Abstract
Aim
This study was retrospectively designed to evaluate the influence of healthy lifestyle behaviors on the incidence of chronic kidney disease (CKD) during a 5-year follow-up period in middle-aged and older males.
Methods
The subjects included 252 males without a history of cardiovascular disease, stroke, renal dysfunction and/or dialysis treatment who were not taking any medications. Their lifestyle behaviors were evaluated using a standardized self-administered questionnaire and defined as follows: (1) habitual moderate exercise, (2) daily physical activity, (3) fast walking speed, (4) slow eating speed, (5) no late-night dinner, (6) no bedtime snacking and (7) no skipping breakfast. The participants were divided into four categories, which were classified into quartile distributions according to the number of healthy lifestyle behaviors (7–6, 5, 4 and ≤3 groups).
Results
After 5 years, the incidence of CKD [estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 and/or proteinuria] was observed in 23 subjects (9.1 %). The Kaplan–Meier survival curves showed that the cumulative incidence of CKD significantly decreased according to an increase in the number of healthy lifestyle behaviors (log-rank test: p = 0.003). According to a multivariate analysis, habitual moderate exercise [hazard ratio (HR) 0.20, 95 % confidence of interval (CI) 0.06–0.69, p = 0.011] and no bedtime snacking (HR 0.19, 95 % CI 0.08–0.48, p = 0.004) were significantly associated with the incidence of CKD.
Conclusions
These results suggest that the accumulation of healthy lifestyle behaviors, especially those related to habitual moderate exercise and no bedtime snacking, is considered to be important to reduce the risk of CKD.
Electronic supplementary material
The online version of this article (doi:10.1007/s12199-016-0506-6) contains supplementary material, which is available to authorized users.
Keywords: Incidence of CKD, Healthy lifestyle behaviors, Habitual moderate exercise, Bedtime snacking
Introduction
The number of patients with end-stage renal disease (ESRD) in Japan is continuously increasing [1]. Chronic kidney disease (CKD) has been associated with the development of ESRD and cardiovascular disease (CVD) [2, 3]. At the present, the large number of ESRD patients is currently believed to be related to an increasing number of patients with CKD. The risk factors for CKD are reported to be aging, hypertension, diabetes mellitus and metabolic syndrome [4–6].
In addition to hypertension, diabetes mellitus and metabolic syndrome, the incidence of CKD is also closely correlated with unhealthy lifestyle behaviors such as smoking, heavy alcohol intake, obesity, physical inactivity and an unhealthy diet [7–12]. Moreover, it is well known that a decreased physical activity and aerobic capacity are predictive factors for the prognosis in ESRD patients [13, 14]. Thus, lifestyle modifications in the early stages of hypertension, diabetes mellitus, dyslipidemia and metabolic syndrome are considered to be necessary for preventing the development of CKD. However, the association between the adherence to healthy lifestyle behaviors and preventing the onset of CKD has not yet been studied.
The accumulation of healthy lifestyle behaviors has been shown to be associated with the prevention of CVD, stroke, metabolic syndrome, type 2 diabetes, hypertension and dyslipidemia [15–19]. The clarification of the association between the adherence to healthy lifestyle behaviors and the incidence of CKD may help to demonstrate the importance of lifestyle modification in CKD prevention. We therefore hypothesize that healthy lifestyle behaviors may predict the incidence of CKD because the adherence to healthy lifestyle behaviors has been shown to be associated with the prevention of CVD, stroke and several coronary risk factors [15–19]. We focused on the physical activity, exercise and eating habits as lifestyle behaviors because the increases in the daily physical activity and changes in diet are important to be an initial step for the prevention of CVD [20]. This long-term follow-up study was retrospectively designed to evaluate the influence of healthy lifestyle behaviors on the incidence of CKD in middle-aged and older males.
Subjects and methods
Subjects
A total of 773 middle-aged and older adults received their periodic health check-up at a health care center in Fukuoka University in 2008. The study diagram of the participants included in this study is shown in Fig. 1. Among the 434 subjects who provided informed consent, 178 females were excluded in this study to remove the influence of gender. Subjects with a previous history of CVD such as angina and myocardial infarction (n = 4), stroke (n = 2), renal dysfunction [glomerular filtration rate estimated by the Japanese GFR inference formula (eGFR) <60 ml/min/1.73 m2, or proteinuria, or both] and/or dialysis treatment (n = 45), or those taking medications (n = 86), such as anti-hypertensive drugs, statins or hypoglycemic agents, were excluded from the analysis because we focused on the effects of only lifestyle behaviors without the influence of these medications. A total of 252 males [age 51.7 ± 6.8 years, body mass index (BMI) 23.1 ± 2.7 kg/m2, serum creatinine 0.83 ± 0.09 mg/dl and eGFR 78.1 ± 10.1 ml/min/1.73 m2] [21] with no missing information during 5 years were eligible for the present study.
Fig. 1.
Participants included in the study. CVD cardiovascular disease, eGFR estimated glomerular filtration rate
All subjects gave their informed consent for participation after agreeing with the purpose, methods and significance of the study. The study conforms to the Declaration of Helsinki and was approved by the Ethics Committee of Fukuoka University (No. 11-08-01).
