Abstract
Objective
To study whether the effects of olanzapine on gastrointestinal motility is related to the serotonin antagonism and myosin light chain kinase.
Methods
Male Sprague-Dawley rats were randomly divided into four groups. Olanzapine gavage was performed for each treatment group during the course of 30 continuous days, while the same volume of saline was given to the rats in the control group. Defecation of the rats was observed on days 7 and 30 after olanzapine gavage. The effects of olanzapine on contraction of colonic smooth muscles were observed in ex vivo experiments. A Western blot was used to evaluate expression levels of the serotonin transporter (SERT) and MLCK in colon segments of the rats.
Results
ResultsaaCompared to the control group, 5-160 µ M of olanzapine could inhibit dose-dependently the contraction of colonic smooth muscle ex vivo experiments. The maximum smooth muscle contraction effects of 5-HT and acetylcholine significantly decreased after treatment with 40-160 µ M of olanzapine. Constipation was found in the olanzapine-treated rats on day 7 and have sustained day 30 after gavage. Expression of MLCK in olanzapine-treated rats was significantly decreased, whereas the expression of SERT significantly increased on the day 7, then significantly decreased on the day 30 after olanzapine gavage.
Conclusion
SERT and MLCK may involve in the inhibition of colonic contraction induced by olanzapine.
Keywords: Antipsychotic agents, Intestinal motility, Serotonin system, Myosin light chain kinase
INTRODUCTION
Olanzapine is an atypical antipsychotic agent that is used to effectively treat symptomatic and asymptomatic schizophrenia. However, several adverse effects including tiredness, weight increase, and constipation have been reported in clinical practice. Compared to other antipsychotic agents, the incidence rate of constipation is 9–11% higher in patients treated with olanzapine.1 Additionally, a dose-dependent increase in constipation instances has also been reported.2,3 Constipation induced by olanzapine could aggravate to the point of life-threatening complications including: intestinal obstruction, ischemia, and even perforation if no effective treatment has been administered.4 Previous studies suggested that antagonism of the peripheral cholinergic M receptor could be involved in constipation induced by olanzapine. In vivo and in vitro experiments have shown that although olanzapine has a high affinity to the M receptor, the antagonistic effects of olanzapine on M receptor are relatively low.5,6 Therefore, it is hypothesized that there are other mechanisms involved in the high incidence of constipation induced by olanzapine.
Olanzapine may antagonize multiple receptors. Previous studies have shown that olanzapine has affinities for dopamine receptors (D1–D5), 5-HT receptors (5-HT1, 5-HT2, 5-HT3, and 5-HT6), α-adrenergic receptor, histamine H1 receptor, and muscarinic receptors (M1–M5).1,5,7 As an important neurotransmitter, 5-HT could participate in regulating the sensory, motility, and secretion of the digestive system and has been considered as a mediator for gut-brain connection.8 About 95% of the 5-HT in the human body is located in the gastrointestinal tract. For most of people, 5-HT is synthesized and secreted by enterochromaffin cells into gastrointestinal lumens and the blood.9 Released 5-HT could then combine into different kinds of receptors (5HT1–5HT7 receptors) and exert different effects. 5-HT is then transferred back to cells by serotonin transporter (SERT) and made inactive. Previous studies have demonstrated that 5-HT receptor subtypes that could potentially affect the intestinal motilities include 5-HT3, 5-HT4, and 5HT7 receptors,10 while olanzapine can distinctly antagonize 5-HT3 receptor.11 5-HT3 receptor are expressed mainly in gastrointestinal smooth muscle tissue, which could regulate the contraction of the smooth muscles and the secretion of gastric fluid.12 Guinea pig experiments showed that olanzapine could block the contraction of ex vivo ileum induced by 5-HT3 receptor agonists, suggesting that the antagonistic effects of olanzapine on the serotonin system should not be neglected.11 SERT plays an important role in terminating the effects of 5-HT as a neurotransmitter and maintaining the homeostasis of neurotransmitters.10 Previous studies have shown that SERT is involved in the development and progression of irritable bowel syndrome (IBS).13,14 This evidence shows that the antagonism effect of olanzapine on serotonin as well as SERT both play important roles in intestinal motility dysfunctions as well as visceral paraesthesia.10,15
One the other hand, Myosin light chain kinase (MLCK) is an important protein involved in the inositol triphosphate and calmodulin (CaM) signal transduction pathway. Previous studies have shown that MLCK could promote the phosphorylation of myosin light chains (20 KD), which interact with actin to induce the contraction of intestinal smooth muscles. This suggests a close association between MLCK and gastrointestinal motility.16,17 Several animal experiments also show that MLCK expression is decreased within the gastrointestinal mucosa of diabetic rats with gastrointestinal dysfunctions.18 Therefore, the aim of this study was to investigate the potential role of serotonin antagonist and MLCK in olanzapine-induced constipation. The present study examined the effect of olanzapine on intestinal motility induced by 5-HT and MLCK in vivo.
