Abstract
Membrane transporters play an essential role in the transport of endogenous and exogenous compounds, and consequently they mediate the uptake, distribution, and excretion of many drugs. The clinical relevance of transporters in drug disposition and their effect in adults have been shown in drug–drug interaction and pharmacogenomic studies. Little is known, however, about the ontogeny of human membrane transporters and their roles in pediatric pharmacotherapy. As they are involved in the transport of endogenous substrates, growth and development may be important determinants of their expression and activity. This review presents an overview of our current knowledge on human membrane transporters in pediatric drug disposition and effect. Existing pharmacokinetic and pharmacogenetic data on membrane substrate drugs frequently used in children are presented and related, where possible, to existing ex vivo data, providing a basis for developmental patterns for individual human membrane transporters. As data for individual transporters are currently still scarce, there is a striking information gap regarding the role of human membrane transporters in drug therapy in children.
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-015-0328-5) contains supplementary material, which is available to authorized users.
Key Points
| Little is known about the ontogeny of transporters and their roles in pediatric pharmacotherapy. |
| Ex vivo, pharmacokinetic and pharmacogenetic studies suggest transporter-specific changes from the human fetus to the adult. |
| No clear transporter-specific maturation pattern can be deducted at this time, hence, further research is needed. |
Introduction
Plasma membrane transporters play an essential role in the uptake of endogenous compounds into cells and their efflux from cells. They also mediate the absorption, distribution, and excretion of a large number of drugs [1, 2]. In particular, two major transporter superfamilies are the focus of pharmacological studies: the adenosine triphosphate (ATP)-binding cassette (ABC) transporters and the solute carrier (SLC) transporter superfamilies [3, 4]. The nomenclature is presented in Table 1. Numerous studies, mostly in adults, have investigated altered membrane transporter functions due to genetic variants or drug–drug interactions by co-medications [1, 5–9]. Studies on the role of membrane transporters in children are scarce, however. Still, growth and maturation are likely to have an impact on activity of these transporters in light of their role in endogenous processes. Animal studies have indeed shown developmental changes in membrane transporter expression [10]. The aim of this review is to present an up-to-date overview on our current knowledge on the role of human membrane transporters in pediatric drug disposition and effect. For this purpose, a short overview of ex vivo studies is presented after which results from pharmacokinetic and pharmacogenetic studies of relevant membrane transporters are reported that may broaden our insight into developmental patterns for individual human membrane transporters.
Table 1.
Nomenclature of human membrane transporters: selection transporters discussed in this paper [source: NCBI Gene (http://www.ncbi.nlm.nih.gov/gene)]
| Gene | Protein | ||
|---|---|---|---|
| Name | Locus | Name | Synonyms |
| ABC transporters | |||
| ABCB1 | 7q21.12 | ABCB1 | MDR1, P-glycoprotein (P-gp), CLCS, PGY1, ABC20, CD243, GP170 |
| ABCC2 | 10q24 | ABCC2 | MRP2, CMOAT, DJS, cMRP, ABC30 |
| ABCC3 | 17q22 | ABCC3 | MRP3, MOAT-D, cMOAT2, MLP2, ABC31, EST90757 |
| ABCC4 | 13q32 | ABCC4 | MRP4, MOAT-B, MOATB |
| ABCG2 | 4q22 | ABCG2 | BRCP, MXR, MRX, ABCP, BMDP, MXR1, BCRP1, CD338, GOUT1, CDw338, UAQTL1, EST157481 |
| SLC transporters | |||
| SLCO1B1 | 12p | OATP1B1 | OATP2, LST-1, OATP-C, HBLRR, LST1, SLC21A6 |
| SLCO1B3 | 12p12 | OATP1B3 | OATP8, LST-2, LST3, HBLRR, SLC21A8, LST-3TM13 |
| SLCO2B1 | 11q13 | OATP2B1 | OATP-B, SLC21A9 |
| SLC3A2 | 11q13 | 4F2hc | 4F2, CD98, MDU1, 4T2HC, NACAE, CD98HC |
| SLC22A1 | 6q25.3 | OCT1 | HOCT1, oct1_cds |
| SLC22A2 | 6q25.3 | OCT2 | |
| SLC22A6 | 11q12.3 | OAT1 | PAHT, HOAT1, ROAT1 |
| SLC22A7 | 6q21.1 | OAT2 | NLT |
| SLC22A8 | 11q11 | OAT3 | |
| SLC15A1 | 13q32.3 | PEPT1 | HPEPT1, HPECT1 |
| SLC47A1 | 17q11.2 | MATE1 | |
| SLC47A2 | 17q11.2 | MATE2-K | MATE2, MATE2-B |
| Other | |||
| FAAH | 1p35-p34 | FAAH | FAAH-1, PSAB |
| ADRB2 | 5q31-q32 | ADRB2 | BAR, B2AR, ADRBR, ADRB2R, BETA2AR |
| CDH17 | 8q22.1 | HPT1 | CDH16, LI cadherin |
ABC adenosine triphosphate (ATP)-binding cassette, ADRB2 β2-adrenergic receptor, FAAH fatty acid hydrolase, SLC solute carrier
Ex Vivo Studies on the Ontogeny of Human Membrane Transporters
Ex vivo data from pediatric samples may be used to extrapolate existing adult pharmacokinetic data to children, as is done using physiologically based pharmacokinetic (PBPK) modeling [11, 12]. Expression patterns of membrane transporters during human development have been studied in postmortem and surgical tissue samples with the use of different techniques such as immunohistochemistry to visualize tissue localization, reverse transcriptase polymerase chain reaction (RT-PCR) for messenger RNA (mRNA) expression, Western blotting and new liquid chromatography–tandem mass spectrometry (LC–MS/MS) techniques to quantify transporter protein abundance. To the best of our knowledge, transporter activity studies using human pediatric tissue are non-existent. Although animal data may provide valuable insight, potential developmental patterns of membrane transporters in animals are likely to differ from those in humans, as studies on drug metabolizing enzymes (DMEs) have shown [13–15]. Moreover, animal studies do not provide any information when there are no direct orthologs in rodents, as is the case, for example, for human organic anion-transporting polypeptide (OATP) 1B1 and OATP1B3.
From the embryonic and fetal period, most transporter data result from immunohistochemistry and mRNA expression studies. These data, often covering a small age range and/or small sample size, suggest transporter-specific maturation with a low fetal/neonatal or stable expression pattern, but quantification is lacking [16–19]. The ex vivo data from the first years of life consist mainly of hepatic and intestinal mRNA expression data, with the inherent limitation of a possible lack of correlation with protein expression [20–24]. In children from 7 years onwards, protein abundance data generated using LC–MS/MS have been recently published [25–27]. Although a large pediatric age range was covered by this project, the younger age range, where most developmental changes are expected, is lacking in protein abundance data.