Blood sampling, blood pressure and anthropometry measurements
Blood samples were collected early in the morning by venipuncture from an antecubital vein after at least 12 h of fasting. The blood samples were analyzed by Special Reference Laboratories (SRL Inc., Tokyo, Japan). The serum creatinine, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels were measured by the direct method. The triglyceride levels were measured by the enzyme method. The plasma glucose level was measured by an ultraviolet/hexokinase method and hemoglobin A1c (HbA1c) was measured by high performance liquid chromatography. HbA1c is presented as the National Glycohemoglobin Standardization Program (NGSP) value, which was calculated using the conversion equation for HbA1c derived from the Japan Diabetes Society (JDS): HbA1c (NGSP value; %) = 1.02 × JDS value (%) + 0.25 % [22].
The eGFR level was calculated using the Japanese GFR inference formula: eGFR (ml/min/1.73 m2) = 194 × serum creatinine (mg/dl)−1.094 × age (years)−0.287 (if female × 0.739) [23]. The GFR is a more accurate measure of the renal function [24] and identifies patients with mild renal impairment despite normal or nearly normal creatinine levels. Moreover, the eGFR is a strong predictor of cardiovascular events and is more useful for this purpose than serum creatinine [25, 26]. A urinalysis was performed using a dipstick, and the urine test results were classified as (−), (±), (1+), (2+) and (3+) [27]. In this study, the CKD was defined according to definition of the Japanese Society of Nephrology [eGFR <60 ml/min/1.73 m2, proteinuria positive (1+ or greater), or both] [21]. The breakdown of subjects’ CKD grade at baseline [21] was as follows: G1 (eGFR ≥90 ml/min/1.73 m2), n = 26 (10.3 %); and G2 (eGFR 60–89 ml/min/1.73 m2), n = 226 (89.7 %).
Blood pressure was measured in the right arm with the subject sitting in a chair, after more than 5 min of rest, and was expressed by an average of duplicate measurements. The height and body weight were measured, and the BMI was calculated as the ratio of the body weight (kg) to height squared (m2). The waist circumference was measured at the level of the umbilicus.
Assessment of lifestyle behaviors
The subjects’ drinking and smoking habits and their lifestyle behaviors regarding exercise, physical activity and diet were selected for the present study based on the standardized self-administered questionnaire of the National Health Promotion Program, which was started in Japan in the fiscal year of 2008 and aimed at preventing CVD, stroke and metabolic syndrome (see Supplemental File) [28, 29]. Previous studies have noted that the combination of these lifestyle behaviors is related to mortality and the incidence/prevalence of CVD, metabolic syndrome, type 2 diabetes, hypertension and dyslipidemia [17–19]. The subjects’ drinking and smoking habits and their lifestyle behaviors regarding the physical activity, exercise and eating habits were determined based on their responses to the following questionnaire items: (1) habitual moderate exercise; ≥30 min at one time and ≥2 times per week (yes or no); (2) physical activity equal to walking at least 1 h per day (yes or no); (3) walking speed; compared with people of the same sex and age-group (fast or slow); (4) eating speed; compared with others (fast or slow); (5) late-night dinner; ≥3 times per week (yes or no); (6) bedtime snacking; ≥3 times per week (yes or no); (7) skipping breakfast; ≥3 times per week (yes or no). The subjects drinking and smoking habits were assessed by the following questionnaire items (with “yes” or “no” responses): drinking habit (not drinking everyday); and smoking habit (recently not smoking). In this study, the total number of healthy lifestyle behaviors related to the physical activity, exercise, eating habits [i.e., (1) habitual moderate exercise, (2) physical activity, (3) fast walking speed, (4) slow eating speed, (5) no late-night dinner, (6) no bedtime snacking and (7) no skipping breakfast] was calculated for each subject. In this study, we focused on the physical activity, exercise and eating habits as lifestyle behaviors because the increases in the daily physical activity and changes in diet are important to be an initial step for the prevention of CVD [20].
Statistical analysis
The biochemical analysis, blood pressure and anthropometry measurements, and assessment of lifestyle behaviors were evaluated every year, while the classification of CKD was also assessed according to the results of eGFR values. As a result, the present study only analyzed data from participants who received their periodic health check-up during 5 years. The data were expressed as the means and the standard deviation (SD). The StatView J-5.0 software package (SAS Institute, NC, USA) was used for all of the statistical analyses. In this study, the subjects’ drinking and smoking habits and their lifestyle behaviors regarding exercise, physical activity and diet were expressed as category variables and other coronary risk factors such as biochemical, blood pressure and anthropometric indices shown as continuous variables. The participants were divided into four categories, which were defined by the quartile distributions of the number of healthy lifestyle behaviors (7–6, 5, 4 and ≤3 groups). The Kaplan–Meier survival curves were compared among the number of healthy lifestyle behaviors (7–6, 5, 4 and ≤3 groups) using the log-rank test. A Cox proportional hazards model was used for predicting the incidence of CKD using the parameters as categorical variables. Variables with a significant difference in the univariate analyses were entered into a multivariate analysis. The differences in the changes in the eGFR level among the number of healthy lifestyle behaviors (7–6, 5, 4 and ≤3 groups) were determined using a two-way repeated measures analysis of variance (ANOVA) for the follow-up and groups × time interactions. Comparisons of the data before and after 5 years were performed using the paired t test for continuous variables. Within group comparisons were determined using the one-way repeated ANOVA and the Tukey–Kramer method for continuous variables and the Chi square test for categorical variables. The linear-by-linear associations were determined using the Jonckheere–Terpstra trend test for continuous variables and the Cochran–Armitage trend test for categorical variables. A probability value <0.05 was considered to be statistically significant.
