% normal goat serum at 4°C for two days. Primary antibodies were detected with Alexa Fluor 568 (A-11036) or 633 (A-21071) goat anti-rabbit (1:500) or Alexa Fluor 633 goat anti-rat (A-21094, 1:300) secondary antibodies (Thermo Fisher Scientific). After a final PBS-Tx wash, embryos and larvae were embedded laterally in low melting-point agarose for imaging.
Image acquisition and processing
Images of anti-5-HT and anti-GABA labeled whole-mount fish were acquired with an Olympus FluoView FV1000 confocal microscope (Center Valley, PA). Z-projections either included neuronal processes or were restricted to exclude processes to accentuate fluorescence in cell somas. Unless noted otherwise, images were collected from a region centered on body segment 15 (midbody). All images are lateral views oriented with dorsal toward the top and rostral to the left. Images were processed using FIJI (Schindelin et al., 2012) and Photoshop CS5 (Adobe Systems, San Jose, CA) to apply layers across entire panels to enhance low-intensity fluorescence.
Quantification of fluorescently-labeled cells and statistical analysis
Anti-5-HT labeled and anti-5-HT/GABA double-labeled Tg(-3.2pet1:EGFP)ne0214 larvae between the ages of 48 and 240 hpf were embedded laterally and confocal Z-stacks were collected through the entire spinal cord with a 441 micron-wide field of view. The number of cells that were positive for each label or combination of labels was quantified in Z-stacks of defined regions centered on body segments 7 (rostral), 15 (midbody), and 23 (caudal). Cells in each region were quantified in 3–6 fish from each age group. Statistical analysis was completed using SigmaPlot 12 (Systat Software, San Jose, CA). Two-way ANOVAs identified significant effects of age on the number of cells that were positive for each label. Holm-Sidak corrected post-hoc t-tests (α = 0.05) were used to compare the number of labeled cells at each timepoint to baseline (48 hpf). Values were expressed as mean (SD).
Results
Tph1a and tph2 are expressed at different stages of spinal cord development
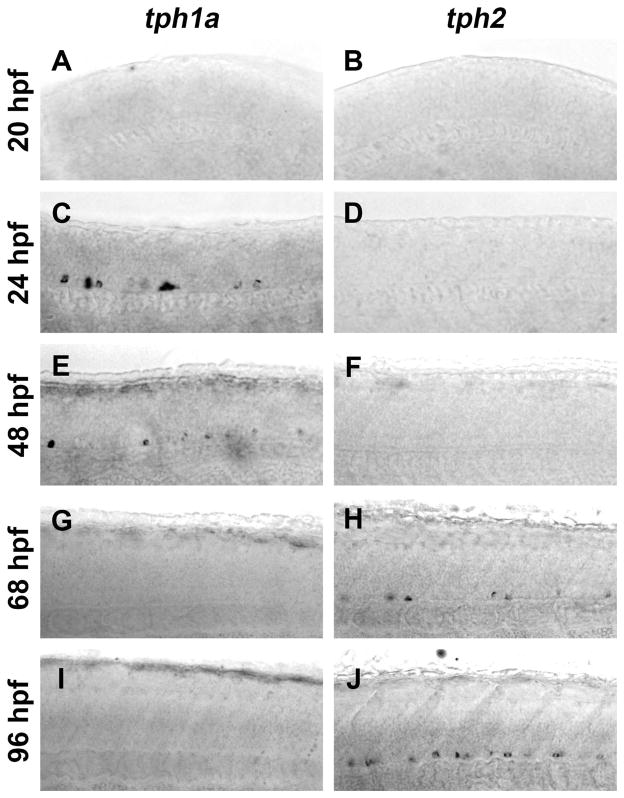
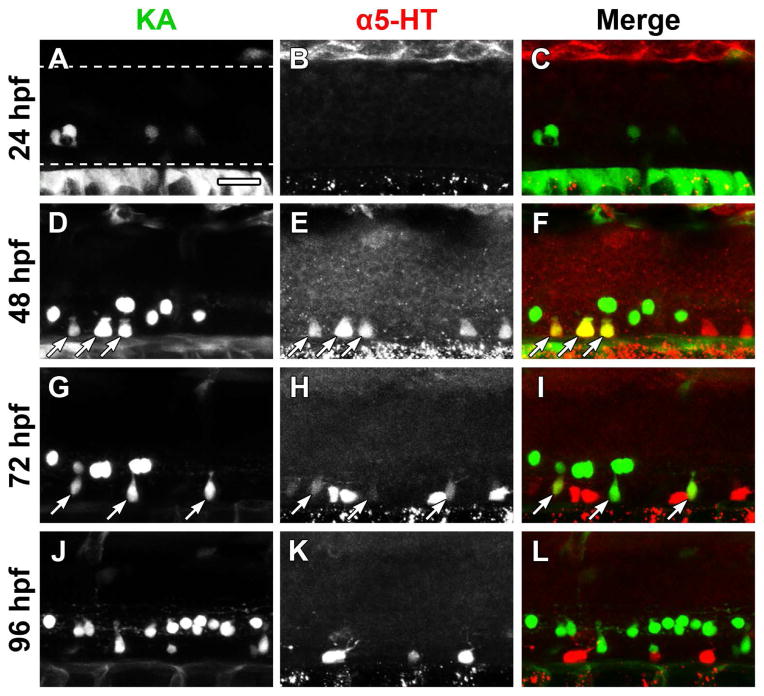
Spinal expression of tph1a is only transient (Bellipanni et al., 2002), yet spinal neurons contain 5-HT at all developmental ages beginning at approximately 32 hpf (Van Raamsdonk et al., 1996; Brustein et al., 2003a; McLean and Fetcho, 2004a). Fragments of the zebrafish pet1 and tph2 promoters have been reported to drive transgene expression in the spinal cord (Lillesaar et al., 2009; Yokogawa et al., 2012), suggesting that Tph2 may be responsible for the persistence of 5-HT in spinal neurons. In situ hybridization was used to determine if there was a developmental change in tph1a and tph2 gene expression in developing spinal cords (Fig. 1). Images were collected from a region centered on the midbody (body segment 15) of laterally-mounted embryos and larvae. Tph gene expression expanded in a rostral to caudal direction, as it was detected near the hindbrain at slightly earlier ages than in the central spinal cord (data not shown). Neither tph1a nor tph2 was detected at 20 hpf (Fig. 1A and B). Consistent with previous gene expression studies (Bellipanni et al., 2002; Thisse and Thisse, 2004), tph1a was transiently expressed in the ventral spinal cord, as it was detected at 24 and 48 hpf (Fig. 1C and E), but not at 68 or 96 hpf (Fig. 1G and I). Conversely, tph2 was not expressed at 24 or 48 hpf (Fig. 1D and F), but was observed in the ventral spinal cord at 68 hpf (Fig. 1H) with labeling becoming more pronounced at 96 hpf (Fig. 1J). Thus, tph1a and tph2 were both expressed in the ventral spinal cord, but at different stages of development, with expression of tph1a preceding expression of tph2.
Figure 1. Expression of tph1a precedes expression of tph2 in the developing spinal cord.