METHODS
Animals
Forty male Sprague-Dawley (SD) rats (body weight of 180–220 g, Vital River Laboratory Animal Technology Co. Ltd., Beijing, China) were randomly divided into 4 groups of 10 and named: control group, low dose (olanzapine) group (0.5 mg/kg/day), moderate dose group (2 mg/kg/day), and high dose group (8 mg/kg/day). Procedures for the animals were performed according to the "Guide for the Care and Use of Laboratory Animals" (NIH Publication No.80-23) issued by National Institute of Health (NIH). Gavage of olanzapine was performed at 7 o'clock every morning for the treatment groups, while gavage of normal saline was performed for the control group at the same time. The overall volume of gavage was 2 mL every day. All the animals were treated for 30 continuous days.
Reagents
Olanzapine crude ingredient (light yellow powder, purity of 98%) was purchased from Yibang Biomedical Technology Co., Ltd (Guangzhou, China). The crude ingredient was dissolved in an appropriate volume of 5% glacial acetic acid (V/V), and then pH adjusted to 5.5 with NaOH. Klein's solution (mmol/L) was prepared by mixing NaCl (114.0), KCl (4.7), MgCl2 (1.2), CaCl2 (2.5), HT2PO4-1 (1.8), glucose (11.5), and NaHCO3(18.0) before being pH adjusted to 7.4±0.5.
Experimental design
Preparation of ex vivo colonic smooth muscle and measurement of the tension19
The rats were sacrificed and their colons were collected. A segment of colon about 1–2 cm of length was quickly obtained and preserved in an ice-bath for 5–8 hours with ventilation. One end of the colon segment was fixed on the hook of an L-shaped tube, while the other end of the colon segment was hung on the hook. The tension of the colon segment was adjusted to ensure the preload of the smooth muscle was 1.0 g before contraction. Then a recorder was connected through a tension sensor and the muscle tension curve was recorded for 50 minutes.
Dose-response curve of olanzapine
After the contraction of the colonic smooth muscles stabilized, different doses (5, 10, 20, 40, 80, and 160 µM) of olanzapine were added, and then changes in smooth muscle contraction were examined. For each dose of olanzapine, the tension curve was recorded for at least one minute. All the drug administration were completed within 20 minutes.
Influences of olanzapine on the colonic motility-promoting effects of Ach and 5-HT
After the contraction of the colonic smooth muscles stabilized, 40 µM of olanzapine was added and incubated for 5 minutes. Then different doses (5, 10, 20, 40, 80, and 160 µM) of acetylcholine (ACh) were added and changes in the smooth muscle contraction were examined. For each dose of olanzapine, the tension curve was recorded for at least one minute. All of the drug administration were completed within 20 minutes, and the dose-response curve of ACh was obtained. The colon segment was changed, and then a dose-response curve of 5-HT was obtained using the same method.
Expression of SERT and MLCK
Five rats from each group were sacrificed on day 7 and day 30, respectively. Colons of the rats were obtained and the expression of SERT was measured. In addition, MLCK expression was also measured on the day 30 by Western blot. The total proteins were extracted using kits (Qiagen), and then separated by electrophoresis (JM-250, Dalian, China) with a protein loading quantity of 50 µg. After electrophoresis, a semi-dry transfer (ST-I, Dalian, China) was used to transfer proteins for 1.5 hours. Then the PVDF membrane (containing protein bands) was incubated with MLCK antibody (1:1000) (Abcam, Hong Kong) overnight. A multispectral imaging system (UVP, Cambridge, UK) was used for the determination and quantification of the bands. Quantification of SERT protein in the colonic tissues was performed using a similar method as in the quantification of MLCK protein, except that the antibody used was SERT antibody (1:1000) (Abcam, Hong Kong). The mean value of each band was calculated as follows: value of the target protein=gray-scale value of the target protein/gray scale value of the internal reference protein.