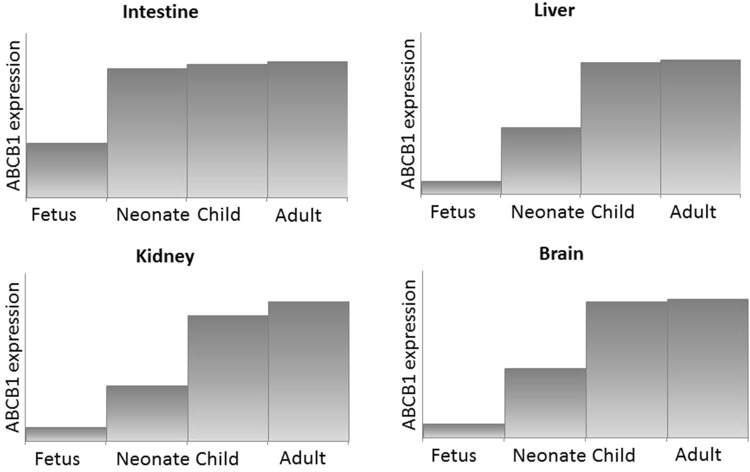
The studies referenced above comprise the most significant studies investigating the maturation of human membrane transporters, with an emphasis on the clinically relevant transporters ABCB1, ABCC2, OATP1B1, and OATP1B3. The best-studied transporter during human development is ABCB1 (Fig. 1). Interestingly, its developmental pattern seems organ-specific. In fetal intestinal samples (16th to 20th week of gestation), ABCB1 could be visualized [16] and intestinal mRNA data suggests stable ABCB1 expression from the neonate up to the adult [22, 24]. In the liver, mRNA expression data suggest a pattern of low ABCB1 expression in fetuses, neonates, and infants until 12 months of age, after which it increases to adult levels [21, 22]. ABCB1 protein abundance measured using LC–MS/MS was quite variable (4.8-fold) in 64 subjects in the age range 7–70 years, but this variation could not be explained by either age or sex [25]. In fetal human brain samples ranging from 7 to 28 weeks of gestational age, ABCB1 immunostaining was detected in only one sample from a 28-week fetus [16]. In contrast, in postmortem central nervous system tissue from neonates (n = 28) of 22–42 weeks of gestational age and from adults (n = 3), immunohistochemistry showed increasing ABCB1 staining with gestational age [28]. ABCB1 gene expression was also detected in the brain of fetuses of 15, 27, and 42 weeks of gestational age [18]. Very recently, the ABCB1 protein was shown to be limited at birth and to increase postnatally to reach adult levels by 3–6 months of age [29]. Renal ABCB1 mRNA expression appears to be related to maturity of nephrons. A trend towards lower expression in fetuses and neonates than in adults was observed. ABCB1 protein has been identified as early as the 5.5th week of gestation [16, 17, 21].
Fig. 1.
Suggested ontogeny of ABCB1 expression in intestine, liver, kidney, and brain. ABC adenosine triphosphate (ATP)-binding cassette
ABCC2 ontogeny shows similarities to ABCB1. Small intestine ABCC2 mRNA expression is stable in neonatal surgical patients compared to adults. While, hepatic ABCC2 mRNA expression is much lower in the fetus, neonate, and young infant (up to 200-fold lower) than the adult [22, 27], in children from 7 years onwards its protein expression appears stable [27]. On the protein level, both the localization pattern and intensity of ABCC2 protein staining appear to change during fetal life, in concert with fetal liver maturation [19, 23].
Hepatic mRNA expression of OATP1B1 and OATP1B3 appears to show a different developmental pattern than ABCB1 and ABCC2. Although fetal expression was 2- to 30-fold lower than adult expression, neonatal and infant expression appeared to be even lower (up to 600-fold) [19, 22]. This pattern appears to be supported by protein data (Western blotting) for OATP1B3 but not for OATP1B1. In one study, OATP1B1 protein expression already appears at adult levels in neonates, while in another OATP1B1 only increases after the age of 6 years [30, 31]. Again, for both OATP1B1 and OATP1B3, protein expression appears stable at adult levels from 7 years onwards [25].
Pharmacokinetic and Pharmacogenetic Studies of Relevant Membrane Transporter Substrates
Pharmacokinetic and pharmacogenetic studies may provide insight into the impact of selected drug transporters in vivo. We identified 16 drugs frequently prescribed to children and that are known substrates of one or more specific membrane transporters (Table 2; see the Electronic Supplementary Material for the search strategy). Age-related differences in pharmacokinetic or pharmacogenetic studies may point to maturational changes in the transporter involved. On the other hand, concordance between adult and pediatric pharmacogenomic studies may support the presence and potentially similar expression of the involved transporters in children as in adults. To further support a potential developmental pattern, we compared the in vivo data with relevant ex vivo data of the individual transporters. As many drugs are also substrates of DMEs and/or multiple transporters, the presented data must be interpreted in the context of the interplay with metabolizing enzymes. It can be speculated that when a specific DME is developmentally low at a certain age, while the transporter is already mature, this may impact the disposition of a drug by potentially altering the absorption, distribution, metabolism, and excretion (ADME) pathway from largely DME based to transporter based. Where possible, we have only included data that are highly supportive of a role for the transporter(s) involved. Table 2 provides a summary of the pharmacokinetic studies in children and the relationship with transporters. For detailed genetic information on individual transporters, the reader is referred to The Pharmacogenomics Knowledgebase (www.pharmgkb.org) and recent reviews [7, 8, 32–34].
Table 2.