Spinal expression of tph1a (A, C, E, G, and I) and tph2 (B, D, F, H, and J) were compared by in situ hybridization in whole-mount embryos and larvae. A and B: Whole-mount in situ hybridization did not detect tph1a (A) or tph2 (B) transcripts in 20 hpf larvae. C–F: At 24 and 48 hpf tph1a (C and E), but not tph2 (D and F), was detected in the ventral spinal cord. G–J: Expression of tph1a was not observed after 68 hpf (G and I). Tph2-expressing cells were observed in the ventral spinal cord at 68 hpf (H) and labeling became more prominent at 96 hpf (J).
Pet1:EGFP transgene expression colocalizes with tph2
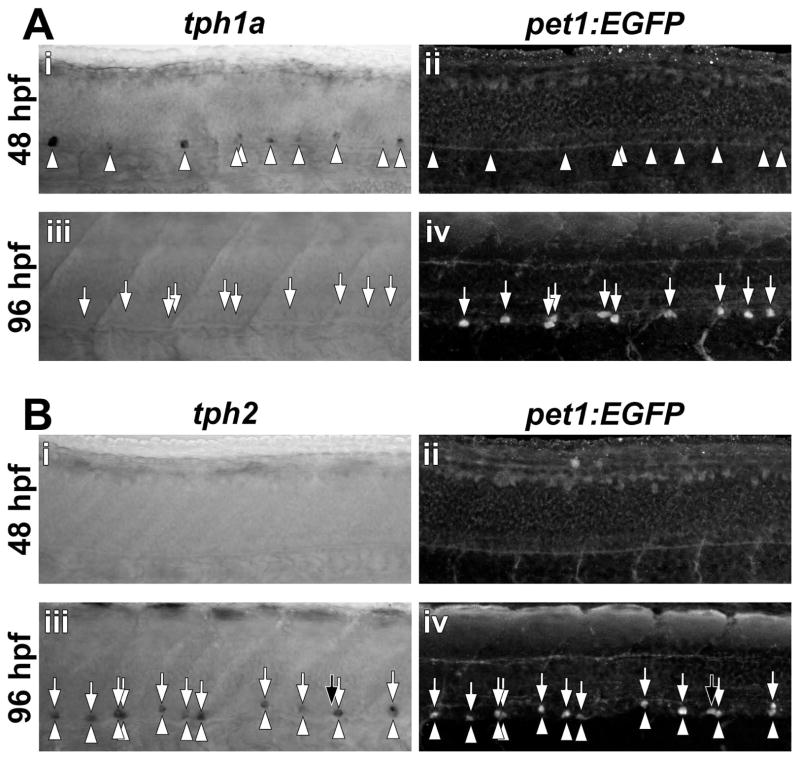
Expression of the pet1 Ets-domain transcription factor precedes expression of tph2 in certain post-mitotic serotonergic cell clusters in the brain (Lillesaar et al., 2007). Because the Tg(-3.2pet1:EGFP)ne0214 line exhibits spinal expression of EGFP (Lillesaar et al., 2009); Supporting Information Fig. S1), we confirmed that pet1:EGFP and tph2 were coexpressed in the spinal cord (Fig. 2 and Supporting Information Fig. S2). Tph transcripts and EGFP were detected in whole-mount Tg(-3.2pet1:EGFP)ne0214 embryos and larvae by sequential in situ hybridization and anti-EGFP immunohistochemistry, respectively. Expression of tph1a (arrowheads) was observed prior to pet1:EGFP at 48 hpf (Fig. 2Ai and ii) and did not temporally correspond with pet1:EGFP expression (arrows) at 96 hpf (Fig. 2Aiii and iv). Tph2 (arrowheads) and pet1:EGFP (arrows) were not detected at 48 hpf (Fig. 2Bi and ii) but were coexpressed in ventral cell bodies at 96 hpf (Fig. 2Biii and iv, vertically aligned arrows and arrowheads indicate colocalization; 29 of 30 cells were double-positive). We identified a single cell in which pet1:EGFP, but not tph2, staining was discernable (Fig. 2Biii and iv, black arrows), consistent with pet1 expression preceding tph2 (Lillesaar et al., 2007). Therefore, the Tg(-3.2pet1:EGFP)ne0214 line selectively labeled tph2-expressing neurons in the 96 hpf spinal cord.
Figure 2. Spinal expression of pet1:EGFP corresponds to tph2, but not tph1a expression.
Transcripts for tph1a (A) and tph2 (B) were detected by in situ hybridization (arrowheads) and EGFP was detected by immunohistochemistry (arrows) in whole-mount Tg(-3.2pet1:EGFP)ne0214 larvae. A: At 48 hpf, in situ hybridization revealed tph1a mRNA that was localized to cells distributed along the ventral spinal cord (Ai) and preceded pet1:EGFP transgene expression (Aii). Spinal tph1a expression was not observed at 96 hpf (Aiii) and EGFP was detected in single cells in the ventral spinal cord (Aiv). B: Neither tph2 nor pet1:EGFP was expressed at 48 hpf (Bi and Bii). At 96 hpf, the majority of labeled cells were double-positive for tph2 and pet1:EGFP (Biii and Biv; Colabeling indicated by the presence of vertically aligned arrowheads and arrows). Black arrows indicate a single cell that expressed pet1:EGFP, but not tph2.