Results
Table 1 compares the subjects’ characteristics and the coronary risk factors at baseline among the four healthy lifestyle behavior groups. The body weight, BMI, waist circumference and HbA1c levels were significantly lower in the 7–6 group than in the ≤3 group (p for trend ≤0.05, respectively). There were no significant differences in the other coronary risk factors among the four groups. Table 2 compares the subjects’ lifestyle behaviors at baseline among the four healthy lifestyle behavior groups. The prevalence of each healthy lifestyle behavior was significantly higher in the 7–6, 5, 4 and ≤3 groups, in increasing order of prevalence (p for trend ≤0.05, respectively).
Table 1.
Differences in the characteristics of the subjects at baseline among the four healthy lifestyle behavior groups
| All (n = 252) | Number of healthy lifestyle behaviors | |||||
|---|---|---|---|---|---|---|
| 7–6 (n = 61) | 5 (n = 60) | 4 (n = 74) | ≤3 (n = 57) | p for trend | ||
| eGFR (ml/min/1.73m2) | 77.9 ± 10.3 | 79.2 ± 9.5 | 77.6 ± 11.7 | 78.8 ± 10.7 | 76.5 ± 7.9 | 0.271 |
| Serum creatinine (mg/dl) | 0.83 ± 0.09 | 0.81 ± 0.09 | 0.84 ± 0.10 | 0.83 ± 0.10 | 0.84 ± 0.08 | 0.122 |
| Age (years) | 51.6 ± 6.8 | 53.3 ± 7.7 | 51.2 ± 6.6 | 51.0 ± 6.3 | 51.2 ± 6.5 | 0.089 |
| Body weight (kg) | 66.7 ± 8.8 | 65.1 ± 8.9 | 66.2 ± 8.5 | 66.6 ± 8.2 | 68.9 ± 9.56 | 0.023 |
| BMI (kg/m2) | 23.1 ± 2.6 | 22.4 ± 2.7b | 23.0 ± 2.4 | 23.0 ± 2.5 | 24.0 ± 3.0a | 0.002 |
| Waist circumference (cm) | 82.6 ± 7.2 | 81.1 ± 7.0b | 82.9 ± 6.8 | 82.3 ± 7.5 | 84.9 ± 7.5a | 0.013 |
| Systolic blood pressure (mmHg) | 124.5 ± 15.4 | 126.1 ± 15.9 | 124.8 ± 16.3 | 124.1 ± 15.0 | 124.3 ± 15.4 | 0.489 |
| Diastolic blood pressure (mmHg) | 81.6 ± 10.9 | 80.8 ± 11.8 | 80.8 ± 10.8 | 82.2 ± 11.0 | 82.6 ± 10.5 | 0.270 |
| LDL-C (mg/dl) | 116.7 ± 28.4 | 116.7 ± 28.4 | 119.1 ± 29.1 | 121.4 ± 29.8 | 119.8 ± 21.9 | 0.440 |
| HDL-C (mg/dl) | 58.8 ± 13.9 | 60.2 ± 14.9 | 57.5 ± 13.1 | 60.2 ± 13.5 | 56.4 ± 13.8 | 0.328 |
| Triglyceride (mg/dl) | 109.9 ± 70.1 | 107.6 ± 101.2 | 114.5 ± 61.5 | 105.0 ± 55.3 | 112.0 ± 47.8 | 0.949 |
| Fasting glucose (mg/dl) | 98.7 ± 15.9 | 96.5 ± 15.4 | 96.5 ± 11.1 | 98.8 ± 13.9 | 99.1 ± 10.0 | 0.176 |
| HbA1c (NGSP values; %) | 5.5 ± 0.7 | 5.4 ± 0.8b | 5.5 ± 0.3 | 5.5 ± 0.5 | 5.7 ± 0.5a | 0.002 |
| Smoking habit (yes/no; n, %) | 56 (22.2)/196 (77.8) | 13 (21.3)/48 (78.7) | 12 (20.0)/48 (80.0) | 14 (18.9)/60 (81.1) | 17 (29.8)/40 (70.2) | 0.229 |
| Drinking habit (yes/no; n, %) | 197 (78.2)/55 (21.8) | 49 (80.3)/12 (19.7) | 45 (75.0)/15 (25.0) | 59 (79.7)/15 (20.3) | 44 (77.2)/13 (22.8) | 0.356 |
The data are expressed as the mean ± SD and the number of subjects
eGFR estimated glomerular filtration rate, BMI body mass index, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, HbA 1 c hemoglobin A1c, NGSP National Glycohemoglobin Standardization Program, NS not significant
a p < 0.05, compared to the 7–6 group
b p < 0.05, compared to the ≤3 group
Table 2.