Defecation of the rats20
The amount of the feces from the rats were recorded before gavage every day. The number was recorded as the number of feces after the last gavage but before the present gavage. For the rats with diarrhea, a smudge was considered to be one feces. The number of feces within 8 hours after the gavage at 7 o'clock was then recorded, and the frequency of defection was calculated as follows: number of feces/8 (hours)/rat number. Fresh feces from the rats were weighed as the wet weight, and the weight after the feces were dried was recorded as the dry weight. Water content in the feces was calculated as follows: (wet weight-dry weight)/wet weight.
Statistical analysis
SPSS 17.0 software was used for the statistical analysis. All the quantitative data were described as means±standard divisions (χ̄±s). One-way analysis of variance was used for the comparison of the means among different groups, while LSD test was used for the pairwise comparisons. p<0.05 was considered statistically significant.
RESULTS
Effects of olanzapine on the contraction of the smooth muscles in ex vivo colon
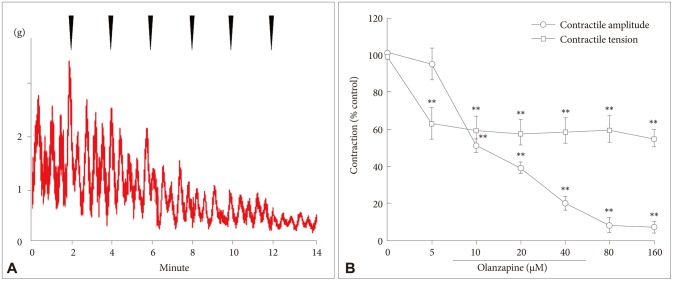
Compared with the control group, the contraction of smooth muscles in the ex vivo colon tissues was significantly inhibited by 5–160 µM of olanzapine, and the effect was dose dependent (Figure 1A). The EC50 of olanzapine was 40 µM (Figure 1B). After incubation with 160 µM of olanzapine, the contraction amplitude of the colonic smooth muscles decreased from 100.0% (±13.4) to 15.4% (±2.6) (p<0.01; n=6 tissues), and the contractile tension decreased from 100.0% (±5.6) to 70.1% (±4.8) (p<0.01; n=6 tissues) (Figure 1B).
Figure 1. Effects of olanzapine on the contraction of smooth muscles in the ex vivo colons. A: Meant the contraction of smooth muscles weaken accompanying with olanzapine effect on the ex vivo colons. B: Meant different doses of olanzapine added to the smooth muscle was indicated by the arrows above. The x-axis means the time of olanzapine injection. The stable recorder for smooth muscle without olanzapine was as comparison. Data were expressed as mean±standard division, N=6. The value in the control group was considered as 100%. **p<0.01.
Influences of olanzapine on the colonic motility-promoting effects of ACh and 5-HT
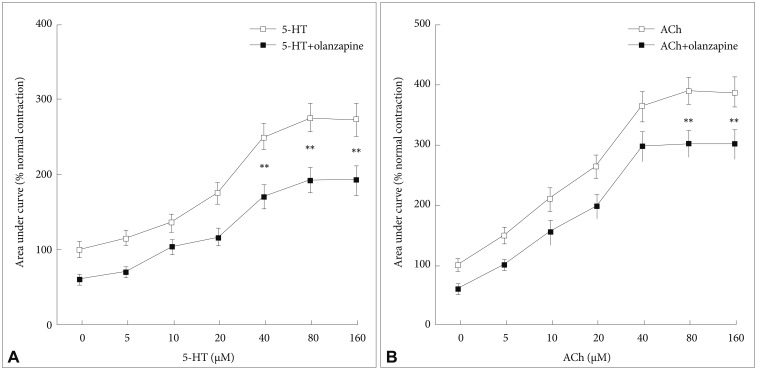
As shown in Figure 2, after incubation with 40 µM of olanzapine, the maximal effects of 5-HT and ACh in improving the contraction of the smooth muscles of the colon decreased significantly (p<0.01). In detail, the contraction of the smooth muscles in the colon treated with 5-HT decreased from 371.2% (±22.8) to 237.1% (±17.9) (Figure 2A), while the contraction of the smooth muscles in the colon treated with Ach decreased from 263.2% (±17.6) to 155.1% (±11.4) (Figure 2B). Both the dose-response curves of 5-HT and ACh shifted significantly upward.