The early population of ISNs is distinct from the later-developing population of pet1:EGFP-expressing ISNs
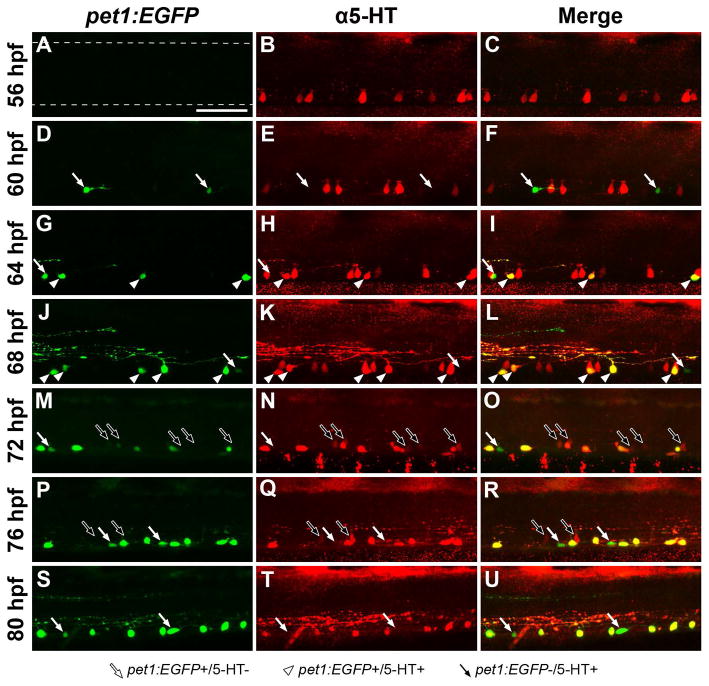
We detected two groups of tph-expressing neurons in the ventral spinal cord; tph1a-expressing cells that did not coexpress pet1:EGFP and tph2-expressing cells that did coexpress pet1:EGFP (Fig. 2). It was not clear if tph1a-expressing cells reduced tph1a levels and upregulated tph2 and pet1:EGFP, or if the tph2/pet1:EGFP coexpressing cell population was entirely distinct from the tph1a-expressing population. Therefore, we investigated the relationship between spinal pet1:EGFP expression (pet1:EGFP-expressing, pet1:EGFP+; pet1:EGFP non-expressing; pet1:EGFP−) and 5-HT immunoreactivity (5-HT positive, 5-HT+; 5-HT negative, 5-HT−) in the midbody region of the spinal cord at developmental stages that coincided with the transition from tph1a to tph2 expression (Fig. 3 and 4C). Pet1:EGFP fluorescence was not observed at 56 hpf, yet 5-HT+ cells were found throughout the ventral spinal cord (Fig. 3A–C). Pet1:EGFP fluorescence was first observed at 60 hpf (white arrows; 5.7 cells (SD 1.2) in midbody region); however, few pet1:EGFP+ cells were 5-HT+ (Fig. 3D–F and 4C; 10% (SD 9)). The number of pet1:EGFP+ cells increased at 64 and 68 hpf (Fig. 3G–L and 4C; 10.3 cells (SD 0.6) and 13.3 cells (SD 1.2) in midbody region at 64 and 68 hpf, respectively; both p < 0.05). The majority of 64 and 68 hpf pet1:EGFP+ cells colabeled with 5-HT antibodies (white arrowheads; 58% (SD 10) and 85% (SD 7), respectively), while a smaller proportion were 5-HT− (white arrows). Pet1:EGFP− ISNs persisted at 72 and 76 hpf, although the number of these cells was reduced (Fig. 4C; 6.0 cells (SD 3.7) and 6.2 cells (SD 6.0) in midbody region, respectively compared to 18.0 cells (SD 4.0) at 68 hpf; both p < 0.05), as was 5-HT antibody labeling intensity (Fig. 3M–R, black arrows). 5-HT antibody labeling became restricted to pet1:EGFP+ cells, as pet1:EGFP− cells were rarely detected with 5-HT antibodies after 80 hpf (Fig. 3S–U and 4C; 0.5 pet1:EGFP−/5-HT+ cells (SD 1.2) in midbody region). Specificity of 5-HT antibody labeling to pet1:EGFP+ cells was verified through 240 hpf (Fig 4C; 100% colabeled).
Figure 3. Spinal 5-HT antibody labeling transitions from pet1:EGFP− to pet1:EGFP+ cells during development.
Tg(-3.2pet1:EGFP)ne0214 larvae were labeled with antibodies to 5-HT every four hours between 56 and 80 hpf. Confocal Z-stacks were collected from the midbody region of the spinal cord (centered on body segment 15; dashed lines in A represent the dorsal and ventral boundaries of the spinal cord, approximately the same location in all panels). White arrows indicate cells that expressed pet1:EGFP and were not labeled with 5-HT antibodies, white arrowheads indicate cells in which pet1:EGFP expression and 5-HT colocalized, and black arrows indicate cells that did not express pet1:EGFP but were faintly labeled with 5-HT antibodies. A–C: 5-HT (B) was detected in cells in the ventral spinal cord prior to expression of pet1:EGFP (A, merge in C). D–F: Pet1:EGFP expression (D) was initially observed at 60 hpf in cells that did not colabel with 5-HT (E, merge in F; white arrows). G–L: At 64 and 68 hpf, a subset of pet1:EGFP+ cells (G, J) were 5-HT+ (H, K, merge in I, L; white arrowheads). 5-HT antibodies continued to detect pet1:EGFP− cells in the ventral spinal cord at 64 and 68 hpf (red cells in merged panels I, L). M–R: Colabeling of cells with pet1:EGFP (M, P) and 5-HT antibodies (N, Q) continued at 72 and 76 hpf (merge in O, R). The number of pet1:EGFP−/5-HT+ cells detected, and the intensity of 5-HT antibody labeling in these cells, were both reduced (black arrows). S–U: 5-HT antibody labeling (T) of cell somas was restricted to pet1:EGFP+ cells (S) in the ventral spinal cord at 80 hpf (merge in U). Scale Bar = 50 μm.
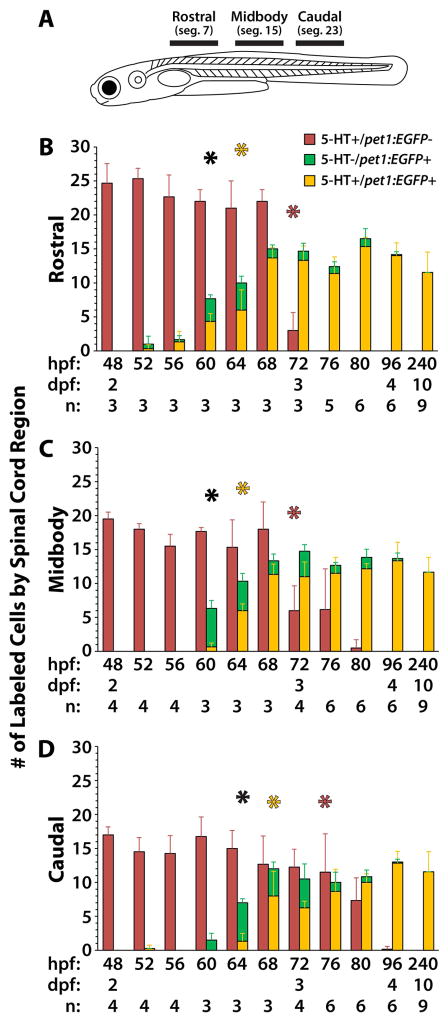
Figure 4. Quantification of 5-HT antibody labeled cells in pet1:EGFP transgenic larvae reveals a rostrocaudal progression of ISN development.
Tg(-3.2pet1:EGFP)ne0214 larvae were fixed and labeled with antibodies to 5-HT every four hours between the ages of 48 and 80 hpf and at 96 and 240 hpf. A: Confocal Z-stacks were collected from regions of the spinal cord (represented by black bars in A) centered on body segments 7 (rostral), 15 (midbody), and 23 (caudal). B–D: The number of 5-HT+ cells that did not express pet1:EGFP (red bars), 5-HT− cells that expressed pet1:EGFP (green bars), and cells that were 5-HT and pet1:EGFP double-positive (yellow bars) were quantified in each region of the spinal cord. Black asterisks indicate the earliest age at which there was a significant increase in the total number of pet1:EGFP+ cells (combined pet1:EGFP+/5-HT− and pet1:EGFP+/5-HT+) compared to 48 hpf. Yellow asterisks indicate the earliest age at which there was a significant increase in the number of pet1:EGFP/5-HT double-positive cells compared to 48 hpf. Red asterisks indicate the earliest age at which there was a significant decrease in the number of pet1:EGFP−/5-HT+ cells compared to 48 hpf. Error bars represent SD.