Differences in the lifestyle behaviors of the subjects at baseline among the four healthy lifestyle behavior groups
| All (n = 252) | Number of healthy lifestyle behaviors | |||||
|---|---|---|---|---|---|---|
| 7–6 (n = 61) | 5 (n = 60) | 4 (n = 74) | ≤3 (n = 57) | p for trend | ||
| Habitual moderate exercise: ≥30 min/once and ≥2 times/week (yes/no; n, %) | 97 (38.5)/155 (61.5) | 51 (83.6)/10 (16.7) | 23 (38.3)/37 (61.7) | 19 (25.7)/55 (74.3) | 4 (7.0)/53 (93.0) | <0.0001 |
| Physical activity equal to walking at least 1 h/day (yes/no; n, %) | 100 (39.7)/152 (60.3) | 55 (90.2)/6 (9.8) | 26 (43.3)/34 (56.7) | 17 (23.0)/57 (77.0) | 2 (3.5)/55 (96.5) | <0.0001 |
| Walking speed: compared with people of the same sex and age-group (fast/slow; n, %) | 153 (60.7)/99 (39.3) | 53 (86.9)/8 (13.1) | 46 (76.7)/14 (23.3) | 36 (48.6)/38 (51.4) | 18 (31.6)/39 (68.4) | <0.0001 |
| Eating speed: compared with others (fast/slow; n, %) | 92 (36.5)/160 (63.5) | 8 (13.1)/53 (86.9) | 15 (25.0)/45 (75.0) | 34 (45.9)/40 (54.1) | 35 (61.4)/22 (38.6) | <0.0001 |
| Late-night dinners: ≥3 times/week (yes/no; n, %) | 81 (32.1)/171 (67.9) | 9 (14.8)/52 (85.2) | 13 (21.7)/47 (78.3) | 22 (29.7)/52 (70.3) | 37 (64.9)/20 (35.1) | <0.0001 |
| Bedtime snacking: ≥3 times/week (yes/no; n, %) | 30 (11.9)/222 (88.1) | 4 (6.6)/57 (93.4) | 2 (3.3)/58 (96.7) | 8 (10.8)/66 (89.2) | 16 (28.1)/41 (71.9) | 0.0003 |
| Skipping breakfast: ≥3 times/week (yes/no; n, %) | 26 (10.3)/226 (89.7) | 1 (1.6)/60 (98.4) | 5 (8.3)/55 (91.7) | 8 (10.8)/66 (89.2) | 12 (21.1)/45 (78.9) | 0.0013 |
The data are expressed as the number of subjects who indicated an unhealthy lifestyle behavior for each status
NS not significant
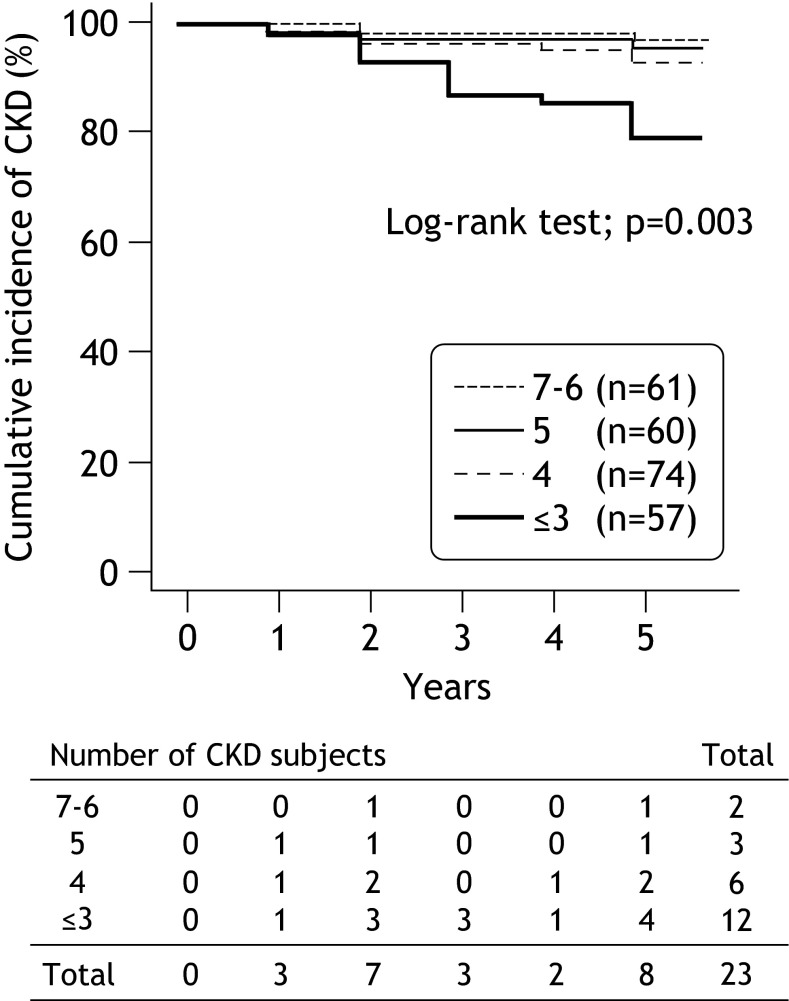
After 5 years, the incidence of CKD (eGFR <60 ml/min/1.73 m2 and/or proteinuria) was observed in 23 subjects (9.1 %). The breakdown of the subjects by the CKD grade [21] after 5 years was as follows: G1 (eGFR ≥90 ml/min/1.73 m2), n = 11 (4.4 %); G2 (eGFR 60–89 ml/min/1.73 m2), n = 218 (86.6 %); and G3a (eGFR 45–59 ml/min/1.73 m2), n = 23 (9.1 %; including two with proteinuria). The Kaplan–Meier survival curve showed that the cumulative incidence of CKD significantly decreased with an increase in number of healthy lifestyle behaviors (3.3, 5.0, 8.1 and 21.4 % for 7–6, 5, 4, and ≤3 groups, respectively, log-rank test: p = 0.003, Fig. 2).