Summary pharmacokinetic and pharmacogenetic studies of relevant membrane transporter substrates
| Drug | Relevant transporters involved in transport of drug | PK and PGx results in children |
|---|---|---|
| Digoxin | ABCB1 | Higher bodyweight-corrected digoxin clearance in term neonates and young children [36–38]. Renal clearance of digoxin in young children may be more dependent on ABCB1-mediated tubular secretion than in adults [39] |
| Tacrolimus | ABCB1 | PGx studies of ABCB1 in relation to tacrolimus PKs appear contradictory [42, 43, 46, 47]. In pediatric liver transplant recipients, high intestinal ABCB1 mRNA expression was associated with a twofold higher tacrolimus clearance [48] |
| Daptomycin | ABCB1 | Higher body size-corrected renal daptomycin clearance in neonates and younger infants [51–53] |
| Fexofenadine | OATP2B1, ABCB1, MRP2 | Apparent bodyweight-corrected oral clearance was 1.5-fold lower in children 6 months to 6 years than in children 6–12 years [58] |
| Morphine | OCT1, ABCB1, ABCC2, ABCC3, OATP1B1 | Neonates and infants have low morphine clearance in the first 10 days of life, increasing thereafter, largely due to immature UGT2B7 metabolism, but transporters may contribute [64, 65]. Neonates are more prone to morphine-related respiratory depression [66]. ABCB1 genotype was associated with respiratory depression in older children, in contrast to an adult study [62]. Also, ABCB1 genotype affects the M3G-formation and OCT1 genotype is associated with variation in morphine clearance and glucuronide-metabolites formation [68] |
| Pravastatin | OATP1B1, OATP2B1, OATP1B3, ABCB1, ABCC2 | Children with hypercholesterolemia and the SLCO1B1 –11187GA variant had lower mean pravastatin AUCs than those with the wild-type, in contrast to an adult study where the opposite effect was found [71, 72]. No age-related variability in pravastatin PKs from children aged 5 years onwards [73] |
| Atorvastatin | OATP1B1, BCRP | Atorvastatin PKs in older children similar to adult PKs [74] |
| Bosentan | OATP1B1, OATP1B3, OATP2B1 | In children, an exposure limit was found at a much lower dose than in adults, which might be due to intestinal OATP2B1 saturation [80] |
| Ondansetron | OCT1 | Ondansetron PKs and clinical efficacy have been correlated with OCT1 genotypes in adults [82]. Ondansetron clearance increased with age in children aged 1–48 months [83] |
| Metformin | OCT, MATE1, MATE2 K | Metformin PKs in children from 9 years of age onwards were comparable with adult PKs, suggesting stable OCT and MATE activity [91] |
| Cimetidine | OCT2, MATE1, MATE2 K, OAT2 | In neonates and children, cimetidine (and metabolites) renal clearance accounts for 80–90 % of total clearance, whereas in adults it accounts for 60 % of total clearance [93–95]. The relatively high renal clearance suggests mature OCT2 activity at birth in the presence of immature GFR [99] |
| Tramadol | OCT1 | In adults, OCT1 genotype was related to metabolite plasma concentrations and prolonged miosis [101]. Tramadol and metabolite PKs show age-related changes in neonates [102] |
| Methotrexate | OATP1B1, ABCC2 | Increased renal toxicity in children 0–3 months old compared with infants 7–12 months [106]. From 1 year of age onwards, body size-corrected methotrexate clearance decreased linearly with age [107]. SLCO1B1 genotype was associated with increased AUC and was a predictor for toxicity [107] |
| Mycophenolate mofetil | MRP2 | In pediatric patients, ABCC2 rs717620 allele has been associated with reduced exposure to MPA, more adverse effects, and rejection [114] |
| Acyclovir/valacyclovir | 4F2hc, HPT1, OAT1, OAT3 | In neonates, the IV acyclovir bodyweight-corrected clearance showed a twofold increase from 25 to 41 weeks of gestational age [118]. In older children, 1 month to 5 years, apparent oral clearance of valacyclovir in children less than 3 months of age was 50 % than that in older children [119] |
| Adefovir | OAT1, MRP4 | Adefovir is partly renally cleared (45 %) [120, 121]. In 45 children (age range 2–17 years) receiving oral adefovir dipivoxil, weight-corrected mean apparent clearance and renal clearance were higher in younger children [122] |
ABC adenosine triphosphate (ATP)-binding cassette, AUC area under the plasma concentration–time curve, BCRP breast cancer resistance protein, GFR glomerular filtration rate, HPT human oligopeptide transporter, IV intravenous, M3G morphine-3-glucuronide, MATE multidrug and toxin extrusion protein, MPA mycophenolic acid, mRNA messenger RNA, MRP multidrug resistance-associated protein, OAT organic anion transporter, OATP organic anion-transporting polypeptide, OCT organic cation transporter, PGx pharmacogenetics, PK pharmacokinetic, UGT uridine 5′-diphospho-glucuronosyltransferase
Digoxin-ABCB1
The cardiac glycoside digoxin is a well-known ABCB1 substrate (Fig. 2). Its US Food and Drug Administration (FDA) drug label warns about pharmacokinetic interactions with intestinal or renal ABCB1 inducers or inhibitors. Digoxin is mainly renally cleared as unchanged drug: 80 % by glomerular filtration and 20 % by tubular secretion [35]. Pharmacokinetic studies in children show clear age-related differences. In a population pharmacokinetic analysis in 71 neonates (age range 2–29 days), oral digoxin clearance increased non-linearly with increasing bodyweight and gestational age [36]. The estimated clearance of digoxin in a term-born 3 kg newborn is 0.338 L/kg/h at a serum concentration of 1 ng/mL. A population pharmacokinetic study in older infants [n = 117, mean age (range) 0.76 (0.08–4.43) years] also found increased oral digoxin clearance with increasing bodyweight [37]. In this study, the simulated apparent oral clearance (CL/F) of an 8 kg infant was 0.43 L/h/kg at a target concentration of 1 ng/mL. Interestingly, digoxin clearance normalized for bodyweight appears to be much higher in term neonates and younger children than in adults (0.17 L/h/kg). This observation is also in line with higher (per kg) dosing recommendations in term neonates and infants. However, as the drug is mainly renally cleared, and glomerular filtration is still immature at birth, one would expect a lower body size-corrected clearance. This was indeed the case in preterm infants (<2.5 kg) whose digoxin bodyweight-corrected clearance was much lower than that of term infants (0.064 vs. 0.1 L/h/kg), in line with lower dosing recommendations for this age group [38]. Thus, in preterm newborns, both the glomerular filtration rate (GFR) and ABCB1 may be immature at birth, while in term infants ABCB1 activity may already be more mature and compensate for developmentally low GFR.
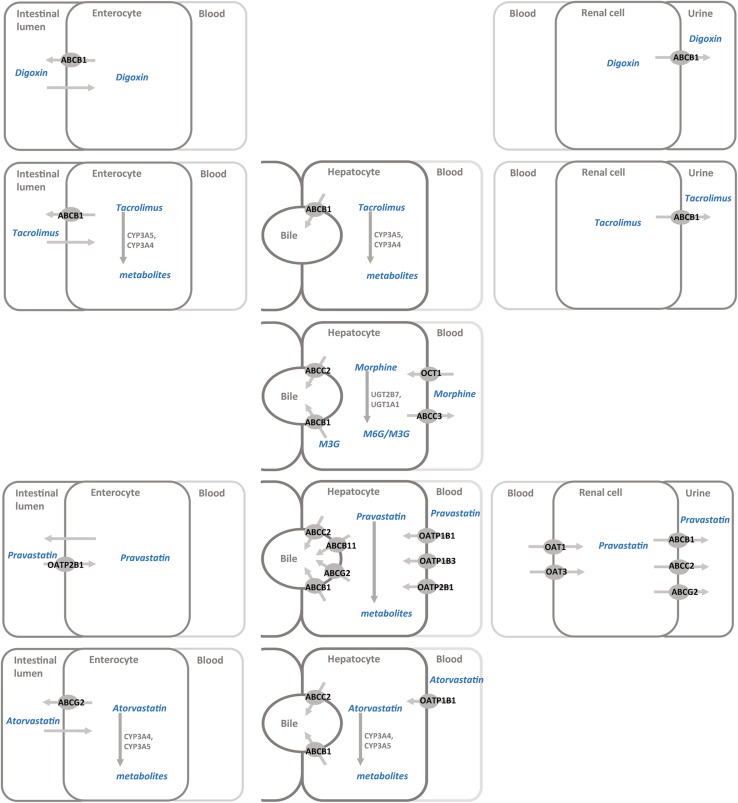
Fig. 2.