To examine the transition of 5-HT antibody labeling from pet1:EGFP− to pet1:EGFP+ ISNs throughout the spinal cord, the number of cells that were labeled in Tg(-3.2pet1:EGFP)ne0214 spinal cords with 5-HT antibodies, pet1:EGFP transgene expression, or both, were quantified at different ages and in different rostrocaudal positions (Fig. 4 and Supplemental Information Fig. S3). Confocal Z-stacks of spinal cord regions that included approximately five body segments were collected (Fig. 4A, regions represented by horizontal black bars) centered on segments 7 (Fig. 4B, rostral), 15 (Fig. 4C, midbody), and 23 (Fig. 4D, caudal). Three types of cells were considered in the statistical analysis: pet1:EGFP−/5-HT+ (red bars), pet1:EGFP+/5-HT− (green bars), and pet1:EGFP+/5-HT+ (yellow bars). There was a statistically significant effect of age on the number of cells of all three types (Two-way ANOVA; all F > 100; all p < 0.001), due to a reduction in the number of pet1:EGFP−/5-HT+ cells over time and an increase in the number of pet1:EGFP+/5-HT− and pet1:EGFP+/5-HT+ cells. The number of cells in each group was compared to baseline (48 hpf) to determine the age at which a significant difference in cell abundance first occurred in each region. A significant reduction in the number of pet1:EGFP−/5-HT+ cells (Fig. 4B–D, red asterisks) was found at 72 hpf in the rostral and midbody regions (corrected t-test; all t > 7.5; all p < 0.001), and at 76 hpf in the caudal region (corrected t-test; t = 3.4; p = 0.03). A significant increase in the total number of pet1:EGFP+ cells (Fig. 4B–D, black asterisks; combined pet1:EGFP+/5-HT− and pet1:EGFP+/5-HT+) was found at 60 hpf in the rostral and midbody regions (corrected t-test; all t > 4.3; all p < 0.001), and at 64 hpf in the caudal region (corrected t-test; t = 4.7; p < 0.001). Finally, a significant increase in the number of pet1:EGFP+/5-HT+ cells was found (Fig. 4B–D, yellow asterisks) at 64 hpf in the rostral and midbody regions (corrected t-test; all t > 3.7; all p < 0.01), and at 68 hpf in the caudal region (corrected t-test; t = 5.4; p < 0.001). The post-hoc comparisons revealed two trends. First, changes in cell abundance began earlier in the rostral region than the caudal region, evidence of rostrocaudal progression of development of the serotonergic cell types. Second, the number of pet1:EGFP+/5-HT+ cells increased before the number of pet1:EGFP−/5-HT+ decreased (rostral and midbody: 64 v. 72 hpf, caudal: 68 v. 76 hpf). Altogether, this supported the hypothesis that the pet1:EGFP+/5-HT+ and pet1:EGFP−/5-HT+ cells are separate neuronal populations, rather than different stages in the development of a single population.
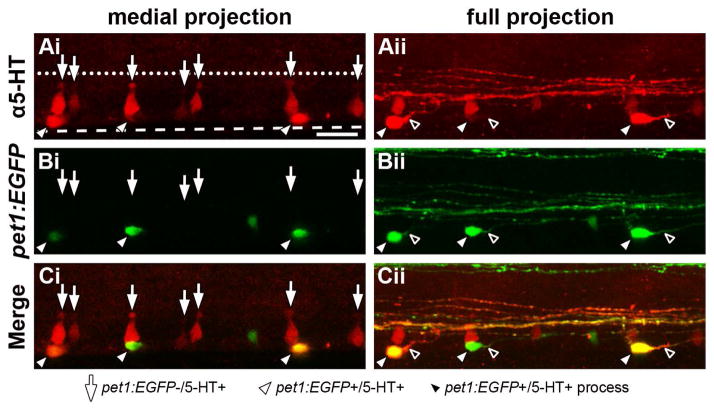
Because the pet1:EGFP+ and pet1:EGFP− ISNs were ostensibly separate populations, we predicted that these populations would possess distinctive morphological characteristics. 5-HT antibodies were used to label both populations of ISNs in 64 hpf Tg(-3.2pet1:EGFP)ne0214 whole-mount larvae to determine if the populations were morphologically distinguishable (Fig. 5). A confocal Z-stack was collected from a laterally-mounted larva and projected to only include the medial spinal cord (Fig. 5Ai–Ci; medial projection) and to include the entire width of the spinal cord (Fig. 5Aii–Cii; full projection) in order to emphasize the features of neuronal groups with differing mediolateral positions. 5-HT antibodies labeled cell somas that possessed either a conical, dorsoventrally elongated shape (arrows) or a more rounded, ovoid morphology (white arrowheads, Fig. 5Ai and 5Aii). The apical ends of the former group of ISNs possessed structures located near the central canal that were suggestive of cerebrospinal fluid (CSF) contacting ciliated terminals (Vigh-Teichmann and Vigh, 1983). The latter group of cells characteristically possessed a single, prominent projection originating from the soma (black arrowheads), which was consistent with previous descriptions of zebrafish ISN morphology (Brustein et al., 2003a; McLean and Fetcho, 2004a). When 5-HT immunofluorescence (Fig. 5Ai and 5Aii) and pet1:EGFP expression (Fig. 5Bi and 5Bii) were overlayed (Fig. 5Ci and 5Cii), pet1:EGFP expression was restricted to the group of cells that possessed rounded or ovoid cell somas (white arrowheads) and not the elongated conically-shaped cells with putative ciliated terminals (arrows). Consequently, the presence or absence of pet1:EGFP expression predicted the morphology of cells that were labeled with 5-HT antibodies. Furthermore, the pet1:EGFP+ ISNs occupied a slightly more lateral position than pet1:EGFP− ISNs, as their cell somas were more distinguishable in the full projection (Fig. 5Bii and Cii) than in the medial projection (Fig. 5Bi and Ci).
Figure 5. ISNs that express pet1:EGFP are morphologically distinct from those that do not express pet1:EGFP.
A 64 hpf Tg(-3.2pet1:EGFP)ne0214 larva was labeled with antibodies to 5-HT, mounted laterally, and a confocal Z-stack was collected through the mediolateral extent of the spinal cord. The morphological structure of ISN somas (red) was examined in a confocal projection restricted to the medial spinal cord (Ai–Ci, medial projection) and a projection through the entire width of the spinal cord (Aii–Cii; full projection). A: 5-HT was detected in cell bodies near the ventral boundary of the spinal cord (dashed line, same position in all panels) that possessed two distinct shapes; dorsoventrally elongated conical cell bodies with apical terminals (Ai, arrows) and round or ovoid cell bodies (Aii, white arrowheads) that each possessed a single, prominent projection (Aii, black arrowheads). Apical terminals of the conically-shaped cells were positioned near the central canal (represented by a dotted line, same position in all panels). B and C: Pet1:EGFP (B, green) was expressed in ISNs with ovoid (white arrowheads), but not conical somas (arrows; merge in C). Scale bar = 20 μm.