Fig. 2.
Comparison of the Kaplan–Meier survival curves using the log-rank test. The cumulative incidence of CKD significantly decreased with an increase in the number of healthy lifestyle behaviors (3.3, 5.0, 8.1 and 21.4 % for 7–6, 5, 4, and ≤3 groups, respectively)
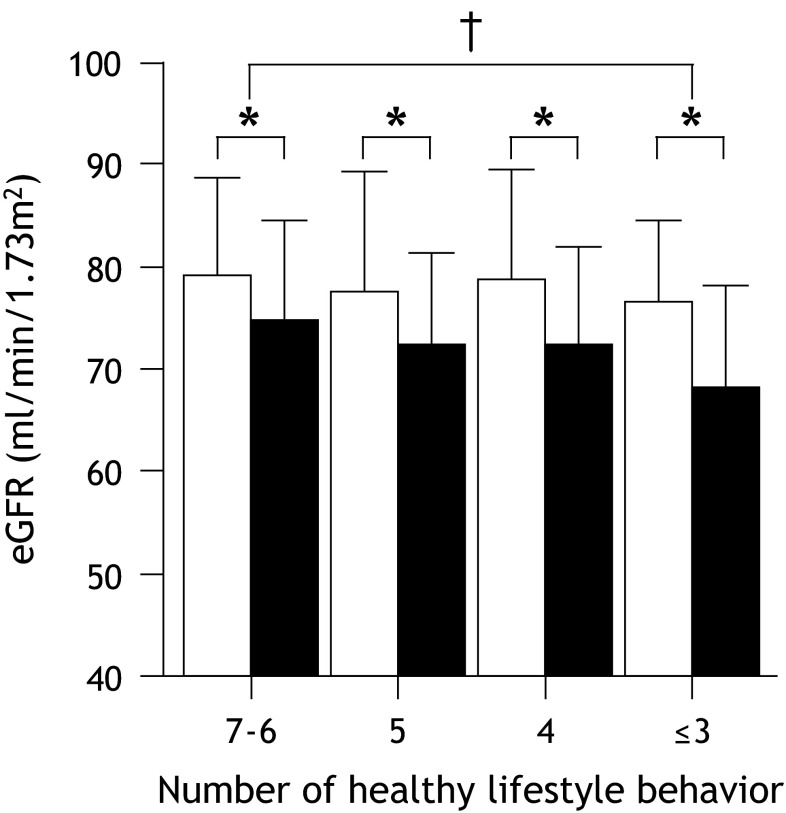
Figure 3 shows the comparisons of the eGFR level at baseline and after the 5-year follow-up period among the four groups. After 5 years, the eGFR level decreased in all of the groups (79.2 ± 9.5–74.7 ± 9.7 ml/min/1.73 m2 in the 7–6 group, 77.6 ± 11.7–72.3 ± 9.1 ml/min/1.73 m2 in the 5 group, 78.8 ± 10.7–72.4 ± 9.5 ml/min/1.73 m2 in the 4 group and 76.5 ± 7.9–68.3 ± 9.8 ml/min/1.73 m2 in the ≤3 group, p < 0.05, respectively). However, a significant interaction effect for group × time was observed in the eGFR level between the 7–6 and ≤3 groups (p = 0.047).
Fig. 3.