Membrane transporters and their relationship with commonly prescribed drugs to children: digoxin, tacrolimus, morphine, pravastatin, and atorvastatin. ABC adenosine triphosphate (ATP)-binding cassette, CYP cytochrome P450, OATP organic anion-transporting polypeptide, OCT organic cation transporter, UGT uridine 5′-diphospho-glucuronosyltransferase
Additionally, clearance decreases non-linearly with increasing concentrations in the range of 0.2 and 2 ng/mL in children up to 4.5 years of age, whereas non-linearity in adults is only found from a serum concentration of 7 ng/mL onwards. We can only speculate as to the underlying mechanism. Earlier transporter saturation due to immature ABCB1 activity in intestine and kidney contradicts the observation of higher bodyweight-corrected clearance in young children than in adults.
A clinically relevant interaction was found in eight children who were co-administered the ABCB1 inhibitor carvedilol. Digoxin clearance decreased twofold, while the digoxin clearance to GFR ratio decreased by 45 %, supporting intestinal and renal ABCB1-mediated inhibition [39]. In contrast, the same drug–drug interaction resulted in only a mild decrease in digoxin clearance in adults. These findings support our hypothesis that renal clearance of digoxin in young children may be more dependent on ABCB1-mediated tubular secretion than in adults.
Interestingly, the hypothesis of higher renal ABCB1 expression after birth is supported by a mouse study showing a relationship between renal Abcb1 expression and digoxin clearance in young mice, but not by the limited human data from neonates [21, 40].
Tacrolimus-ABCB1
The calcineurin inhibitor tacrolimus inhibits synthesis of cytotoxic lymphocytes and so prevents transplant rejection. Tacrolimus is a substrate for intestinal and hepatic ABCB1 (Fig. 2) [41]. Weight-normalized oral tacrolimus clearance, which is also dependent on cytochrome P450 (CYP) 3A4/5 metabolism, is higher in infants between 1 and 6 years of age than in older children and adults [42, 43]. CYP3A4 activity matures in the first year of life, while CYP3A5 activity, when present, appears stable from fetus to adult [44].
Pharmacogenetic studies of ABCB1 in relation to tacrolimus disposition appear contradictory [45]. In pediatric heart and kidney transplant recipients no relation was found between ABCB1 genotype and tacrolimus dosing requirements or concentration/dose ratio [42, 43]. In pediatric liver transplant patients, homozygous ABCB1 1236TT/2677TT/3435TT carriers needed higher tacrolimus doses than non-carriers both early and later after transplantation [42, 46]. In a population pharmacokinetic analysis in 114 pediatric liver transplant recipients, the ABCB1 2677G>T allele was associated with a higher pre-dose and concentration/dose ratio at day 1 after transplantation [47]. Such associations were not found for recipient or donor ABCB1 1199G>A and 3435C>T variants. These findings can be understood from a combined ex vivo/population pharmacokinetic study in 130 pediatric liver transplant recipients. High intestinal ABCB1 mRNA expression was associated with an almost twofold higher tacrolimus clearance early after transplantation, indicative of a switch from primarily intestinal to hepatic tacrolimus clearance upon graft recovery [48].
The results from ex vivo studies suggesting stable ABCB1 mRNA intestinal expression and lower hepatic ABCB1 expression in young infants may explain the pharmacogenetic findings and the impact of the recipient intestinal ABCB1 on tacrolimus disposition [20, 22, 24, 48].
Daptomycin-ABCB1
The antibacterial daptomycin is used to treat infections caused by Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Daptomycin is excreted primarily by the kidney and is an ABCB1 substrate. In 23 adult Caucasian patients, the ABCB1 3435T single nucleotide polymorphism (SNP) was associated with a higher intravenous daptomycin dose-normalized area under the plasma concentration–time curve (AUC) and lower steady state clearance [49, 50]. In 24 children (age range 3–24 months), intravenous daptomycin clearance in younger infants (approximately 20 mL/h/kg) was higher than previously reported for older children and adults (8–13 mL/h/kg) [51, 52]. Likewise, in 20 preterm and term infants (32–40 weeks of gestational age and 0–85 days’ postnatal age, the mean clearance was approximately 20 mL/kg/h) [53]. No relationship with gestational or postnatal age was found, possibly due to the small sample size. The maturation pattern of daptomycin pharmacokinetics resembles that of digoxin pharmacokinetics, with higher clearance values early in life. This pattern is not consistent with immature GFR, but may reflect a compensatory role of ABCB1-mediated renal tubular secretion in young children. As discussed earlier, this may be the result of increased renal ABCB1 expression in young infants, but these findings need to be confirmed [16, 18, 21].
Fexofenadine-OATP2B1
The antihistamine fexofenadine is mainly excreted through bile as parent drug. Only a minor part is metabolized by intestinal microflora and CYP3A4. Its disposition appears to be subject to membrane transport by the uptake transporter OATP2B1, the efflux transporter ABCB1, and possibly ABCC2 [54–56]. In 14 healthy men, fexofenadine clearance was related to OATP2B1 polymorphisms and simultaneous apple juice ingestion [57]. This latter finding of a potential food–drug interaction concerning OATP2B1 substrates may be even more relevant for children, as heavy consumers of apple juice. In a population pharmacokinetic study in 515 Japanese children (6 months to 16 years), CL/F was stable across the age groups (1 L/h/kg) with the exception of the 6- to 12-year-olds, whose clearance was 1.5-fold higher (1.5 L/h/kg) [58]. In another population pharmacokinetic study including 46 Caucasian and 31 non-Caucasian children (6 months to 12 years) and 138 adults, apparent bodyweight-normalized oral clearance was lower in children less than 1 year of age than in older children and adults [59]. Interestingly when comparing ethnicities, the CL/F was slightly higher in the 6- to 12-year-old Japanese children (1.5 L/kg/h) than in 14 non-Japanese children (0.8 L/h/kg) (13 Caucasian and one non-Caucasian), but this difference may be due to the small sample size rather than ethnicity [58].
Ex vivo data in these age groups are lacking, but OATP2B1 gene expression in 15 neonatal intestinal samples obtained during surgery was nearly three times higher than in adult samples [22]. This may imply higher oral absorption of fenofexadine in neonates and young infants.
Morphine-ABCB1 and OCT1
The opioid morphine is almost completely metabolized by uridine 5′-diphospho-glucuronosyltransferase (UGT) 2B7 and UGT1A1 to morphine-3-glucuronide and morphine-6-glucuronide. The disposition of both morphine and/or its metabolites is subject to membrane transport by the uptake transporters organic cation transporter (OCT) 1 and OATP1B1 and by the efflux transporters ABCB1, ABCC2, and ABCC3 (Fig. 2) [60, 61]. Hepatic uptake of morphine appeared to be OCT1-mediated in an adult volunteer study and carriers of loss-of-function SLC22A1 gene polymorphisms showed higher morphine AUCs [61]. In other healthy adult volunteers, co-administration of the ABCB1 inhibitor quinidine altered both morphine pharmacokinetics and its opioid effects after oral but not after intravenous morphine administration, suggesting a limited role for ABCB1 in the disposition of morphine at the liver and blood–brain barrier [62]. In adult cancer patients receiving oral morphine, pain relief was more prominent in homozygous carriers of the ABCB1 3435T/T SNP, probably due to higher intestinal uptake [63].