A subset of KA neurons transiently contains 5-HT
ISNs that were detected at 48 hpf were a distinct population from those present at later stages of development (Figs. 3–5). The characteristic structure located at the apical end of pet1:EGFP− ISNs (Fig. 5, arrows) and their position within the ventral spinal cord were consistent with the morphology and position of GABAergic KA″ neurons (Dale et al., 1987b; Bernhardt et al., 1992; Park et al., 2004; Yang et al., 2010; Huang et al., 2012). To determine if 5-HT was present in the KA neurons, the Et(-1.5hsp70l:Gal4-VP16)s1003t and Tg(UAS-E1b:Kaede)s1999t lines were crossed to produce progeny that expressed Kaede in the KA neurons (Scott et al., 2007). Transgene expression in the Et(-1.5hsp70l:Gal4-VP16)s1003t line is not completely penetrant (Wyart et al., 2009) and this variegated expression pattern may have been further impacted by generational silencing of the UAS:Kaede transgene (Halpern et al., 2008; Goll et al., 2009; Akitake et al., 2011). ISNs were labeled with antibodies to 5-HT (Alexa Fluor 633 secondary antibody was used to prevent spectral overlap with Kaede) between 24 and 96 hpf (Fig. 6). Prior to detection of 5-HT, Kaede expression was observed in a small number of KA neurons at 24 hpf (Fig. 6A–C). At 48 hpf, 5-HT antibodies labeled cells along the ventral border of the spinal cord and often colocalized with KA neurons (Fig. 6D–F, arrows). 5-HT antibody labeling persisted in KA neurons at 72 hpf (arrows), but labeling intensity appeared reduced (Fig. 6G–I, arrows), which suggested that the KA neurons were being depleted of 5-HT over time. Meanwhile, non-KA neurons (that did not express Kaede) were more strongly labeled with 5-HT antibodies (Fig. 6G–I). Finally, 5-HT antibody labeling ceased to colocalize with KA neurons at 96 hpf (Fig. 6J–L). This suggested that at least a subset of early, pet1:EGFP− ISNs were KA neurons; however, the proportion of ISNs that possessed a KA neuronal identity could not be determined due to the lack of transgene penetrance in the Et(-1.5hsp70l:Gal4-VP16)s1003t line.
Figure 6. Kolmer-Agduhr (KA) neurons are 5-HT immunoreactive at 48 and 72 hpf.
The Et(-1.5hsp70l:Gal4-VP16)s1003t and Tg(UAS-E1b:Kaede)s1999t lines were crossed to express Kaede in KA neurons (green) of progeny, which were immunolabeled for 5-HT (red). The Et(-1.5hsp70l:Gal4-VP16)s1003t line did not drive transgene expression in all KA neurons. A–C: Kaede expression (A), but not 5-HT antibody labeling (B, merge in C), was detected at 24 hpf (dashed lines represent the dorsal and ventral boundaries of the spinal cord, approximately the same location in all panels). D–F: A subset of ventral KA neurons (D) were labeled with 5-HT antibodies (E, merge in F; arrows indicate colocalization). G–I: 5-HT antibody labeling (H) of KA neurons (G) was faint at 72 hpf (arrows) and Kaede-negative cells were more strongly labeled with 5-HT antibodies (H, merge in I). J–L: Kaede expression (J) and 5-HT antibody labeling (K) did not overlap at 96 hpf (L). Scale bar = 20 μm.
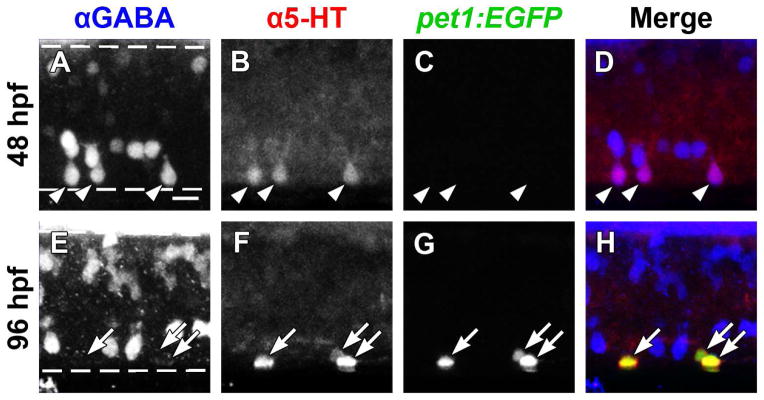
We were unable to use the Et(-1.5hsp70l:Gal4-VP16)s1003t line to confirm that all early (48 hpf, pet1:EGFP−) ISNs possessed a KA identity or that all late (96 hpf, pet1:EGFP+) ISNs did not possess a KA identity (Fig. 6). Because KA neurons are the most ventral GABAergic cell class in the zebrafish spinal cord (Bernhardt et al., 1992), GABA and 5-HT antibody double-labeling was used to examine the relationship between the GABAergic KA neurons and ISN populations in the midbody region of Tg(-3.2pet1:EGFP)ne0214 larvae (Fig. 7). At 48 hpf, prior to expression of pet1:EGFP, all spinal 5-HT immunolabeling was restricted to the GABA-positive neurons that were adjacent to the ventral border of the spinal cord (Fig. 7A–D, arrowheads; 114 of 114 5-HT+ cells colabeled with GABA, n = 5). At 96 hpf, 5-HT antibody labeling rarely colocalized with GABA (1 of 71 5-HT+ cells colabeled with GABA, n = 5), as it became restricted to the pet1:EGFP+ cell population (Fig. 7E–H, arrows; colocalization of 5-HT and pet1:EGFP quantified in Fig. 4C). GABA was not detected in pet1:EGFP+ ISNs. The position of the 5-HT+ KA neurons along the floor of the spinal cord suggested that only the double-prime subpopulation of KA neurons contained 5-HT and not the more dorsally-located KA single prime (KA″) or ventral longitudinal descending (VeLD) neurons (Bernhardt et al., 1992; Park et al., 2004). Thus, the early ISN population was identified as GABAergic KA″ neurons, which were distinct from the later-appearing pet1:EGFP+ ISNs.
Figure 7. GABA and 5-HT immunolabeling overlap in ISNs that do not express pet1:EGFP.
Whole-mount Tg(-3.2pet1:EGFP)ne0214 larvae were double-labeled with antibodies to GABA (blue) and 5-HT (red; dashed lines represent the dorsal and ventral spinal cord boundaries, same in A–D and E–H). A–D: A subset of ventral GABA-positive neurons (A) colabeled with 5-HT antibodies (B, merge in D; arrowheads indicate colocalization of GABA and 5-HT) at 48 hpf. Expression of pet1:EGFP (green) was not detected at 48 hpf (C). E–H: GABA (E) ceased to colocalize with 5-HT (F) at 96 hpf, as 5-HT became restricted to non-GABAergic pet1:EGFP+ (G; merge in H; arrows indicate colocalization of pet1:EGFP and 5-HT). Scale bar = 10 μm.