Comparisons of the eGFR at baseline and after the 5-year follow-up period among the four lifestyle behavior groups. The data are expressed as the mean ± SD. Open square baseline, filled square after 5 years. *p < 0.0001, compared to the values at baseline and after the 5 years in each group. † p < 0.05, a significant interaction effect for group × time
Figure 4 shows the influence of healthy lifestyle behaviors on the incidence of CKD. In this analysis, each healthy lifestyle behavior was a dependent variable and the incidence of CKD was an independent variable. In a univariate analysis, habitual moderate exercise [hazard ratio (HR) 0.10, 95 % confidence of interval (CI) 0.03–0.41, p = 0.001], slow eating speed (HR 0.33, 95 % CI 0.12–0.96, p = 0.041) and no bedtime snacking (HR 0.21, 95 % CI 0.08–0.53, p = 0.001) were significantly correlated with the incidence of CKD. In a multivariate analysis, age, BMI, smoking habit, drinking habit, the eGFR and HbA1c levels, and systolic and diastolic blood pressure at baseline were entered as adjusted factors, because age, BMI, smoking habit, drinking habit and HbA1c levels, and systolic and diastolic blood pressure potentially influence on both healthy lifestyle behaviors and renal function (Table 1). After adjusting for age, BMI, smoking habit, drinking habit, the eGFR and HbA1c levels, and systolic and diastolic blood pressure at baseline, habitual moderate exercise (HR 0.20, 95 % CI 0.06–0.69, p = 0.011) and no bedtime snacking (HR 0.19, 95 % CI 0.08–0.48, p = 0.004) were found to be significantly associated with the incidence of CKD.
Fig. 4.

Influence of the healthy lifestyle behaviors on the incidence of CKD. The data are expressed as the hazard ratio [95 % confidence interval (CI)]. Open circle univariate model. Filled circle multivariate model: adjusting for age, BMI, smoking habit, drinking habit, the eGFR and HbA1c levels, and systolic and diastolic blood pressure at baseline. In this analysis, each healthy lifestyle behavior was a dependent variable and the incidence of CKD was an independent variable
Discussion
The major findings of our study were that the cumulative incidence of CKD significantly decreased with an increase in the number of healthy lifestyle behaviors. Furthermore, although the eGFR level decreased in all groups, a significant interaction effect for group × time was observed in the eGFR level between the 7–6 and ≤3 groups.
It is well known that the adherence to healthy lifestyle behaviors is associated with the reduced incidence of non-communicable diseases, including metabolic syndrome, type 2 diabetes, hypertension and dyslipidemia [15–19]. We speculate that the relationship between healthy lifestyle behaviors and CKD has similar mechanisms, and the accumulation of healthy lifestyle behaviors plays a pivotal role in the prevention of these diseases. However, at present, the association between the accumulation of healthy lifestyle behaviors and the prevention of CKD remains unknown. In a 1-year follow-up study, Wakasugi et al. [30] investigated the effect of a combination of health lifestyle factors on the incidence of proteinuria and showed that the incidence of proteinuria significantly decreased with an increase in the healthy lifestyle scores. Recently, our cross-sectional study [31] also observed that the accumulation of unhealthy lifestyle behaviors, especially those related to lack of habitual moderate exercise, late-night dinner and bedtime snacking may be independent risk factors for the development of CKD. In the present study, the cumulative incidence of CKD significantly decreased with an increasing number of healthy lifestyle behaviors, which is consistent with previous studies. Therefore, the results of the present study support the possibility that the accumulation of healthy lifestyle behaviors is important for preventing the development of CKD. According to these findings, not only individual lifestyle behaviors, but also the accumulation of healthy lifestyle behaviors, may be critical for preventing the development of CKD, ESRD and CVD.
According to the multivariate analysis, the healthy lifestyle behaviors related to habitual moderate exercise and no bedtime snacking were significantly associated with the incidence of CKD. However, there were no significant relationships between other lifestyle behaviors and the incidence of CKD. Recently, several studies have demonstrated that individual lifestyle behaviors relating to exercise, physical activity and diet correlate with the renal function. Robinson-Cohen et al. [32] observed that each 60-min increment in weekly physical activity was associated with a 0.5 % slower decline of the eGFR per year. Howden et al. [33] reported that lifestyle modifications focusing on exercise training in patients with CKD produced improvements in the cardiorespiratory fitness, body composition and cardiac diastolic function. In addition, a previous meta-analysis showed that the lack of habitual exercise and decreased physical activity influence the incidence of CKD through obesity, hypertension and type 2 diabetes [10]. Moreover, it has been reported that an unhealthy diet including high levels of dietary animal fat, sodium and soft drink consumption leads to the development of renal dysfunction [34–36]. Mekary et al. [37] showed additional snack intake beyond the three main meals (breakfast, lunch and dinner) were associated with an increased risk for type 2 diabetes. According to these findings, habitual moderate exercise and no bedtime snacking may be important for preventing the incidence of CKD.
Previous studies have noted that habitual moderate exercise and no bedtime snacking were related to mortality and the incidence/prevalence of CVD, metabolic syndrome, type 2 diabetes, hypertension and dyslipidemia [36, 38]. The current findings suggest that habitual moderate exercise and bedtime snacking may be predictive factors for the incidence of CKD and may indirectly contribute to the prevention of the development of future ESRD, CVD or the introduction of dialysis in middle-aged and older males. Therefore, we consider that the assessment of healthy lifestyle behaviors, with a particular focus on regular habitual exercise and healthy eating habits such as avoiding bedtime snacking, is necessary when performing lifestyle counseling to prevent the early stages of CKD.