Figure 2. Influences of olanzapine on the colonic motility-promoting effects of 5-HT (A) and ACh (B). All the data were expressed as means±standard divisions, N=6. The value in the control group was considered as 100%. **p<0.01. 5-HT: serotonin, ACh: acetylcholine.
Effects of olanzapine on defecation of the rats
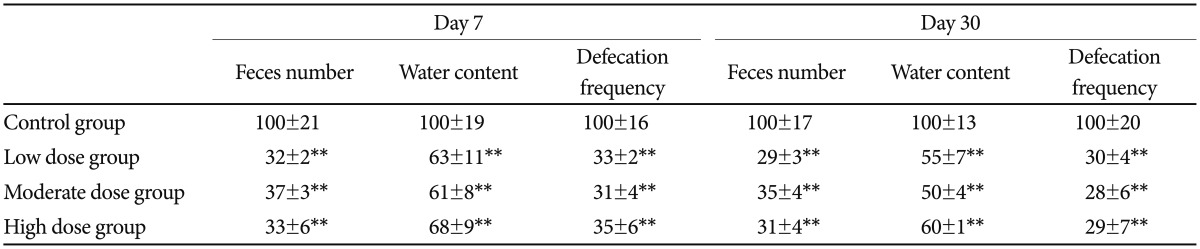
In contrast to the control group, constipation was found in the rats from each treatment group one week after the gavage of olanzapine. The presentations included a significant decrease in the number of feces, defecation frequency, and water content (p<0.01). The rats in each treatment group were found to have constipation by day 30 (Table 1).
Table 1. Effects of olanzapine on defecation of the rats.
All the data were expressed as mean±standard division, N=6. The value in the control group was considered as 100% (**p<0.01)
Effects of olanzapine on SERT expression
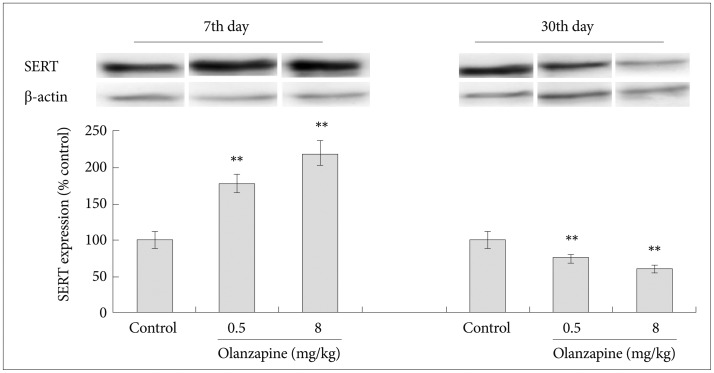
As shown in the Figure 3, the SERT level increased on day 7 after olanzapine gavage. The SERT level in the high dose group was 210.2% (±19.3), which was significantly higher than the level in the control group 100.0% (±11.2) (p<0.01). In contrast, SERT level in the treatment groups was lower than the levels in the control group on the day 30, after olanzapine gavage. The SERT level in the high dose group was 61.2% (±5.5), which were significantly lower than the level in the control group 100.0% (±9.8) (p<0.01).
Figure 3. Effects of olanzapine on SERT expression. All these data were expressed as means±standard divisions, N=4. The value in the control group was considered as 100%. **p<0.01. β-actin was used as the internal reference. SERT: serotonin transporter, MLCK: myosin light chain kinase.