Discussion
Until now, zebrafish ISNs have been considered a single, homogeneous population throughout development (Brustein et al., 2003a; McLean and Fetcho, 2004a; Airhart et al., 2007; Airhart et al., 2012). 5-HT-immunoreactive cells are initially detected in the spinal cord between 24 and 32 hpf (McLean and Fetcho, 2004a; Sallinen et al., 2009), although they do not achieve a mature innervation pattern until 96 hpf (McLean and Fetcho, 2004a). This age corresponds to the onset of beat-and-glide swimming and effective pharmacological perturbation of locomotor output with 5-HT receptor agonists and antagonists (Brustein et al., 2003a) and selective 5-HT reuptake inhibitors (Airhart et al., 2007). Taken together, this represents a 48–72 hour delay between the onset of 5-HT synthesis in the ISNs and serotonergic modulation of motor output. Further, only tph1a expression has been described in the zebrafish spinal cord and this expression only persists until approximately 48 hpf (Bellipanni et al., 2002), bringing the source of 5-HT in older fish into question. We present three major findings that improve our understanding of ISN development. First, tph2 is expressed subsequent to tph1a (Fig. 1 and 2), accounting for the presence of 5-HT in older larvae, which do not express tph1a (Bellipanni et al., 2002). Next, we demonstrated that there are two populations of ISNs; an early-appearing and transiently 5-HT+ population that does not express pet1:EGFP, and a later-appearing, persistent population that does express pet1:EGFP (Fig. 3 and 4). Finally, we identified the early-appearing population of ISNs as CSF-contacting KA″ interneurons (Fig. 5–7), which are thought to play a role in producing spontaneous locomotion (Wyart et al., 2009). Altogether these findings (summarized in Fig. 8) progress our understanding of the development of the spinal 5-HT system, while also introducing new questions about potential functions of 5-HT in KA neurons.
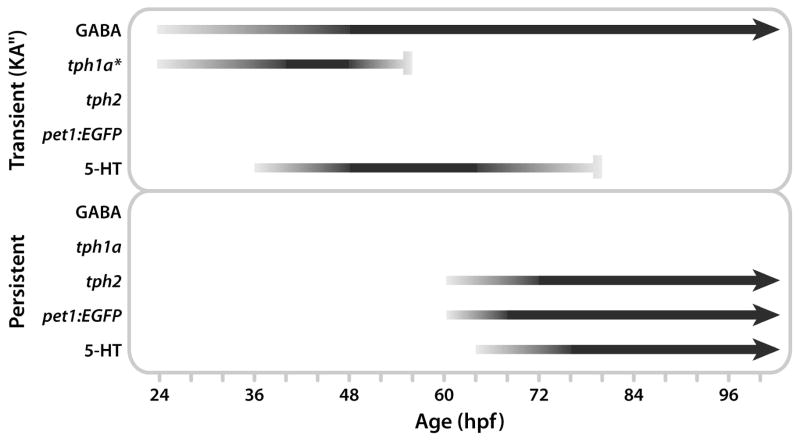
Figure 8. Schematic model of marker labeling in transient and persistent serotonergic spinal cell populations.
Horizontal lines indicate marker labeling and line intensity represents labeling intensity (black represents strongest labeling). Arrowheads indicate the hypothesized persistence of a label beyond the ages included in this study (10 dpf), and vertical bars indicate the last age that a marker was detected in a cell population. Top: Tph1a was expressed from 24 hpf until approximately 56 hpf (detected at 48, but not 68 hpf) and GABAergic KA neurons contained 5-HT from 36 hpf (estimate based on previous reports) until 80 hpf. The model predicts that tph1a is expressed in KA neurons, although localization of tph1a to the KA neurons has not yet been demonstrated (indicated by the asterisk). Bottom: Tph2 and pet1:EGFP expression began at 60 hpf and 5-HT was first detected in a second population of ISNs at 64 hpf. Colabeling of these makers persisted until at least 10 dpf.
Tph2 is upregulated as tph1a expression is lost
Spinal expression of tph1a between 24 and 48 hpf (Bellipanni et al., 2002) is consistent with the detection of 5-HT with antibodies at this age (McLean and Fetcho, 2004a). The persistence of 5-HT in cells in the ventral spinal cord well after tph1a expression ceased was initially confusing (Lillesaar, 2011). Although the existence of spinal tph2 transcripts had not been described, the observation that the tph2 (Yokogawa et al., 2012) and pet1 (Lillesaar et al., 2009) promoters drove transgene expression in the spinal cord suggested that Tph2 may be responsible for synthesizing 5-HT in the ISNs at later ages. To verify our hypothesis that both tph1a and tph2 were expressed in the larval zebrafish spinal cord, and to determine the developmental stage at which each tph gene was expressed, we conducted a developmental time-course study of tph gene expression. Consistent with previous work (Bellipanni et al., 2002), we detected tph1a transcripts in the ventral spinal cord between 24 and 48 hpf, but not at 68 or 96 hpf (Fig. 1). This tph1a expression transitioned to expression of tph2 at 68 and 96 hpf and both transcripts were localized to the ventral spinal cord (Fig. 1). The presence of tph2 transcripts after the loss of tph1a expression presented a plausible explanation for the persistence of 5-HT antibody labeling in older larval and adult spinal cords and also hinted at underlying developmental mechanisms explaining the delay between the initial appearance of ISNs and their morphological and functional maturation. This provided evidence that ISNs may not be a homogeneous population in terms of gene expression or identity.
Zebrafish possess two temporally-distinct ISN populations
We considered two hypotheses to account for the spatial and temporal tph1a and tph2 gene expression patterns. First, as tph1a and tph2 transcripts were both detected in the ventral spinal cord, it was plausible that tph1a-expressing cells down-regulated tph1a and later up-regulated tph2; that is, tph1a and tph2 were expressed in the same cells, but at different developmental stages. Alternatively, tph1a may have been expressed transiently in one population of cells, with tph2 expression arising in a distinct ventral population. Our data supported the latter hypothesis. First, the pet1:EGFP transgene labeled only a subset of ISNs, which were morphologically distinct from ISNs that did not express pet1:EGFP at 64 hpf (Fig. 5). Pet1:EGFP+ ISNs were morphologically similar to previous characterizations of ISNs, possessing ovoid somas and a single, prominent process (Brustein et al., 2003a; McLean and Fetcho, 2004a). Meanwhile, pet1:EGFP− ISNs appeared more comparable to earlier descriptions of CSF-contacting KA neurons (Vigh-Teichmann and Vigh, 1983; Dale et al., 1987a; Dale et al., 1987b; Bernhardt et al., 1992; Harper and Roberts, 1993). Antibody detection of spinal 5-HT preceded expression of the pet1:EGFP transgene at 48 hpf and a portion of pet1:EGFP+ cells did not contain 5-HT between 60 and 80 hpf (Fig. 3 and 4). This suggested that preexisting ISNs did not express the transgene; instead, pet1:EGFP was likely expressed in a separate neuronal population. Finally, cell counts demonstrated that the total number of ISNs increased between 64 and 68 hpf as pet1:EGFP+ ISNs were added to the extant population of pet1:EGFP− ISNs (Fig. 4). Altogether this evidence supported the conclusion that an early population of ISNs produced 5-HT between approximately 24 and 48 hpf and were followed by the appearance of a separate pet1:EGFP+ population of ISNs beginning at approximately 60 hpf (Fig. 8). 5-HT was restricted to pet1:EGFP+ cells through 10 dpf (Fig. 4); however, it has not been determined if 5-HT is localized to pet1:EGFP and tph2-expressing cells in adult spinal cords. The existence of separate ISN populations in embryos and larvae may have previously been unnoticed due to their temporal overlap and similar location in the ventral spinal cord. Finally, while we focused on the midbody region of the spinal cord, we did observe a clear rostral to caudal progression of development (Fig. 4 and Supplemental Information Fig. S3) which may account for the differences in the reported onset time of ISN labeling with 5-HT antibodies (McLean and Fetcho, 2004a; Sallinen et al., 2009).
