Abstract
AIM: To investigate patient-reported outcomes from, and adherence to, a low FODMAP diet among patients suffering from irritable bowel syndrome and inflammatory bowel disease.
METHODS: Consecutive patients with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD) and co-existing IBS fulfilling the ROME III criteria, who previously attended an outpatient clinic for low FODMAP diet (LFD) dietary management and assessment by a gastroenterologist, were invited to participate in a retrospective questionnaire analysis. The questionnaires were sent and returned by regular mail and gathered information on recall of dietary treatment, efficacy, symptoms, adherence, satisfaction, change in disease course and stool type, and quality of life. Before study enrolment all patients had to sign an informed written consent.
RESULTS: One hundred and eighty patients were included, 131 (73%) IBS and 49 (27%) IBD patients. Median age was 43 years (range: 18-85) and 147 (82%) were females. Median follow-up time was 16 mo (range: 2-80). Eighty-six percent reported either partial (54%) or full (32%) efficacy with greatest improvement of bloating (82%) and abdominal pain (71%). The proportion of patients with full efficacy tended to be greater in the IBD group than in the IBS group (42% vs 29%, P = 0.08). There was a significant reduction in patients with a chronic continuous disease course in both the IBS group (25%, P < 0.001) and IBD group (23%, P = 0.002) along with a significant increase in patients with a mild indolent disease course of 37% (P < 0.001) and 23% (P = 0.002), respectively. The proportion of patients having normal stools increased with 41% in the IBS group (P < 0.001) and 66% in the IBD group (P < 0.001). One-third of patients adhered to the diet and high adherence was associated with longer duration of dietary course (P < 0.001). Satisfaction with dietary management was seen in 83 (70%) IBS patients and 24 (55%) IBD patients. Eighty-four percent of patients lived on a modified LFD, where some foods rich in FODMAPs were reintroduced, and 16% followed the LFD by the book without deviations. Wheat, dairy products, and onions were the foods most often not reintroduced by patients.
CONCLUSION: These data suggest that a diet low in FODMAPs is an efficacious treatment solution in the management of functional bowel symptoms for IBS and IBD patients.
Keywords: Low FODMAP, Irritable bowel syndrome, Inflammatory bowel disease, Adherence, Disease course
Core tip: This is a retrospective study based on patient-reported questionnaires to evaluate the low FODMAP diet (LFD) dietary course of patients with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD). Effect was reported by 86% of patients with greatest relief of abdominal pain and bloating. Long-term IBS disease course and stool type improved significantly after dietary intervention. One-third of patients were adherent and the majority was satisfied with the treatment. These are the first data on changes of long-term IBS disease course following LFD treatment and the longest FU to date of IBS and IBD patients treated with the LFD with a median FU of 16 mo.
INTRODUCTION
Irritable bowel syndrome (IBS) is a highly prevalent functional gastrointestinal disorder characterised by abdominal pain or discomfort in association with altered bowel habits and no organic disease. IBS symptoms are prevalent in about 10%-20% of the general population, with a female gender predilection and a high proportion of undiagnosed sufferers, making IBS a major health issue[1-3]. Furthermore, IBS-like symptoms are common in patients with inflammatory bowel disease (IBD) and are present in between 30%-40% of patients in clinical remission[4-6].
Treatment of IBS is a challenging task for primary care physicians and gastroenterologists due to the heterogeneity of the disorder, a lack of reliable outcome measures, and high placebo response rates. In the last decades, a diet excluding foods high in short-chained carbohydrates, termed FODMAPs (fermentable oligo-, di-, and monosaccharides and polyols), has proven effective in the treatment of functional gastrointestinal symptoms[7]. FODMAPs are poorly absorbed in the small intestine and are passed on to the colon, where they exert an osmotic effect, drawing fluid into the lumen and, furthermore, causing an increase in gas production (mainly hydrogen and methane) due to the excess delivery of fermentable substrates to the colonic microflora[8]. These mechanisms can lead to abdominal pain, bloating, flatulence, and diarrhoea in susceptible subjects[9,10]. There is strong evidence supporting the low FODMAP diet (LFD) as an effective therapeutic tool in the management of IBS with an overall response rate of 75[7,9,11-13]. The diet also seems to reduce functional bowel symptoms in patients with inflammatory bowel disease[14,15] and patients without a colon[8].
Adherence to the diet is key to its success and according to previous studies, adherence can be expected in up to 75% of patients[12,14,16]. However, many struggle with implementing the LFD in daily life due to its complexity. Currently, studies of long-term efficacy of the LFD are lacking, and no one has yet investigated the dietary impact on IBS disease course.