Age-related morphine clearance in neonates and infants is very low in the first 10 days of life and increases thereafter [64]. Although this pattern was explained by UGT2B7 maturation, an impact of the maturation of the relevant transporters cannot be ruled out. Interestingly, in follow-up studies in which morphine doses were adjusted to the age-related clearance and similar exposure was reached across the first year of age, pain relief was adequate in neonates (<10 days of age), but the older children still needed high doses of rescue morphine [65]. Neonates are more prone to respiratory depression, which may be explained by increased exposure to morphine when doses are not adjusted to the age-related changes in its disposition. In addition, immature ABCB1 activity at the blood–brain barrier, as shown very recently, cannot be ruled out to contribute as well and deserves further study [29].
Sadhasivam and co-workers [66, 67] have extensively studied the impact of genetics of transporters in a large cohort of infants and children receiving intravenous morphine for tonsillectomy. In 220 infants, OCT1 homozygous genotypes (SLC22A1 1365GAT>del/1498G>C) were associated with lower morphine clearance and lower morphine glucuronide formation [68]. ABCC3 homozygous –211 CC carriers showed (approximately 40 %) higher metabolite transformation, indicating increased efflux of metabolites into plasma. ABCB1 polymorphisms (3435 C>T) only affected morphine-3-glucuronide formation, not morphine or morphine-6-glucuronide pharmacokinetics. It should be noted that only pharmacokinetic data up to 45 min post-dosing were available and, hence, the full pharmacokinetic profile of morphine and its metabolites could not be determined. In 263 children of the same cohort, the ABCB1 G allele of the rs9282564 polymorphism was associated with respiratory depression, resulting in prolonged hospital stay (odds ratio 4.7; 95 % confidence interval 2.1–10.8, P = 0.0002) [66]. This study contrasts with the adult study in which ABCB1 inhibition did not influence the effect of morphine after intravenous administration [62]. In the extended cohort, now including 347 children, the interaction of genetic variants of ABCB1 and two other genes, the fatty acid hydrolase (FAAH, which has been associated with opioid use and addiction acting via cannabinoid receptor type 1), and the β2-adrenergic receptor (ADRB2, receptor blockade has been associated with pain and pain relief), helped discriminate low and high risk for morphine-related postoperative respiratory depression [67].
As hepatic ABCB1 gene expression only appears to reach adult levels after the first year of life [20, 22, 24, 48], immature hepatic and possibly also blood–brain barrier ABCB1 expression may play a role in higher morphine plasma and brain exposure and the higher risk for respiratory depression in neonates.
Pravastatin-OATP1B1
Clearance of the cholesterol synthesis inhibitor pravastatin is mainly dependent on non-CYP450-mediated drug metabolism and several uptake and efflux transporters, such as OATP1B1, OATP2B1, OATP1B3, ABCB1, and ABCC2 (Fig. 2) [69]. In adults, SLCO1B1, but not ABCC2, ABCB1, or ABCG2, gene variants were associated with inter-individual variability in pravastatin pharmacokinetics, suggesting a major role of SLCO1B1 in pravastatin disposition [70, 71].
Intriguingly, in one small pharmacogenetic study (n = 20; mean ± standard deviation age 10.3 ± 2.9 years), children with familiar hypercholesterolemia with the SLCO1B1 –11187GA genotype had lower mean pravastatin AUCs than children with the wild type [72]. The opposite effect was found in an adult study (n = 41) [71]. These results, which should be interpreted with care in view of the small sample size, suggest age-related post-translational differences in OATP1B1 expression. The pharmacokinetics of pravastatin in children (aged 5–16 years) were similar to adults, which suggests no major impact of age-related variability in OATP1B1 or other transporter activity from 5 years onwards [73]. These results appear to be in line with the ex vivo results of stable hepatic OATP1B1 expression in older children [25].
Atorvastatin-OATP1B1
Another cholesterol synthesis inhibitor increasingly used in children is atorvastatin. Like the other statins (HMG-CoA reductase inhibitors), atorvastatin is extensively metabolized, largely by CYP3A4, and is a substrate for OATP1B1 (hepatic uptake) and ABCG2 (oral absorption) (Fig. 2). In a population pharmacokinetic study in pediatric hypercholesterolemia patients aged 6–17 years, atorvastatin CL/F was described as a function of bodyweight. When scaled allometrically, CL/F was similar to values reported for adults [74]. Atorvastatin metabolism is probably mature from 6 years onwards. The stable clearance and the existing ex vivo data of OATP1B1 suggest similarly mature transporter activity, although an age-related change in the relative contribution of individual transporters cannot be ruled out [25, 26]. We could not identify pediatric pharmacogenetic atorvastatin studies, although a comprehensive study in adults clearly indicated the SLCO1B1 variant 388A>G as a major determinant for atorvastatin pharmacokinetics [75].
Bosentan-OATP2B1
Bosentan is metabolized by CYP3A4 and CYP2C9, with both the parent compound and one metabolite being pharmacological active, but is also subject to hepatic uptake by OATP1B1, OATP1B3, and maybe OATP2B1 [76]. Next to the developmental pattern of CYP3A4, CYP2C9 already shows an increase prenatally, with stable, though variable, activity after the first week of postnatal age [77, 78]. Hence, the impact of the maturation of the transporters may be compounded by CYP3A4 and CYP2C9 maturation in the first year of life, but age-related variation as observed later in life may be more likely due to transporter maturation. At oral doses of approximately 2 mg/kg, bosentan plasma exposures for 19 children (aged 3–15 years) were similar to those for healthy adults [79]. In children [n = 36, median (range) age 7.0 (2–11) years], plasma concentrations did not further increase at doses higher than 2 mg/kg (exposure limit), while in adults the exposure limit was 7 mg/kg, with no difference in the other pharmacokinetic parameters [80]. As bosentan appears to be an OATP2B1 substrate, intestinal saturation due to immature OATP2B1, and perhaps other anatomical or physiological age-related differences in oral absorption, may explain this observation. This result does not correspond with the OATP2B1 mRNA expression results, suggesting higher expression in neonates than in adults [22]. In a pediatric population pharmacokinetic–pharmacogenetic study [n = 46, mean (range) age 3.8 years (25 days–16.9 years)], no relationship between bosentan pharmacokinetics and genetic polymorphisms of SLCO1B1, SLCO1B3, SLCO2B1, or CYP3A5 was found [81].
Ondansetron-OCT1
The anti-emetic ondansetron is mainly metabolized by CYP2D6, which contributes to genetic variation in its disposition and effect. In addition to this CYP2D6 effect, the pharmacokinetics (n = 45) and clinical efficacy (n = 222) of ondansetron in adults (age range 18–83 years) have also been correlated with SLC22A1 genotypes [82]. OCT1 deficiency potentially limits the hepatic uptake and increases plasma concentrations of ondansetron [82]. In a population pharmacokinetic analysis of 124 patients in the age range 1–48 months, ondansetron bodyweight-normalized clearance was reduced by 76 % in a 1-month-old patient and by 31 % in a 6-month-old patient, compared with the older children [83]. This contrasts with evidence from both in vitro and in vivo studies suggesting maturity of CYP2D6 activity as early as 1–12 weeks postnatal [84–86]. Thus, we hypothesize that lower ondansetron clearance in the first year of life may be related to immature OCT1 activity.