Kolmer-Agduhr neurons are transiently serotonergic
We confirmed the existence of a previously undescribed population of transiently 5-HT+ spinal neurons and next determined the identity and fate of these cells. The morphological characteristics of the pet1:EGFP− ISNs were similar to those of GABAergic KA neurons (Fig. 5) and accordingly, these cells colocalized with KA neuronal markers at 48 hpf (Fig. 6 and 7). Because 5-HT antibody labeling gradually transitioned from pet1:EGFP− to pet1:EGFP+ ISNs between 48 and 96 hpf (Fig. 3 and 4), it was consistent that 5-HT antibody labeling transitioned from KA to non-KA neurons over the same developmental timespan (Fig. 6 and 7). Only GABAergic neurons whose somas contacted the ventral border of the spinal cord contained 5-HT (Fig. 7). This was consistent with the location of the KA″ subclass of KA neurons, which are distinct from the KA″ subclass and VeLD neurons, both spatially (Bernhardt et al., 1992) and in their gene expression profiles (Park et al., 2004; Yang et al., 2010; Huang et al., 2012). Further, the loss of 5-HT labeling in KA″ neurons (pet1:EGFP− ISNs) ostensibly resulted from reduced 5-HT synthesis and not developmentally programmed cell death, as the KA″ neurons have been described in adult zebrafish (Djenoune et al., 2014).
We propose that KA″ neurons express tph1a to mediate 5-HT synthesis. Although the lack of available markers prevented us from verifying that tph1a is expressed in KA″ neurons, we have shown that tph1a is expressed in cells that share a similar location along the ventral spinal cord (Fig. 1 and 2) at developmental timepoints that correspond to the presence of 5-HT in KA″ neurons (Fig. 6 and 7). Further, all 5-HT+ cells at 48 hpf were GABAergic (Fig. 7). If tph1a were expressed in non-KA spinal neurons, we would have expected to detect these cells (GABA-negative) with 5-HT antibodies at 48 hpf. Therefore, tph1a-mediated production of spinal 5-HT likely occurs in only KA″ neurons, or a subset of them. Verification of this hypothesis would be a suitable direction for further study as additional markers are developed.
The prospect of 5-HT being produced in KA neurons presents a number of compelling, yet challenging directions for future studies. Work could focus on identifying the functional roles of KA-derived 5-HT and determining its method, or methods, of signal transmission. 5-HT is a modulator of numerous physiological processes, including digestion, cardiovascular function, and locomotion (Barnes and Sharp, 1999; Berger et al., 2009), and it also provides trophic influences on developmental processes, such as cellular proliferation and differentiation, neurite growth, and synaptogenesis (McMahon, 1974; Lauder and Krebs, 1978; Lauder, 1993; Hodges and Richerson, 2008; Neckameyer, 2010). Because 5-HT is only present in the KA neurons during a narrow, temporally-defined window of development, during which the zebrafish nervous system undergoes rapid change, it is feasible that KA-derived 5-HT participates in these developmental changes. In agreement with this hypothesis, systemic depletion of 5-HT between 24 and 48 hpf resulted in decreased body length in zebrafish larvae (Airhart et al., 2012). Further, the connection of KA neurons to the central canal introduces the possibility that 5-HT is released from the KA neurons into the central canal and is transmitted through the CSF.
Conclusion
This study underscores the difficulties that are intrinsic to functional studies in a developing model system. Zebrafish locomotor networks undergo significant developmental change until 96 hpf, when they exhibit a mature swimming pattern (Drapeau et al., 2002; Brustein et al., 2003b), hunting behavior (Buss and Drapeau, 2001), and modulation of locomotion by neuromodulators such as dopamine (Thirumalai and Cline, 2008; Lambert et al., 2012) and 5-HT (Brustein et al., 2003a; Airhart et al., 2007). Previous studies have shown that perturbing the 5-HT system prior to 96 hpf does not produce locomotor defects and suggest that the ISNs are involved in modulating locomotion in 96 hpf and older larvae (Brustein et al., 2003a; Airhart et al., 2007). Our data show that the earlier (prior to 96 hpf) perturbations likely affected transiently synthesized, tph1a-derived 5-HT (in addition to non-spinal 5-HT) and not 5-HT originating from the persistent population of tph2-expressing ISNs. We have demonstrated that: 1) tph2 is expressed in the larval zebrafish spinal cord, 2) two populations of ventral ISNs arise during development of embryos/larvae, and 3) the KA″ interneurons are transiently serotonergic before the appearance of a persistent population of tph2-expressing ISNs. Thus, understanding the functional roles of transient 5-HT in KA″ neurons and persistent 5-HT in tph2-expressing ISNs is a compelling direction for future investigation.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by National Institutes of Health (http://www.nih.gov/) grants R01 NS065054 (MAM), F31 NS083110 (TDW), and R25 NS083059 (LMR). JM was supported by the University of Minnesota Bob Allison Ataxia Research Center (BAARC). CL is funded by the Program Chancengleichheit für Frauen in Forschung und Lehre from the Bayerische Staatsregierung and Julius-Maximilians-Universität Würzburg.
We thank Dr. Manfred Schartl for generously providing laboratory and fish facility space to CL. We appreciate the insightful suggestions and comments regarding this project from Dr. Claire Wyart and members of her lab. We thank Dr. Harold Burgess for sharing the Tg(tph2:nfsB-mCherry)y226 line, Dr. Laure Bally-Cuif for sharing the Tg(-3.2pet1:EGFP)ne0214 line, and Marc Tye and the staff of the University of Minnesota Zebrafish Core Facility for animal care and maintenance. We also thank the members of the Masino Lab for their contributions to the project and assistance in preparing this manuscript.