The present retrospective study aimed to examine patient-reported long-term effects of the LFD, dietary adherence, and dietary impact on disease course in patients with IBS and patients with IBD and co-existing IBS.
MATERIALS AND METHODS
Study design
A retrospective, cross-sectional study was conducted to investigate long-term adherence and effect on disease course in IBS and IBD patients treated with the LFD.
Study population
Consecutive patients with IBS or IBD and co-existing IBS fulfilling the Rome III criteria[17] for IBS and having received LFD education followed by a dietary course of varying duration in the period 2009-2013, were invited to participate in the study. All patients had initially been treated by their general practitioner and subsequently been referred to Herlev University Hospital (HUH) for dietary management of IBS with clinical dieticians.
All study participants, or their legal guardian, provided informed written consent prior to study enrolment. Participants gave written informed consent for data sharing.
Prior to dietary consultation, gastroenterologists had assessed all patients and the majority of patients presented normal colonoscopy results, and were tested for lactose intolerance and celiac disease, among other relevant tests.
Patients were excluded from the study if they had significant gastrointestinal co-morbidities such as abdominal cancer or ileo-/colostomy. IBD patients were not tested for level of disease activity at follow-up.
eHealth: A web-program for IBS and IBD patients
Some of the recruited patients (103, 57%) had earlier been engaged in other LFD studies at HUH involving eHealth web-program monitoring and a dietary course of six weeks with follow-up evaluation[15,17,18]. The program has been described in previous papers[19]. The majority of IBD patients (40, 82%) included here participated in these eHealth studies, while patients with moderate to severe disease activity (assessed by HBI or SCCAI) were excluded.
Data collection
Patients eligible for the study received a letter containing an invitation, an informed consent form, and a questionnaire regarding the dietary treatment, adherence to diet, disease severity and course, stool pattern, and quality of life. If the patients did not reply to the invitation, reminders were sent by regular mail. Before accessing electronic patient files for extraction of additional demographic data, an informed written consent for data sharing had to be signed by the patients in accordance with the Danish health authority regulations.
Dietary advice
Four experts in clinical nutrition and the LFD performed the dietary consultations. One dietician was FODMAP-certified at King’s College, London, United Kingdom[20]. The initial consultation lasted 60-90 min and included IBS education, dietary history, LFD education, and individualisation of LFD advice in order to facilitate implementation of the diet. Patients were to stay on the diet for 6-8 wk and then review the treatment response with support from the dietary expert. If the response was considered satisfactory, patients continued the restriction of FODMAPs, but with reintroduction of small amounts of foods high in FODMAPs in order to determine individual tolerance level and ensure variety in diet. The patients were offered follow-up consultations either in clinic or by telephone and were also able to email the experts.
Questionnaires
At follow-up, all patients were asked to complete a questionnaire analysis including four self-developed questionnaires and three or four internationally validated questionnaires.
The first questionnaire, developed in cooperation with clinical dieticians, consisted of 23 questions with limited answering options or visual analogue scales (VAS) addressing efficacy of diet, dietary management, and compliance. The FODMAP Adherence Report Scale (FARS) was constructed to evaluate dietary adherence and was inspired by the validated Medication Adherence Report Scale by Byrne et al[21]. The scale consists of five questions (see Table 1), each question offering five possible answers (always, often, sometimes, rare, and never) scoring from one to five points with a maximum score of 25 points. A total score of at least 20 points (≥ 80%) was considered as adherence to the diet. Furthermore, a questionnaire previously applied in a study by Pedersen et al[17] at HUH was included in order to assess satisfaction with the dietary treatment. Its six questions were accompanied by VAS scales, with a scoring range of 0-100 points (i.e., 1 cm = 10 points), and covered the following items: dietary consultations, distributed written material, flavour of diet, compliance/adherence to diet, and availability of appropriate foods in supermarkets. The maximum score was 600 points and a total score of a minimum of 360 points (≥ 60%) was considered as satisfaction with the dietary treatment.
Table 1.