Study limitations and clinical implications
There are several limitations associated with this study. First, the limited study population resulted in a small number of male subjects, who were predominantly middle-aged and older, not taking any medications and did not have any health complications. Thus, there is potential selection bias in this study, because our limited study population may include more CKD subjects with slowly declining renal function than CKD subjects with rapid deterioration. Therefore, it remains unclear whether our findings are generalizable to females, patients with ESRD, or subjects with taking medications and other complications. Second, the follow-up period was insufficient to clarify the association between healthy lifestyle behaviors and the incidence of CKD. Third, because the renal function is influenced by the dietary electrolytes, the present study could not confirm the dietary total energy, sodium and potassium intake of the subjects. Therefore, we could not clarify the influence of dietary electrolytes using the subjects’ dietary records. Fourth, we assessed the eGFR as calculated using the Japanese GFR inference formula [23] and proteinuria as an index of the renal function. To more fully evaluate the influence of healthy lifestyle behaviors on the renal function, other indices of renal function such as urinary protein excretion, or microalbuminuria, or cystatin C should be simultaneously assessed. We could not measure any additional markers of renal function because this study was performed within the constraints of the health check-up. Finally, the standardized self-administered questionnaire aimed at preventing the development of CVD, stroke and metabolic syndrome. Therefore, it is unclear whether all these questionnaire items are related to general health.
However, this study is the first report to evaluate the association between the accumulation of healthy lifestyle behaviors and the incidence of CKD. We consider that our results are helpful to clarify that the accumulation of healthy lifestyle behaviors can prevent the incidence of CKD, although this questionnaire aimed at preventing CVD, stroke and metabolic syndrome. Moreover, the serum creatinine level can be easily measured as part of a routine clinical evaluation, the eGFR is a strong predictor of cardiovascular events and is more useful for this purpose than the serum creatinine level [25, 26]. In several recent studies, it has been clearly demonstrated that the incidence of CKD is also closely correlated with unhealthy lifestyles behaviors, such as smoking, heavy alcohol intake, obesity, physical inactivity and unhealthy diet [7–12]. Therefore, the results of the present study demonstrate a link between the accumulation of healthy lifestyle behaviors and the incidence of CKD and may confirm the hypothesis that the accumulation of healthy lifestyle behaviors may thus help to prevent the development of CVD and ESRD. According to our results, we believe that it is necessary to provide lifestyle counseling, particularly with a focus on regular habitual exercise and healthy eating habits, such as avoiding bedtime snacking, to reduce the incidence of CKD. Additionally, type 2 error may be present in this study because the study population was limited, resulting in a small number of male subjects. Further investigation in a large number of subjects, including subjects taking medications and other complications is necessary to more precisely clarify the mechanisms, clinical implications and associations between the changes in lifestyle behaviors and the incidence of CKD following long-term intervention.
Conclusions
This study was retrospectively designed to evaluate the influence of healthy lifestyle behaviors on the incidence of CKD in middle-aged and older males. We found that the cumulative incidence of CKD significantly decreased with an increase in the number of healthy lifestyle behaviors. Furthermore, after adjusting for age, BMI, smoking habit, drinking habit, the eGFR and HbA1c levels, and systolic and diastolic blood pressure at baseline, habitual moderate exercise and no bedtime snacking were found to be a significantly associated with the incidence of CKD. These results suggest that the accumulation of healthy lifestyle behaviors, especially those related to habitual moderate exercise and no bedtime snacking, is considered to be important to reduce the risk of CKD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Drs. Masaki Munekiyo, Kazunori Mine, Tatsuhiko Kawarabayashi, Eiichi Yoshimura, Noriko Takeda, Tomoe Horita and the members of the Laboratory of Exercise Physiology and Health Care Center of Fukuoka University for their assistance with the data evaluation. We are grateful to the participants of this study. This work was performed with the support of the Fukuoka University Institute for Physical Activity via a Technology Scientific Research Budget Basic Research Grant (No. A19200049, Strategic Research Infrastructure) from the Japanese Government’s Ministry of Education, Culture, Sports, Science and Technology.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest in association with this study.
References
- 1.The Japanese Society for Dialysis Therapy. Current status of dialysis therapy in Japan. http://docs.jsdt.or.jp/overview/pdf2014/p003.pdf. Accessed 2 Dec 2015 (in Japanese).