References
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–664. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG, Monaco PJ. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol Teratol. 2012;34:152–160. doi: 10.1016/j.ntt.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Akitake CM, Macurak M, Halpern ME, Goll MG. Transgenerational analysis of transcriptional silencing in zebrafish. Dev Biol. 2011;352:191–201. doi: 10.1016/j.ydbio.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Mech Dev. 2002;119(Suppl 1):S215–220. doi: 10.1016/s0925-4773(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, Patel CK, Wilson SW, Kuwada JY. Axonal trajectories and distribution of GABAergic spinal neurons in wildtype and mutant zebrafish lacking floor plate cells. J Comp Neurol. 1992;326:263–272. doi: 10.1002/cne.903260208. [DOI] [PubMed] [Google Scholar]
- Brustein E, Chong M, Holmqvist B, Drapeau P. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J Neurobiol. 2003a;57:303–322. doi: 10.1002/neu.10292. [DOI] [PubMed] [Google Scholar]
- Brustein E, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Drapeau P. Steps during the development of the zebrafish locomotor network. J Physiol Paris. 2003b;97:77–86. doi: 10.1016/j.jphysparis.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol. 2001;86:197–210. doi: 10.1152/jn.2001.86.1.197. [DOI] [PubMed] [Google Scholar]
- Dale N, Roberts A, Ottersen OP, Storm-Mathisen J. The development of a population of spinal cord neurons and their axonal projections revealed by GABA immunocytochemistry in frog embryos. Proc R Soc Lond B Biol Sci. 1987a;232:205–215. doi: 10.1098/rspb.1987.0069. [DOI] [PubMed] [Google Scholar]
- Dale N, Roberts A, Ottersen OP, Storm-Mathisen J. The morphology and distribution of ‘Kolmer-Agduhr cells’, a class of cerebrospinal-fluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proc R Soc Lond B Biol Sci. 1987b;232:193–203. doi: 10.1098/rspb.1987.0068. [DOI] [PubMed] [Google Scholar]
- De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Djenoune L, Khabou H, Joubert F, Quan FB, Nunes Figueiredo S, Bodineau L, Del Bene F, Burckle C, Tostivint H, Wyart C. Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front Neuroanat. 2014;8:26. doi: 10.3389/fnana.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Brustein E. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Fuiman LA, Webb PW. Ontogeny of Routine Swimming Activity and Performance in Zebra Danios (Teleostei, Cyprinidae) Animal Behaviour. 1988;36:250–261. [Google Scholar]
- Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Goll MG, Anderson R, Stainier DY, Spradling AC, Halpern ME. Transcriptional silencing and reactivation in transgenic zebrafish. Genetics. 2009;182:747–755. doi: 10.1534/genetics.109.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Rhee J, Goll MG, Akitake CM, Parsons M, Leach SD. Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish. 2008;5:97–110. doi: 10.1089/zeb.2008.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CE, Roberts A. Spinal cord neuron classes in embryos of the smooth newt Triturus vulgaris: a horseradish peroxidase and immunocytochemical study. Philos Trans R Soc Lond B Biol Sci. 1993;340:141–160. doi: 10.1098/rstb.1993.0053. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huang P, Xiong F, Megason SG, Schier AF. Attenuation of Notch and Hedgehog signaling is required for fate specification in the spinal cord. PLoS Genet. 2012;8:e1002762. doi: 10.1371/journal.pgen.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lambert AM, Bonkowsky JL, Masino MA. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J Neurosci. 2012;32:13488–13500. doi: 10.1523/JNEUROSCI.1638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Lillesaar C. The serotonergic system in fish. J Chem Neuroanat. 2011;41:294–308. doi: 10.1016/j.jchemneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Stigloher C, Tannhauser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. J Comp Neurol. 2009;512:158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Tannhauser B, Stigloher C, Kremmer E, Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn. 2007;236:1072–1084. doi: 10.1002/dvdy.21095. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004a;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. J Comp Neurol. 2004b;480:57–71. doi: 10.1002/cne.20281. [DOI] [PubMed] [Google Scholar]
- McMahon D. Chemical messengers in development: a hypothesis. Science. 1974;185:1012–1021. doi: 10.1126/science.185.4156.1012. [DOI] [PubMed] [Google Scholar]
- Muller UK, Stamhuis EJ, Videler JJ. Hydrodynamics of unsteady fish swimming and the effects of body size: comparing the flow fields of fish larvae and adults. J Exp Biol. 2000;203:193–206. doi: 10.1242/jeb.203.2.193. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. A trophic role for serotonin in the development of a simple feeding circuit. Dev Neurosci. 2010;32:217–237. doi: 10.1159/000304888. [DOI] [PubMed] [Google Scholar]
- Norton WH, Mangoli M, Lele Z, Pogoda HM, Diamond B, Mercurio S, Russell C, Teraoka H, Stickney HL, Rauch GJ, Heisenberg CP, Houart C, Schilling TF, Frohnhoefer HG, Rastegar S, Neumann CJ, Gardiner RM, Strahle U, Geisler R, Rees M, Talbot WS, Wilson SW. Monorail/Foxa2 regulates floorplate differentiation and specification of oligodendrocytes, serotonergic raphe neurones and cranial motoneurones. Development. 2005;132:645–658. doi: 10.1242/dev.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004;131:5959–5969. doi: 10.1242/dev.01456. [DOI] [PubMed] [Google Scholar]
- Rauch GJ, Lyons DA, Middendorf I, Friedlander B, Arana N, Reyes T, Talbot WS. Submission and Curation of Gene Expression Data. ZFIN Direct Data Submission. 2003 ( http://zfin.org)
- Ren G, Li S, Zhong H, Lin S. Zebrafish tyrosine hydroxylase 2 Gene Encodes Tryptophan Hydroxylase. J Biol Chem. 2013 doi: 10.1074/jbc.M113.485227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen V, Sundvik M, Reenila I, Peitsaro N, Khrustalyov D, Anichtchik O, Toleikyte G, Kaslin J, Panula P. Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J Neurochem. 2009;109:403–415. doi: 10.1111/j.1471-4159.2009.05986.x. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods. 2007;4:323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Russell C, Regan J, Chandrasekhar A, Concha ML, Yokoyama R, Higashi K, Take-Uchi M, Dong W, Hiraga T, Holder N, Wilson SW. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J Neurobiol. 2004;60:275–288. doi: 10.1002/neu.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J Neurophysiol. 2008;100:1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission. 2004 ( http://zfin.org)
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Van Dongen PA, Hokfelt T, Grillner S, Verhofstad AA, Steinbusch HW. Possible target neurons of 5-hydroxytryptamine fibers in the lamprey spinal cord: immunohistochemistry combined with intracellular staining with Lucifer yellow. J Comp Neurol. 1985;234:523–535. doi: 10.1002/cne.902340409. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk W, Bosch TJ, Smit-Onel MJ, Maslam S. Organisation of the zebrafish spinal cord: distribution of motoneuron dendrites and 5-HT containing cells. Eur J Morphol. 1996;34:65–77. doi: 10.1076/ejom.34.2.65.13021. [DOI] [PubMed] [Google Scholar]
- Vigh-Teichmann I, Vigh B. The system of cerebrospinal fluid-contacting neurons. Arch Histol Jpn. 1983;46:427–468. doi: 10.1679/aohc.46.427. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Rastegar S, Strahle U. Regulatory interactions specifying Kolmer-Agduhr interneurons. Development. 2010;137:2713–2722. doi: 10.1242/dev.048470. [DOI] [PubMed] [Google Scholar]
- Yokogawa T, Hannan MC, Burgess HA. The dorsal raphe modulates sensory responsiveness during arousal in zebrafish. J Neurosci. 2012;32:15205–15215. doi: 10.1523/JNEUROSCI.1019-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.