Dietary adherence at follow-up, estimated by the FODMAP adherence report scale n (%)
| FARS questions | All patients | IBD | IBS |
| “I change the content of a LFD meal despite the recommended content” | 57 (35) | 12 (28) | 45 (37) |
| “I follow a modified LFD compared to the recommended LFD” | 61 (37) | 15 (34) | 46 (38) |
| “I replace a LFD meal with a regular meal containing FODMAPs” | 90 (55) | 27 (63) | 63 (53) |
| “I forget to follow the LFD” | 111 (68) | 33 (75) | 78 (65) |
| “I stop taking the LFD for a period of time” | 107 (65) | 34 (77) | 73 (61) |
Each of the FARS questions had five possible responses, scoring 1-5 points. A score of ≥ 4 points was regarded as adherence to the LFD. FARS: FODMAP adherence report scale; IBD: Inflammatory bowel disease; IBS: Irritable bowel syndrome; LFD: Low FODMAP diet; FODMAPs: Fermentable oligo-, di- and monosaccharides and polyols.
The last questionnaire developed addressed changes in IBS disease course prior to, and after, dietary intervention and consisted of four figures depicting different types of disease courses, the Copenhagen IBS disease courses (see Figure 1). The figures were constructed based on several years of clinical experience in IBS management and studies of pattern recognition of IBD disease courses[22,23]. The mild indolent disease course was considered the preferred type, as disease activity decreased over time. The patients had to choose one figure representing their disease course before and after dietary management.
Figure 1.
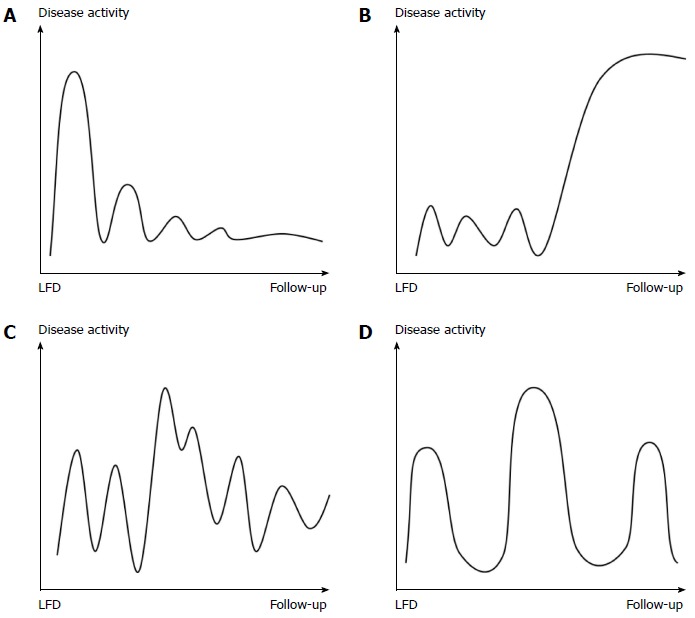
Copenhagen irritable bowel syndrome disease courses. The four figures each depict a different type of IBS disease course with varying disease activity over time. The time span is from introduction of the LFD until follow-up. The same figures were used from time of diagnosis to introduction of the LFD (not shown). A: Mild IBS with indolent course: The disease activity fades over time; B: Mild IBS with aggressive course: The disease activity increases over time; C: Chronic IBS with continuous course: There is constant disease activity without remission periods; D: Chronic IBS with intermittent course: The disease activity appears in relapses with remission periods in between. LFD: Low FODMAP diet; IBS: Irritable bowel syndrome.
The Bristol Stool Chart (BSC)[24,25] illustrates seven different stool types representing constipation (type 1-2), normal stools (type 3-4), and diarrhoea (type 5-7), and was used retrospectively to assess stool type prior to and after dietary treatment. The IBS Severity Scoring System (IBS-SSS)[26] was applied to measure IBS disease severity at follow-up and consists of five questions combined with VAS-scales, with the maximum score being 500 points. Remission/mild disease is classified as a score less than 175 points, moderate disease as a score between 175 and 300 points, and above 300 points the disease is severe. Quality of life was evaluated at follow-up using the IBS Quality of Life questionnaire (IBS-QoL)[27] that contains 34 questions, each with five possible answers and a scoring range of 34-170 points. Due to the absence of an official cut-off for good quality of life, we arbitrarily set the bar at ≤ 102 points (50%).
Finally, the Short IBD Questionnaire (SIBDQ)[28] is composed of 10 questions and was used to measure quality of life at follow-up for IBD patients only. A total score of 50 points or more was considered to indicate a good quality of life[29].