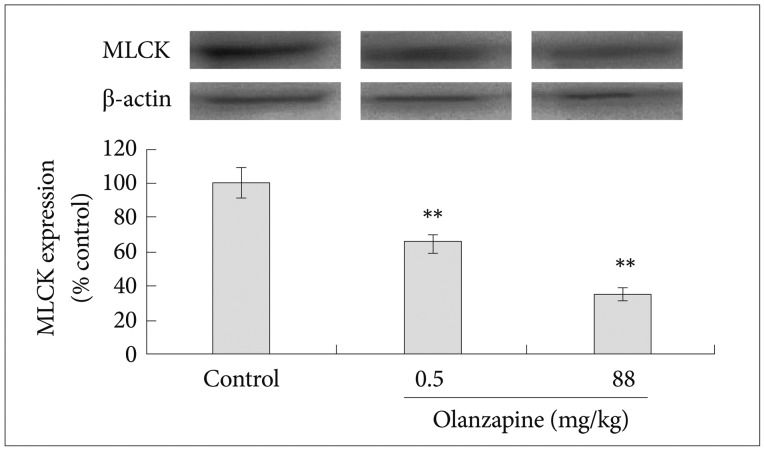
Effects of olanzapine on MLCK expression
MLCK expression decreased significantly after olanzapine gavage (Figure 4). The MLCK level in the high dose group was 53.2% (±2.9), which was significantly lower than the level in the control group 100.0 (±13.6)% (p<0.01).
Figure 4. Effects of olanzapine on MLCK expression. All these data were expressed as means±standard divisions, N=4. The value in the control group was considered as 100%. **p<0.01. β-actin was used as the internal reference. MLCK: myosion light chain kinase.
DISCUSSION
Previous opinions suggested that olanzapine could antagonize peripheral M2 and M3 receptors to reduce gastric secre-tions, thus inhibiting the motility of gastrointestinal smooth muscles, and resulting in constipation.21 However, the antagonistic effects of olanzapine on M receptor are relatively low, thus there may be other mechanisms involved in the development of olanzapine induced constipation. Amount of previous studies demonstrated that 5-HT3 receptors were mainly expressed in the intestinal and colonic smooth muscles, where olanzapine could effectively block the 5-HT3 receptor.10,11,12 For this reason, olanzapine has ever been used in clinical practices for the treatment of IBS.22
In the present study, ex vivo experiments showed that olanzapine could inhibit dose-dependently the contraction of ex vivo colonic smooth muscle in rats and reverse the activation of smooth muscles induced by 5-HT and ACh. It suggested that the inhibitory effect of olanzapine on rat colonic motility correlated with the antagonisms of 5-HT and ACh. The in vivo experiments in the present study showed that all rats treated with different doses of olanzapine developed constipation on day 30 after olanzapine gavage, which further suggested that olanzapine could antagonize certain peripheral receptors, probably 5-HT and ACh receptors, leading to constipation. SERT was distributed at the mucosal layer of the intestinal tract and the enteric nervous system. It could transport the 5-HT from the synaptic cleft to adjacent cells for further oxidative metabolism and thus regulate the conductive activity of serotonin nerves.23 However, these effects were associated with the expression of SERT on the membrane.24 The findings of the present study showed that the expression of SERT in the colonic smooth muscles increased one week after olanzapine gavage compared with control group, but significantly decreased on day 30 after olanzapine gavage. Increased expression of SERT could decrease 5-HT concentration in the synaptic clefts and thus inhibit colonic motility. The significant decrease of SERT could theoretically increase the transportation of 5-HT and thus improve the colonic motility, which could in turn decrease the risk of constipation. Although this theory was not directly supported by the present results, previous studies indicated that the increase of 5-HT in synaptic clefts increased sharply instead of gradually increasing;25 and among the multiple 5-HT receptor family members, the 5-HT3 receptor is the only ligand-gated ion channel receptor and closely related to contraction of the smooth muscles and the secretion of gastric fluid, which makes it with high susceptible to desensitization.26 The significantly decreased expression of SERT in the rats received long-term olanzapine gavage could decrease the re-uptake of 5-HT. Therefore, large amounts of 5-HT would aggregate at the target site and thus induced desensitization of 5-HT3 receptor, which in turn decreased the colonic motility and induced dysfunction.14,27,28 Based on the above theory, we indirectly speculated that olanzapine probably antagonize the 5-HT3 receptor besides ACh receptor to cause constipation in rats. Some other studies also found that the expression of SERT in the intestinal mucosa of patients with chronic constipation was reduced, while constipation could not induce compensative changes in the 5-HT signaling pathway within the intestinal tract through feedback.28 These findings suggested that constipation and 5-HT3 were essential prerequisites for each other's dysfunction, and they cooperate with each other to induce the development and progression of reduced colonic contraction. In addition, several other studies also found that 5-HT3 receptor antagonist could increase the absorption of fluid in the jejunum.29 which could also be one of the mechanisms involved in the constipation risk increase caused by olanzapine.