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 4.Imai E, Horio M, Iseki K, Yamagata K, Watanabe T, Hara S, et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the NDRD equation modified by a Japanese coefficient. Clin Exp Nephrol. 2007;11:156–163. doi: 10.1007/s10157-007-0463-x. [DOI] [PubMed] [Google Scholar]
- 5.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 6.Ninomiya T, Kiyohara Y, Kubo M, Yonemoto K, Tnizaki Y, Doi Y, et al. Metabolic syndrome and CKD in a general Japanese population: the Hisayama study. Am J Kidney Dis. 2006;48:383–91. [DOI] [PubMed]
- 7.Nagasawa Y, Yamamoto R, Rakugi H, Isaka Y. Cigarette smoking and chronic kidney diseases. Hypertens Res. 2012;35:261–265. doi: 10.1038/hr.2011.205. [DOI] [PubMed] [Google Scholar]
- 8.White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transplant. 2009;24:2464–2472. doi: 10.1093/ndt/gfp114. [DOI] [PubMed] [Google Scholar]
- 9.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 10.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, et al. Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study. Am J Kidney Dis. 2014;64:204–213. doi: 10.1053/j.ajkd.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu W, Luying S, Haiyan W, Xiaomei L. Importance and benefits of dietary sodium restriction in the management of chronic kidney disease patients: experience from a single Chinese center. Int Urol Nephrol. 2012;44:549–556. doi: 10.1007/s11255-011-9986-x. [DOI] [PubMed] [Google Scholar]
- 13.O’Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41:447–454. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 14.Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int. 2004;65:719–724. doi: 10.1111/j.1523-1755.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi E, Iso H, Tanabe N, Wada Y, Yatsuya H, Kikuchi S, Japan Collaborative Cohort Study Group et al. Healthy lifestyle behaviours and cardiovascular mortality among Japanese men and women: the Japan collaborative cohort study. Eur Heart J. 2012;33:467–477. doi: 10.1093/eurheartj/ehr429. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi E, Iso H, Wada Y, Kikuchi S, Watanabe Y, Tamakoshi A, Japan Collaborative Cohort Study Group Parental history and lifestyle behaviors in relation to mortality from stroke among Japanese men and women: the Japan Collaborative Cohort Study. J Epidemiol. 2012;22:331–339. doi: 10.2188/jea.JE20110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tajima M, Lee JS, Watanabe E, Park JS, Tsuchiya R, Fukahori A, et al. Association between changes in 12 lifestyle behaviors and the development of metabolic syndrome during 1 year among workers in the Tokyo metropolitan area. Circ J. 2014;78:1152–1159. doi: 10.1253/circj.CJ-13-1082. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Yatsuya H, Iso H, Tamakoshi K, Toyoshima H. Incidence of metabolic syndrome according to combinations of lifestyle factors among middle-aged Japanese male workers. Prev Med. 2010;51:118–122. doi: 10.1016/j.ypmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Nagahama S, Kurotani K, Pham NM, Nanri A, Kuwahara K, Dan M, et al. Self-reported eating rate and metabolic syndrome in Japanese people: cross-sectional study. BMJ Open. 2014;4:e005241. doi: 10.1136/bmjopen-2014-005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Society of Nephrology Evidence-based clinical practice guideline for CKD 2013. Clin Exp Nephrol. 2014;18:346–423. doi: 10.1007/s10157-014-0949-2. [DOI] [Google Scholar]
- 22.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Committee on the Standardization of Diabetes Mellitus Related Laboratory Testing of Japan Diabetes Society, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. [DOI] [PMC free article] [PubMed]
- 23.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, CKD Prognosis Consortium et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo K, Inoue T, Node K. Estimated glomerular filtration rate as a predictor of secondary outcomes in Japanese patients with coronary artery disease. J Cardiol. 2009;53:232–239. doi: 10.1016/j.jjcc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Harrison NA, Rainford DJ, White GA, Cullen SA, Strike PW. Proteinuria—what value is the dipstick? Br J Urol. 1989;63:202–208. doi: 10.1111/j.1464-410X.1989.tb05166.x. [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Health, Labour and Welfare. Standardized health check-up and intervention program, 2007. http://www.mhlw.go.jp/bunya/kenkou/seikatsu/pdf/02.pdf. Accessed 23 Dec 2014 (in Japanese).
- 29.Kohro T, Furui Y, Mitsutake N, Fujii R, Morita H, Oku S, et al. The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int Heart J. 2008;49:193–203. doi: 10.1536/ihj.49.193. [DOI] [PubMed] [Google Scholar]
- 30.Wakasugi M, Kazama JJ, Yamamoto S, Kawamura K, Narita I. A combination of healthy lifestyle factors is associated with a decreased incidence of chronic kidney disease: a population-based cohort study. Hypertens Res. 2013;36:328–333. doi: 10.1038/hr.2012.186. [DOI] [PubMed] [Google Scholar]
- 31.Michishita R, Matsuda T, Kawakami S, Kiyonaga A, Tanaka H, Morito N, et al. The association between unhealthy lifestyle behaviors and the prevalence of chronic kidney disease (CKD) in middle-aged and older males. J Epidemiol (accepted). [DOI] [PMC free article] [PubMed]
- 32.Robinson-Cohen C, Littman AJ, Duncan GE, Weiss NS, Sachs MC, Ruzinski J, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25:399–406. doi: 10.1681/ASN.2013040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol. 2013;8:1494–1501. doi: 10.2215/CJN.10141012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Hu FB, Curhan GC. Associations of diet with albuminuria and kidney function decline. Clin J Am Soc Nephrol. 2010;5:836–843. doi: 10.2215/CJN.08001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98:2621–2628. doi: 10.1161/01.CIR.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160–166. doi: 10.2215/CJN.03260410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95:1182–1189. doi: 10.3945/ajcn.111.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitzmann MF, Park Y, Blair A, Ballard-Barbash R, Mouw T, Hollenbeck AR, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med. 2007;167:2453–2460. doi: 10.1001/archinte.167.22.2453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.