Statistical analysis
Statistical analysis was performed using the SAS v. 9.3 (NC, United States) and SPSS v. 20 (IL, United States) statistical software packages. Standard descriptive statistics were carried out including frequency distributions for categorical data and calculation of medians and ranges for continuous variables. Fisher’s exact and χ2 tests were used to investigate whether or not the differences in descriptive data between groups were significant. The Wilcoxon ranked test was applied to determine if there had been significant changes in disease course and stool type from baseline to follow-up. Fisher’s exact test and multiple logistic regression were performed to examine the relationship between responses and explanatory variables. All reported P-values are two-sided and tests were performed with a 5% level of significance. The statistical methods of this study were reviewed by Henrik Wachmann, Larix A/S.
RESULTS
Demographic data
Four hundred and three (294 IBS, 109 IBD) patients eligible for the study were identified. Fifteen were excluded due to co-morbidity (eight), migration (four), or uncertain IBS diagnosis (three). Forty patients rejected the invitation. Of the remaining 348 patients, a total of 180 patients (52%), 131 IBS and 49 IBD, answered one or more questionnaires and were included in the study. Demographic characteristics are presented in Table 2. Twenty (11%) patients did not consent to extraction of data from their electronic patient files; therefore, only data from questionnaires were available. There were significant differences in demographic data between the two groups regarding IBS subtypes and IBS severity at follow-up. The IBS-D subtype and IBS-C subtype were more frequent in the IBD (P < 0.01) and IBS (P < 0.01) group, respectively, and the proportion of IBD patients with mild IBS at follow-up was significantly greater when compared to the IBS patients (P = 0.01). The median duration of follow-up from the initial dietary consultation to the completion of the questionnaire analysis was 16 (range: 2-80) months overall, with 15 (range: 2-80) months for the IBS group, and 17 (range: 5-32) mo for the IBD group.
Table 2.
Demographic data at follow-up n (%)
| IBS | IBD | |
| Patients | 131 (73) | 49 (27) |
| Participation in eHealth studies | 63 (48) | 40 (82) |
| IBD type | ||
| UC | 32 (65) | |
| CD | 12 (25) | |
| IBDU | 5 (10) | |
| Female | 107 (82) | 40 (82) |
| Age, median (range) | 43 (18-85) | 44 (19-70) |
| Height, median (range) | 168 (133-189) | 171 (160-189) |
| Weight, median (range) | 65 (43-115) | 75 (49-146) |
| BMI, median (range) | 23 (16-45) | 25 (18-53) |
| Smokers | 15 (14) | 5 (12) |
| IBS subtypes | ||
| IBS-D | 47 (40) | 28 (67) |
| IBS-C | 44 (37) | 6 (14) |
| IBS-M | 17 (14) | 3 (7) |
| IBS-U | 10 (9) | 5 (12) |
| IBS disease severity | ||
| Mild | 41 (32) | 23 (53) |
| Moderate | 56 (43) | 14 (33) |
| Severe | 32 (25) | 6 (14) |
| Lactose intolerance1 | 18 (25) | 1 (6) |
| Gluten intolerance2 | 2 (2) | 1 (8) |
| Food allergy3 | 5 (24) | 5 (42) |
| Dietary consultations | ||
| None | 4 (4) | 0 (0) |
| 1 | 30 (28) | 14 (33) |
| 2 | 33 (30) | 22 (53) |
| 3 or more | 41 (38) | 6 (14) |
| Follow-up time, median (range) | 15 (2-80) | 17 (5-32) |
Diagnosed by lactose intolerance test or genetic test of lactase;
Diagnosed by serologic test for immunoglobulin A tissue transaminase;
Diagnosed by serologic tests for specific immunoglobulins. IBS: Irritable bowel syndrome; IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn’s disease; IBDU: Undetermined inflammatory bowel disease; BMI: Body mass index; IBS-D: IBS with diarrhoea; IBS-C: IBS with constipation; IBS-M: Mixed IBS; IBS-U: Unsubtyped IBS.
Efficacy and symptoms
One hundred and fifty patients (86%) reported either partial (94, 54%) or full (56, 32%) effectiveness of dietary treatment (Figure 2). The proportion of patients experiencing full effectiveness was greater in the IBD group than in the IBS group (42% vs 29%, P = 0.08). The diet showed greatest effect on bloating (82%) and abdominal pain (71%) (Figure 3). Furthermore, 46 (37%) IBS patients and 21 (24%) IBD patients became asymptomatic while following the diet.
Figure 2.