MLCK is very important for gastrointestinal motility. Previous studies showed that a knock-out of the MLCK gene could reduce the gastrointestinal motility and the gastrointestinal tension in mice.16 The findings of the present study showed that olanzapine could significantly reduce expression of MLCK in the rat intestinal tract, suggesting that olanzapine could inhibit the expression of MLCK and thus reduce the contraction of smooth muscles. This in turn reduced the colonic motility and the forces on chyme. Previous studies showed that chlorpromazine, a CaM inhibitor, could widely influence the intracellular calcium-binding proteins and thus interfere the signal transductions mediated by Ca2+.30 Inhibition of CaM could induce inactivation of MCLK and thus influence the contraction of intestinal smooth muscles.31 However, more studies would be needed to investigate whether the effects of olanzapine on the expression of MCLK are also involved in these procedures.
There are some limitations in our present study. Firstly, our study demonstrated that olanzapine inhibited 5-HT-induced colonic motility and constipation ex vivo. However, the involvement of ACh receptor couldn't be excluded, although olanzapine was a weaker antagonist at muscarinic receptor.5 Secondly, the present study couldn't directly concluded which 5-HT receptor subtypes was antagonized by olanzapine to cause abnormality of intestinal motility in rats, although previous evidence showed that the distribution of 5-HT3 receptor in humans and rodents are mainly in gastrointestinal smooth muscle tissue, which could regulate the contraction of the smooth muscles and the secretion of gastric fluid.12 Thirdly, the protein level of CaM or the phosphorylation level of MLCK could well explain the possible involvement of Ca2+-CaM antagonistic action. Nevertheless, we only investigate the expression of MLCK with Western blot. Therefore, further study should focus on the unresolved above questions in the future.
In conclusion, we demonstrated that peripheral 5-HT receptor involved in the constipation induced by olanzapine. Furthermore, the effect of promoting intestinal motility of 5-HT was blocked by olanzapine, combined with the characteristics of the 5-HT receptor subtypes distribution in the gastrointestinal tract of rats and the pharmacological properties of olanzapine. Our results suggest serotonin antagonism and MLCK, besides M receptor, probably participated in the course of pathophysiology of constipation induced by olanzapine.
Acknowledgments
This study was funded National Natural Science Foundation of China (81230027, 81401127), Shanghai Natural Science Foundation (13ZR1460500), Key Laboratory of Psychotic Disorders (13dz2260500-14-K06), Animal special fund of the Science and Technology Commission of Shanghai (13140903400), Shanghai Changhai Hospital Foundation, and Postdoctoral Grant of Secondary Military Medical University. We would like to acknowledge the reviewers for their helpful comments on this paper.