Metformin-OCT1 and MATE1
Several transporters have been implicated in metformin elimination, tissue distribution, and response. OCT1 is a major determinant of the hepatic uptake of metformin, while multidrug and toxin extrusion protein (MATE) 1/MATE2-K determine the efflux of metformin [7, 34]. Recently, the transcription factor hepatocyte nuclear factor 1 was found to regulate OCT1 expression and was related to metformin pharmacokinetics and pharmacodynamics [87, 88]. The combination of SLC22A1 (OCT1) and SLC47A1 (MATE1) genotypes further explains variation in response to metformin in adults [8, 89]. In contrast, in 140 non-obese adolescent girls with androgen excess after precocious pubarche, SLC22A1 genotype was not related to metabolic response at 1 year of metformin treatment [90]. In non-obese 9-year-old girls and diabetic patients aged 12–16 years, pharmacokinetics were comparable with those in adults [91, 92]. This suggests stable OCT1 or MATE activity from the age of 9 years onwards, but this needs further study, especially since data in younger children are lacking.
Cimetidine-OCT2
In adults, renal excretion of the histamine H2-receptor antagonist cimetidine and its metabolites accounts for 60 % of total clearance, versus 80–90 % in children and neonates [93–95]. Cimetidine is partially metabolized and is a substrate of the uptake transporters OCT2 and organic anion transporter (OAT) 2 and the efflux transporters MATE1 and MATE2-K [8, 34, 96, 97]. Cimetidine is a well-known OCT2 inhibitor and therefore could potentially counteract OCT2-driven cisplatin oto- and renal toxicity [98]. Although the drug is now rarely used in pediatric clinical care, this latter promise necessitates a full understanding of its disposition across the pediatric age range, as part of studies to confirm this new indication. The relatively high renal clearance in neonates and children suggests an important role for renal tubular secretion and suggests mature activity already at birth. On the other hand, Ziemniak et al. [94] suggest that the unexplained gap between total and renal clearance in adults could be due to secondary metabolite formation in adults, which maybe missing in neonates due to immature metabolism. This is less likely, however, as the higher renal clearance was also observed in older children, whose drug metabolism is largely at adult levels. Moreover, in a rat study, bile duct ligation increased cimetidine renal tubular secretion by up-regulation of OCT2 (but not MATE1), supporting the hypothesis that the non-renal clearance in adults occurs through hepatic/bile excretion and is not related to unknown secondary metabolite formation [99]. Nevertheless, as analytical methods to measure drugs and metabolites have become more sensitive since the early studies in the mid-1980s, new studies in children of different ages could help elucidate why renal clearance of cimetidine differs between children and adults and give insight into the role of OCT2 in cimetidine disposition.
Tramadol-OCT1
Tramadol is a prodrug of the µ-opioid receptor agonist O-desmethyltramadol. It is metabolized mainly by CYP2D6 to its active O-desmethyltramadol metabolite [100]. The variation in tramadol pharmacokinetics cannot solely be explained by variation in CYP2D6, as was shown by Tzvetkov et al. [101] who showed an additive effect of OCT1 on tramadol disposition variation. Loss-of-function SLC22A1 polymorphisms have been related to higher plasma concentrations of the active O-desmethyltramadol and prolonged miosis, as a surrogate marker of the opioid effect. These effects are likely due to reduced OCT1-mediated hepatic uptake [101]. Allegaert and co-workers [102] showed that maturational clearance of tramadol, driven by CYP2D6 activity, is almost complete by 44 weeks post-menstrual age. In a pooled population pharmacogenetics–pharmacokinetic study covering the age range from preterm to elderly, only part of the variability in O-desmethyltramadol formation clearance could be explained by the CYP2D6 genotype, further supporting a potential role for SLC22A1 genetic variation [86]. A relationship between CYP2D6 genotype and tramadol metabolism was shown in young preterm infants, which is surprising as CYP2D6 is not fully mature at birth, especially not in preterm infants, and a genotype effect may have been obscured. It would be worthwhile, therefore, to study the impact of the SLC22A1 genotype in this young population [10]. In a population pharmacokinetic/pharmacodynamic analysis of 104 older children (2–8 years), age did not clearly contribute to variation in pharmacokinetics or the prediction of response [103].
Methotrexate-OATP1B1 and ABCC2
Methotrexate is a folic acid antagonist used to treat several forms of cancer and anti-inflammatory diseases. Methotrexate undergoes complex hepatic and intracellular metabolism [104]. Many membrane transporters are responsible for its uptake and excretion and for its metabolism to active polyglutamine metabolites and inactive 7-hydroxy-methotrexate [104, 105]. Methotrexate is eliminated primarily by renal excretion through glomerular filtration and renal tubular reabsorption and secretion. Approximately 70–90 % of a dose is excreted unchanged in urine. A small pharmacokinetic study showed only marginally lower methotrexate steady-state clearance (body surface area corrected), but increased renal toxicity, in 0- to 3-month-old infants than in 7- to 12-month-old infants [106, 107]. From 1 year of age onwards, methotrexate clearance (body surface area normalized) decreased linearly with age [107]. A 2014 review concluded that “although there is no pharmacogenetic marker for MTX [methotrexate] in use in the clinic at present, polymorphisms in SLCO1B1 have an important role in MTX pharmacokinetics and toxicity in pediatric ALL [acute lymphoblastic leukemia] patients and show the most consistent and promising results” [105]. For example, in a cohort of almost 500 pediatric acute lymphoblastic leukemia (ALL) patients, the methotrexate AUC from time zero to 48 h (AUC48) increased by 26 % (P < 0.001) per SLCO1B1 rs4149056 C allele and was a significant predictor of overall toxic adverse events during methotrexate courses (R2 = 0.043; P < 0.001), but no relationship was found for ABCC2 [107]. This study confirmed the results of a genome-wide association study (GWAS) in 434 ALL children, the first to identify SCLO1B1 genetic variation as an important marker of methotrexate pharmacokinetics and clinical response, and was recently validated by five different treatment regimens of high-dose methotrexate in ALL treatment protocols at St Jude Children’s Research Hospital (Memphis, TN, USA) [108]. Moreover, a deep sequencing approach for SLCO1B1 demonstrated that rare damaging variants contributed significantly to methotrexate clearance and had larger effect sizes than common SLCO1B1 variants [109, 110]. Other recent studies have detected a relationship between ABCC2 and methotrexate pharmacokinetics and toxicity. In 112 Han Chinese pediatric ALL patients, the ABCC2 –24T allele (rs717620) was associated with significantly higher methotrexate plasma concentrations at 48 h and with significant hematological and non-hematological toxicities. These findings are partially supported by other studies in 127 Lebanese and 151 Spanish pediatric ALL patients [111, 112].