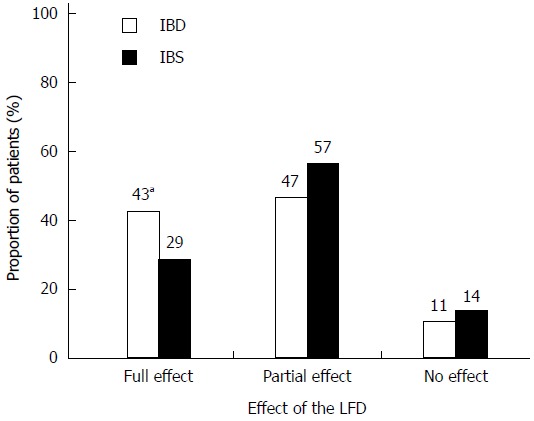
Patient-reported effectiveness of the low FODMAP diet in inflammatory bowel disease and irritable bowel syndrome patients at follow-up. Effectiveness was categorised as full, partial, or no effect. There were more IBD patients with full effect than IBS patients (aP = 0.08). IBD: n = 47; IBS: n = 126. LFD: Low FODMAP diet; IBD: Inflammatory bowel disease; IBS: Irritable bowel syndrome.
Figure 3.
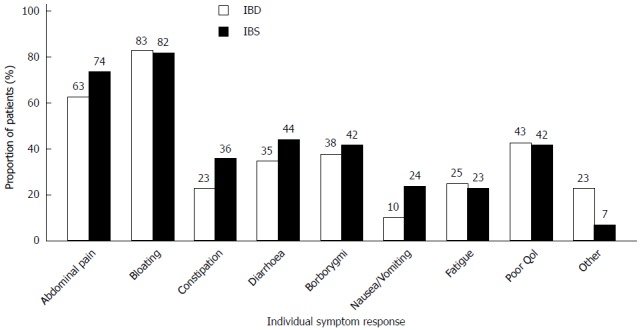
Patient-reported symptom relief for individual symptoms. Patients were able to select as many symptoms as they felt appropriate according to subjective symptom improvement following LFD treatment. The majority experienced relief of abdominal pain and bloating. IBD: n = 40; IBS: n =113. QoL: Quality of life; IBD: Inflammatory bowel disease; IBS: Irritable bowel syndrome.
Disease course and stool type
Figure 4 illustrates changes in IBS disease course related to the LFD. After dietary treatment, the number of patients with a chronic continuous course was significantly reduced in both patient groups (IBS: -25%, P < 0.001; IBD: -23%, P = 0.002), while the mild indolent course became the predominant type (IBS: +37%, P < 0.001; IBD: +23%, P = 0.002). The mild indolent disease course following LFD intervention was associated with good quality of life and normal stool pattern (P < 0.0001). Furthermore, mild indolent disease course prior to LFD was a strong predictor of a disease course persisting beyond the LFD (84%, P < 0.001). Patients starting on one of the three other less favourable disease courses had a probability of about 40% (range: 39%-46%) of transitioning to the mild indolent course after dietary treatment. The baseline variables showed no influence on the probability of changing from one of the three less favourable disease course types to the mild indolent course.
Figure 4.
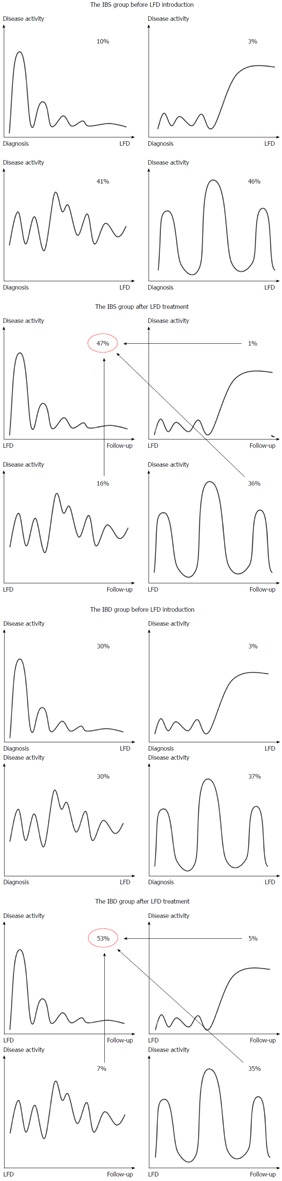
Changes in Copenhagen IBS disease courses after low FODMAP diet treatment. The four different disease courses are depicted before and after the LFD intervention for the IBD and IBS group, separately. Above each figure, the prevalence is denoted as a percentage. The mild indolent course increased significantly following LFD introduction in both patient groups (P < 0.001), while the chronic continuous course and the intermittent course were less common at follow-up. IBD: n = 43; IBS: n = 120. LFD: Low FODMAP diet; IBS: Irritable bowel syndrome; IBD: Inflammatory bowel disease.