References
- 1.Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30:75–82. doi: 10.1177/1049909112441241. [DOI] [PubMed] [Google Scholar]
- 2.Kelly DL, Richardson CM, Yu Y, Conley RR. Plasma concentrations of high-dose olanzapine in a double-blind crossover study. Hum Psychopharmacol. 2006;21:393–398. doi: 10.1002/hup.781. [DOI] [PubMed] [Google Scholar]
- 3.Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Kirshner MA, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res. 2006;88:63–72. doi: 10.1016/j.schres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Rege S, Lafferty T. Life-threatening constipation associated with clozapine. Australas Psychiatry. 2008;16:216–219. doi: 10.1080/10398560701882203. [DOI] [PubMed] [Google Scholar]
- 5.Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, et al. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999;37:107–122. doi: 10.1016/s0920-9964(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 6.Bymaster FP, Rasmussen K, Calligaro DO, Nelson DL, Nelapp NW, Wong DT, et al. In vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug. J Clin Psychiatry. 1997;58(Suppl 10):28–36. [PubMed] [Google Scholar]
- 7.Bhana N, Foster RH, Olney R, Plosker GL. Olanzapine: an updated review of its use in the management of schizophrenia. Drugs. 2001;61:111–161. doi: 10.2165/00003495-200161010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 9.Tamir H, Payette RF, Huang YL, Liu KP, Gershon MD. Human serotonectin: a blood glycoprotein that binds serotonin and is associated with platelets and white blood cells. J Cell Sci. 1985;73:187–206. doi: 10.1242/jcs.73.1.187. [DOI] [PubMed] [Google Scholar]
- 10.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Bymaster FP, Falcone JF, Bauzon D, Kennedy JS, Schenck K, Delapp NW, et al. Potent antagonism of 5-HT(3) and 5-HT(6) receptors by olanzapine. Eur J Pharmacol. 2001;430:341–349. doi: 10.1016/s0014-2999(01)01399-1. [DOI] [PubMed] [Google Scholar]
- 12.Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol. 2014;18:613–621. doi: 10.1007/s10151-013-1106-8. [DOI] [PubMed] [Google Scholar]
- 13.Kerckhoffs AP, Ter Linde JJ, Akkermans LM, Samsom M. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–907. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 14.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 16.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 18.Hu W, Feng P. Myosin light chain kinase is involved in the mechanism of gastrointestinal dysfunction in diabetic rats. Dig Dis Sci. 2012;57:1197–1202. doi: 10.1007/s10620-012-2041-7. [DOI] [PubMed] [Google Scholar]
- 19.Umer A, Lugowska H, Sein-Anand J, Rekowski P, Ruczynski J, Petrusewicz J, et al. The contractile effects of several substituted short analogues of porcine galanin in isolated rat jejunal and colonic smooth muscle strips. Pharmacol Res. 2005;52:283–289. doi: 10.1016/j.phrs.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Xiong YJ, Chen DP, Lv BC, Liu FF, Wang L, Lin Y. The characteristics of genistin-induced inhibitory effects on intestinal motility. Arch Pharm Res. 2013;36:345–352. doi: 10.1007/s12272-013-0053-2. [DOI] [PubMed] [Google Scholar]
- 21.Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL. Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Farthing MJ. Irritable bowel syndrome: new pharmaceutical approaches to treatment. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:461–471. doi: 10.1053/bega.1999.0040. [DOI] [PubMed] [Google Scholar]
- 23.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- 27.Guarino M, Cheng L, Cicala M, Ripetti V, Biancani P, Behar J. Progesterone receptors and serotonin levels in colon epithelial cells from females with slow transit constipation. Neurogastroenterol Motil. 2011;23:575–e210. doi: 10.1111/j.1365-2982.2011.01705.x. [DOI] [PubMed] [Google Scholar]
- 28.Costedio MM, Coates MD, Brooks EM, Glass LM, Ganguly EK, Blaszyk H, et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am J Gastroenterol. 2010;105:1173–1180. doi: 10.1038/ajg.2009.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsen J, Lendorf A, Raskov H, Boesby S. Ketanserin versus placebo in carcinoid syndrome. A clinical controlled trial. Scand J Gastroenterol. 1986;21:816–818. doi: 10.3109/00365528609011123. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura H, Arai M, Togari A. Inhibitory effect of chlorpromazine on RANKL-induced osteoclastogenesis in mouse bone marrow cells. J Pharmacol Sci. 2011;117:54–62. doi: 10.1254/jphs.11006fp. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Andrade M, Mata R, Madariaga-Mazon A, Rodriguez-Sotres R, Del PL, Sosa-Peinado A. Importance of the interaction protein-protein of the CaM-PDE1A and CaM-MLCK complexes in the development of new anti-CaM drugs. J Mol Recognit. 2013;26:165–174. doi: 10.1002/jmr.2261. [DOI] [PubMed] [Google Scholar]