Mycophenolate Mofetil-ABCC2
Mycophenolate mofetil is the prodrug of the active mycophenolic acid (MPA). It is metabolized by carboxylesterase 2 (CES2), after which MPA is further metabolized by several CYPs and UGTs [113]. MPA-glucuronide is excreted in the bile primarily by ABCC2 (encoded by ABCC2) and this transport is essential for enterohepatic circulation. The ABCC2 rs717620 A allele has been associated with reduced exposure to MPA in pediatric renal transplant recipients [114]. In a large multicenter cohort of pediatric heart transplant recipients, ABCC2 rs717620 A allele was also associated with more gastrointestinal intolerance, but with fewer short- and long-term rejection episodes [114]. As ABCC2 is thought to excrete MPA-glucuronide in the bile, carriers of the active A allele, may have increased enterohepatic circulation with an increased concentration of free MPA in the intestine. This is potentially associated with more gastrointestinal intolerance, but simultaneously with higher exposure and efficacy. As SNPs in UGT1A9, UGT2B7, SLCO1B3, and IMPDH have also been associated with altered MPA exposure, the combined effect of these SNPs and potentially interacting co-medication, may define high- and low-risk patients for MPA efficacy and toxicity. Full hepatic ABCC2 maturation appears to occur after infancy, suggesting a lower enterohepatic circulation of MPA, which may result in less gastrointestinal intolerance but potentially also with less efficacy in this age group [22]. This is merely a hypothesis without confirming pharmacokinetic data.
Acyclovir/Valacyclovir-OAT1 and OAT3
The oral bioavailability of the anti-viral agent acyclovir is poor, and therefore its prodrug valacyclovir was developed. A positive association was found between intestinal expression of 4F2hc (SLC3A2, amino acid transporter heavy chain, a membrane glycoprotein), and HPT1 (human oligopeptide transporter) and plasma levels of valacyclovir, but not peptide transporter 1 [PEPT1 (SLC15A1)] or any of the other investigated intestinal organic anion or cation transporters [115].
After hepatic metabolism, both drugs are mainly renally excreted by both glomerular filtration and renal tubular secretion, most likely by OAT1 and OAT3 [116, 117]. In preterm and term neonates (n = 28, median age 30 weeks of gestation), the intravenous acyclovir bodyweight-corrected clearance showed a twofold increase from 25 to 41 weeks of gestational age [118]. In children 1 month to 5 years old with or at risk for herpes infection, the CL/F of valacyclovir (mL/kg/min) in those younger than 3 months was 50 % of that in older children, in whom bodyweight-corrected clearance remained stable [119]. A recent study showed markedly increased acyclovir concentrations when co-administered with benzylpenicillin, which was shown to be due to OAT3 and possibly OAT1 inhibition [116]. This could be a very relevant interaction in septic newborns, who often receive both drugs, as increased acyclovir concentrations are associated with neurological adverse events as well as neutropenia.
Adefovir-OAT1 and ABCC4
Adefovir, the antiviral agent used for treatment of hepatitis B virus or HIV, is 45 % renally cleared through glomerular filtration and OAT1/ABCC4 renal tubular secretion [120, 121]. In 45 children in three age groups (2–6, 7–11, and 12–17 years) receiving oral treatment for hepatitis B virus with the prodrug adefovir dipivoxil, bodyweight-corrected mean apparent clearance and renal clearance were decreasing with increasing age [122]. Similarly, in a phase I study in children (n = 13, age range 6 months to 18 years) receiving oral adefovir dipivoxil for HIV treatment, systemic exposure was lower in children younger than 5 years [123].
Summary and Discussion
In summary, ex vivo, pharmacokinetic and pharmacogenetic studies suggest transporter-specific changes from the human fetus to the adult. At this time, data are very scarce and the impact of these changes on drug therapy in children is still largely unknown. However, despite data scarcity, our review may aid clinical pharmacologists and clinicians in rationale drug prescribing of the drugs presented, not only by showing how pharmacokinetics are probably similar in certain pediatric age groups compared to adults, but also by pointing out where potential age-related changes in individual transporters could impact the drug’s efficacy and safety. It broadens our views on ontogeny of transporters by evaluation of the results of pharmacokinetic and pharmacogenetic studies on relevant transporters. Moreover, this review presents clear information gaps, which may guide future research efforts to elucidate the role of human membrane transporters in the developing child.
For most drugs, the in vivo data to support the ex vivo data in understanding the maturation of individual transporters are limited to older children, and, hence, their usefulness is limited. No clear transporter maturation pattern can be deducted from any of the available pharmacokinetic studies in children. This contrasts with our knowledge from individual DMEs. For example, using midazolam as a phenotyping probe, the developmental expression of CYP3A4/5 from the preterm neonate to the adult has been extensively characterized [124, 125] as has amikacin clearance to display the maturation of GFR in neonates [125]. Specific phenotyping probes to study individual membrane transporters are suboptimal and have only been validated in adults. For many drugs, multiple transporters are involved in their uptake and excretion, which in turn may also compensate for changes in individual transporter activity. In adults, knowledge has also been gained from pharmacogenetic and drug–drug interaction studies.
This review shows that pharmacogenetic variation in membrane transporter activity also impact drug disposition, effect, and toxicity in children. Most pharmacogenetic studies in children are in line with adult data. However, these similar pharmacogenetic relationships should be interpreted with care, especially when it comes to translating these data across the whole pediatric age range. First, most pharmacogenetic study cohorts only contain older children, whereas pharmacokinetic studies in neonates and infants often show clear developmental changes up to 4–6 years of age. Hence, the impact of SNPs may be obscured by more prominent changes due to growth and development. Second, the relationship between SLCO1B1 SNPs and pravastatin disposition in adolescents was found to be the opposite of that in adults. If these results are validated in other studies, a potential impact of hormonal changes on individual transporters needs to be elucidated; this may also provide insight into the physiological role these transporters play during adolescence.
The limited data from ex vivo studies of postmortem or surgical samples support the notion of membrane transporter-specific maturational patterns. It is still difficult, however, to determine definitive patterns for the different transporters. One of the reasons for this is that most studies only used limited samples and limited age ranges. Most fetal and neonatal studies only applied immunohistochemistry or mRNA techniques, while the more quantitative protein expression data are mainly from older children, above the age range in which most developmental changes can be expected. In addition, the quality and interpretation is further challenged as the exact origin, handling, and storage of tissues, including detailed patient characteristics and exact procurement site from organ (e.g., where in the intestine?), is often unknown.
The mechanisms underlying maturational changes in transporters are largely unknown. Recent studies on CYP3A4 show maturational changes in methylation patterns to mirror the maturational expression of CYP3A4, which may point towards similar mechanisms for the transporters [126].
Differences between ethnic groups in DME abundance or ethnicity have been described, even in newborn infants [77, 85, 127]. Like in DMEs, it is credible to believe that ethnicity might have an effect on transporter activity or abundance. Nevertheless, for 27 drug transporters in 95 pathologically normal kidney samples, the expression did not differ between European Americans or African Americans [128].