There was a significant improvement of stool pattern in both patient groups (Figure 5). After dietary intervention, the proportion of patients producing normal stools increased, with 41% in the IBS group (P < 0.001) and 66% in the IBD group (P < 0.001).
Figure 5.
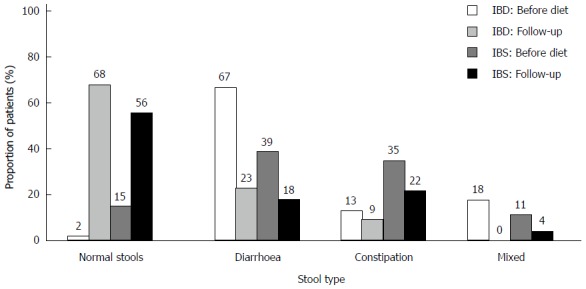
Changes in stool type after low FODMAP diet treatment. The stool types (Bristol stool chart) was categorised as normal stools, diarrhoea, constipation, and mixed. After the LFD, there was a significant increase in the prevalence of normal stools in both IBD and IBS patients (P < 0.001) with decreases in the remaining three categories of stool type, in particular the diarrhoea group (P < 0.001). IBD: n = 43; IBS: n = 121. LFD: Low FODMAP diet; IBS: Irritable bowel syndrome; IBD: Inflammatory bowel disease.
Adherence and satisfaction
In both patient groups, approximately one-third were adherent to the diet according to the FARS. Adherence was highest with regards to remembering to follow the diet and not taking breaks, and poorest when asked if patients followed the diet “by the book” and without making their own modifications (Table 2). Increased adherence was associated with longer duration of dietary treatment (P < 0.001), but otherwise no associations were found. Thirty-two percent of the IBS group and 37% of the IBD group were on the diet for less than three months, while 47% and 50%, respectively, stayed on the diet until follow-up. Fifty-four percent used the LFD on and off depending on symptom severity, while the rest were continuously on the diet. Eighty-four percent lived on a modified LFD, where some foods rich in FODMAPs were reintroduced in varying amounts according to individual tolerance level, whereas the remaining patients followed the LFD by the book without deviations. Wheat, dairy products, and onions were the foods most often not reintroduced by patients. Weight loss occurred in 29% of patients, while 7% gained weight during the dietary course. Thirty-three (26%) IBS patients and 8 (20%) IBD patients answered that they quit the diet before completion of the standard dietary period. The most common reasons for quitting were that the diet was too complicated to follow (50%), too expensive (23%), or bland in taste (15%), and other reasons (53%) were co-morbidities and detrimental effect on functional gastrointestinal symptoms. Satisfaction with dietary management was seen in 83 (70%) IBS patients and 24 (55%) IBD patients.
Disease severity and quality of life
The overall median IBS-SSS score at follow-up was 211 (range: 16-487). Mild IBS was associated with mild indolent disease course prior to LFD (P < 0.01) and, furthermore, was trending towards a normal stool type after LFD (P = 0.068). IBD patients were more likely to have less severe IBS at follow-up than were IBS patients (P < 0.05).
At follow-up, the median IBS-QoL score was 75 (range: 37-145) for the IBS group and 63 (range: 36-126) for the IBD group corresponding to 70 (range: 15-98) and 79 (range: 32-99) on the transformed scale, respectively. For the IBD patients only, the median SIBDQ score was 55 (range: 23-69) and 75 (range: 22-98) on the transformed scale. Good quality of life was associated with normal stool type after dietary therapy (P < 0.001) and duration of dietary course (P < 0.01). A tendency to report good quality of life at follow-up was observed in non-smokers and patients with normal stool pattern before introduction of LFD (P = 0.068, P = 0.060). IBD patients were observed to be more content with life as compared to IBS patients (89% vs 73%, P < 0.05).
DISCUSSION
This study focuses on long-term outcomes of LFD treatment and hopes to increase global awareness of FODMAP restriction. It is the first survey ever to assess the impact of LFD on long-term IBS disease course and, furthermore, the longest follow-up study on IBS and IBD patients treated with the LFD.
Long-term IBS disease courses improved significantly after the LFD period. Mild indolent disease course at baseline was a reliable predictor of remaining on this course after treatment. However, patients starting with one of the less favourable disease courses had a probability of about two in five of transitioning to a mild indolent course. This suggests that patients with more severe chronic courses might be able to improve their disease course with LFD management. Having said that, these data are the first on IBS disease course in relation to LFD and prospective cohort studies are needed for further investigation of whether or not LFD can improve disease course of IBS patients.
The prevalence of a normal stool type increased significantly after dietary therapy. Improvement of stool pattern occurred across all three groups of stool types, particularly in the diarrhoea group. An improved outcome among patients with diarrhoea was also found in a recent prospective study comparing LFD to a normal diet[13]. The enhanced efficacy of LFD in patients with diarrhoea could be explained by the osmotic nature of pooled FODMAPs in a regular diet which contributes to increased water output[8].
Previous studies found adherence to be of paramount importance to the diet’s success[12,14,16]. In this study, only one-third of the patients were adherent to the diet. High adherence was associated with longer duration of dietary treatment. No more than half of patients were still on the diet at follow-up; nevertheless, the majority was satisfied with the dietary treatment and did not quit the dietary course before planned. The probability of patients discontinuing dietary management increases with duration of dietary course, as motivation tends to dwindle. Gearry et al[14] found that use of resources (e.g., cookbooks), a higher educational level, and working fewer than thirty-five hours a week were significantly related to better adherence.
At follow-up, most patients reported good quality of life and had only mild or moderate disease severity. Good quality of life was associated with normal stool type after diet and longer duration of dietary treatment, while mild disease severity was related to having a mild indolent disease course before LFD introduction. Two recent prospective studies by Pedersen et al[15,17] investigated the efficacy of LFD as compared to a normal diet in IBS and IBD patients and demonstrated a significant reduction in IBS disease severity and IBD disease activity, along with a significant increase in quality of life among IBD patients and IBS-D patients during the six weeks of LFD intervention.
A retrospective study is not the ideal way to assess efficacy of therapy and is accompanied by some limitations. The high rate of patients (48%) not replying to the invitation to join the study could have led to selection bias which, together with possible recall bias, might have resulted in type I errors.
In the last decade it has been suggested that FODMAPs increase endothelial barrier permeability and, together with other factors, cause immune activation and low-grade inflammation, which could play a crucial role in the pathogenesis of IBS[30,31]. FODMAPs have been shown to influence on the colonic microbiota, and Halmos et al[32] recently found that diets differing in FODMAP content significantly affected the gut microflora composition and that a low FODMAP intake was associated with reduced absolute abundance of bacteria. As a LFD might lead to detrimental changes in gut microbiota, caution should be taken when recommending the LFD for long-term treatment.
Although there are limitations of this study, the retrospective follow-up provided data supporting the use of LFD in both IBS and IBD with co-existing IBS. A majority of patients reported beneficial effects and satisfaction with the dietary treatment and, furthermore, long-term disease courses and stool pattern improved significantly. Long-term, prospective studies are needed to further investigate those characteristics of patients responding to the diet, dietary impact on IBS disease course, and the safety of long-term FODMAP restriction.
ACKNOWLEDGMENTS
The clinical dieticians, Mette Hestetun and Maria Felding, contributed to the LFD consultations at HUH. The authors thank Henrik Wachmann (Larix A/S) for statistical analysis.
COMMENTS
Background
Treatment of irritable bowel syndrome (IBS) is challenging due to the heterogeneity of the disorder, a lack of reliable outcome measures, and high placebo response rates. The low FODMAP diet excludes foods high in short-chained carbohydrates termed FODMAPs (fermentable oligo-, di-, and monosaccharides and polyols) and has been demonstrated to be effective in the treatment of functional gastrointestinal symptoms. FODMAPs are poorly absorbed in the small intestine and are passed on to the colon, where they exert an osmotic effect, drawing fluid into the lumen and causing an increase in gas production (mainly hydrogen and methane) due to the excess delivery of fermentable substrates to the colonic microflora. These mechanisms can lead to abdominal pain, bloating, flatulence, and diarrhoea in susceptible subjects. In this long-term follow-up study, we evaluated the patient-reported outcomes among those with IBS and inflammatory bowel disease (IBD) after low FODMAP diet (LFD) treatment.
Research frontiers
Currently, studies of long-term efficacy of the LFD are lacking, and no one has yet investigated the dietary impact on IBS disease courses. The results of this study provide long-term patient-reported outcomes of LFD treatment and details on implementing the LFD.
Innovations and breakthroughs
The majority of IBS and IBD patients reported beneficial effects and satisfaction with the dietary treatment, although only one third was adherent. Furthermore, long-term IBS disease courses and stool patterns improved significantly. Eighty-four percent of patients lived on a modified LFD, where some foods rich in FODMAPs were reintroduced, and 16% followed the LFD by the book without deviations. Wheat, dairy products, and onions were the foods most often not reintroduced by patients.
Applications
This study suggests that the LFD is effective in the management of functional gastrointestinal (GI) symptoms in IBS patients and IBD patients in remission.
Peer-review
This study is of relevance and importance given the widespread use of the low FODMAP diet in the management of patients with IBS, and those with functional GI symptoms in IBD. Although there are clearly limitations with such a retrospective study based on patient self-report questionnaires, the study provides some interesting long-term data.
Footnotes
Supported by the Danish patient society of inflammatory bowel disease and irritable bowel syndrome patients, Colitis Crohn Foreningen.
Institutional review board statement: Not needed for a simple questionnaire analysis according to rules of the Ethics Committee of Science, Denmark.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent before study enrolment.
Conflict-of-interest statement: None to declare.
Data sharing statement: Questionnaires and data set are available from the corresponding author at louisemaagaard3@gmail.com. The Danish Data Protection Agency approved the study design. Participants gave informed written consent for data sharing and furthermore, the presented data are anonymised and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 30, 2015
First decision: November 27, 2015
Article in press: January 18, 2016
P- Reviewer: Day AS, Manguso F, van Langenberg DR S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 2.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 3.Krogsgaard LR, Engsbro AL, Bytzer P. The epidemiology of irritable bowel syndrome in Denmark. A population-based survey in adults ≤50 years of age. Scand J Gastroenterol. 2013;48:523–529. doi: 10.3109/00365521.2013.775328. [DOI] [PubMed] [Google Scholar]
- 4.Keohane J, O’Mahony C, O’Mahony L, O’Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788, 1789–1794; quiz 1795. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- 5.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 6.Berrill JW, Green JT, Hood K, Campbell AK. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment Pharmacol Ther. 2013;38:44–51. doi: 10.1111/apt.12335. [DOI] [PubMed] [Google Scholar]
- 7.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–666; quiz 667. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 8.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 10.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 11.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 12.de Roest RH, Dobbs BR, Chapman BA, Batman B, O’Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 13.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis. 2009;3:8–14. doi: 10.1016/j.crohns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen N. EHealth: self-management in inflammatory bowel disease and in irritable bowel syndrome using novel constant-care web applications. eHealth by constant-care in IBD and IBS. Dan Med J. 2015;62:B5168. [PubMed] [Google Scholar]
- 16.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen N, Andersen NN, Végh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20:16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen N, Vegh Z, Burisch J, Jensen L, Ankersen DV, Felding M, Andersen NN, Munkholm P. Ehealth monitoring in irritable bowel syndrome patients treated with low fermentable oligo-, di-, mono-saccharides and polyols diet. World J Gastroenterol. 2014;20:6680–6684. doi: 10.3748/wjg.v20.i21.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkjaer M, Burisch J, Avnstrøm S, Lynge E, Munkholm P. Development of a Web-based concept for patients with ulcerative colitis and 5-aminosalicylic acid treatment. Eur J Gastroenterol Hepatol. 2010;22:695–704. doi: 10.1097/MEG.0b013e32832e0a18. [DOI] [PubMed] [Google Scholar]
- 20.King’s College London. FODMAPs courses. Available from: http://www.kcl.ac.uk/lsm/research/divisions/dns/projects/fodmaps/courses.aspx.
- 21.Byrne M, Walsh J, Murphy AW. Secondary prevention of coronary heart disease: patient beliefs and health-related behaviour. J Psychosom Res. 2005;58:403–415. doi: 10.1016/j.jpsychores.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 23.Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995;30:699–706. doi: 10.3109/00365529509096316. [DOI] [PubMed] [Google Scholar]
- 24.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300:439–440. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 27.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 28.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 29.Burisch J, Weimers P, Pedersen N, Cukovic-Cavka S, Vucelic B, Kaimakliotis I, Duricova D, Bortlik M, Shonová O, Vind I, et al. Health-related quality of life improves during one year of medical and surgical treatment in a European population-based inception cohort of patients with inflammatory bowel disease--an ECCO-EpiCom study. J Crohns Colitis. 2014;8:1030–1042. doi: 10.1016/j.crohns.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21:1399–1409. doi: 10.1111/j.1365-2036.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- 31.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 32.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]