The interpretation of pharmacokinetic studies, to understand maturation of transporters, is complicated by the fact that these transporters are part of the larger system of the ADME processes involved in the disposition of drugs. In contrast to DMEs, where clearance to a specific metabolite can be estimated to understand the DME maturation, studying a single transporter is more difficult. One may not be able to pinpoint one specific membrane transporter involved, and if the dominant transporter is still immature early in life, other transporters may compensate, thereby obscuring individual transporter maturation.
Future Directions
Several approaches are needed to increase our understanding of membrane transporters in the fetus and child (Table 3). First, the impact of transporter maturation on efficacy and toxicity in daily clinical care needs to be elucidated. Pharmacogenetic studies can be a powerful tool to this aim, provided they have adequate power and validation cohorts, the lack of which is a major limitation of currently published studies. Studies need to not only be powered to study the impact of a single SNP in one transporter gene, but at the least, the interaction with age should be part of their designs. Preferably such studies should be designed to study the disposition of a drug in the context of systems pharmacology, also including SNPs in other relevant pharmacokinetic and/or pharmacodynamic genes, as well as pharmacokinetic sampling enabling the separation of different excretion pathways (e.g., GFR vs. tubular secretion vs. bile secretion vs. metabolism). In addition to pharmacogenetic studies, well-designed studies reflecting clinical drug–drug interaction scenarios may improve our understanding of transporter maturation, such as pharmacokinetic studies in which patients’ samples are taken before/during/after co-medication with a potentially interacting drug. For example, the carvedilol–digoxin study [39] or the older cimetidine studies in neonates in which renal clearance by glomerular filtration could be separated from renal tubular secretion [94], have, by their design, provided support for age-related differences in specific renal transporters.
Table 3.
Approaches to future transporter studies
| Ex vivo research Build multidisciplinary research teams for tissue collection and study design, e.g., surgeons, pathologists, clinical study staff, basic researchers Use optimal age distribution, e.g., tissues samples from fetuses, neonates, and young infants, where most developmental changes can be expected, as well as sample number to ensure adequate power to detect age-related changes Establish high-quality tissue collections with detailed tissue handling description and detailed description of patient characteristics Use protein quantification techniques (e.g., LC–MS) and develop tools to study activity with minimal amounts of human tissue |
| PK studies Phenotyping studies with drugs that are clinically used, and consider microdosing studies Blood sampling at similar times as clinical blood draws (opportunistic sampling) Perform population PK analyses Design drug–drug interaction studies |
| PGx studies In pediatric populations, test genetic variants in transporters known to affect PKs or PDs in adults Take into account PGx variation in affected drug-metabolizing enzymes as added variants Include relevant age range: e.g., younger children and neonates Design adequately powered studies |
LC–MS liquid chromatography–mass spectrometry, PD pharmacodynamic, PGx pharmacogenetic, PK pharmacokinetic
Ethical challenges have limited studies in infants and neonates. However, many drugs in our review are regularly prescribed, even for these young children. Therefore, opportunistic sampling or biobanking of left-over samples from children who take these drugs for therapeutic reasons may aid overcoming these ethical barriers.
At this time, endogenous markers to phenotype the activity of individual transporters are lacking. With the increased availability of metabolomics, specific metabolites or metabolite ratios may be identified to reflect transporter activity in vivo. A recent GWAS–metabolomics study detected specific metabolites/metabolite ratios for selected transporters such as OCT1 [129–133]. The feasibility of this approach was recently shown for CY2D6 phenotyping in children [129]. To design drug-dosing regimens in children, population PBPK models are increasingly being used [134]. Modeling and simulation can be used in different ways to increase our understanding and to design dosing regimens. Using a systems approach, modeling of the disposition of a substrate model drug can result in a mathematical description of the maturation of the specific transporter. This maturational description can then be used to simulate dosing guidelines for other transporter substrates. The feasibility of this approach has been used to describe maturation of selected DMEs and GFR clearance [133, 135]. Secondly, PBPK modeling, which incorporates available drug property and physiological information, could be used to simulate the impact of maturation of specific transporters, preferably with actual ex vivo data on transporter expression/activity [134]. An example is a mechanistic PBPK model to predict morphine levels in breast-fed neonates of codeine-treated mothers [136]. A major limitation of these models is the lack of high quality ex vivo data on transporter activity across the pediatric age range and the lack of in vivo validation of these models.
In the design of new studies, the following issues should be considered. The collection of these data can only be achieved by an international effort to collect high-quality tissue in collaboration with surgeons, pathologists, ethicists, clinical researchers, and experts in drug transporter research. The limitations of current tissue collections have been described here and good protocols for tissue collection, preferably in the context of internationally accessible biobanks, should be developed. Newer laboratory techniques should be strongly considered to minimize tissue amounts needed, for example, for laser capture and LC–MS/MS to determine protein abundance. Moreover, a multi-omics approach, including not only genomics but also transcriptomics, proteomics, metabolomics, and microbiomics, may provide greater power to predict drug efficacy and adverse drug reactions [137, 138]. Also, with the fast developments in tissue engineering, the current ethical and practical issues regarding tissue sampling and storage could be overcome using pediatric-engineered tissues. This may even enable transporter activity studies, which are now not available in pediatric tissue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank J. Hagoort for editorial assistance.
Compliance with Ethical Standards
This work was supported in part by the Netherlands Organisation for Health Research and Development (ZonMW SNW Clinical Fellowship, grant 90700304), the German Federal Ministry of Education and Research (Virtual Liver Network grant 2318 0315755), the Robert Bosch Stiftung, Stuttgart, Germany, and the ICEPHA (Interfacultary Centre for Pharmacogenomics and Pharma Research) Graduate School Tübingen-Stuttgart, Germany. MGM, ATN, CAJK, ES, DT, MS and SNW have no conflict of interest.
Contributor Information
Miriam G. Mooij, Email: m.mooij@erasmusmc.nl
Anne T. Nies, Email: anne.nies@ikp-stuttgart.de
Catherijne A. J. Knibbe, Email: c.knibbe@antoniusziekenhuis.nl
Elke Schaeffeler, Email: elke.schaeffeler@ikp-stuttgart.de.
Dick Tibboel, Email: d.tibboel@erasmusmc.nl.
Matthias Schwab, Email: matthias.schwab@ikp-stuttgart.de.
Saskia N. de Wildt, Phone: +31 10 70 36 889, Email: s.dewildt@erasmusmc.nl
References
- 1.International Transporter Consortium. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kell DB, Dobson PD, Oliver SG. Pharmaceutical drug transport: the issues and the implications that it is essentially carrier-mediated only. Drug Discov Today. 2011;16(15–16):704–714. doi: 10.1016/j.drudis.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Moitra K, Dean M. Evolution of ABC transporters by gene duplication and their role in human disease. Biol Chem. 2011;392(1–2):29–37. doi: 10.1515/BC.2011.006. [DOI] [PubMed] [Google Scholar]
- 4.Hediger MA, Clemencon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med. 2013;34(2–3):95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeGorter MK, Xia CQ, Yang JJ, Kim RB. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
- 6.Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65(3):944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 7.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–167. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 8.Emami Riedmaier A, Nies AT, Schaeffeler E, Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacol Rev. 2012;64(3):421–449. doi: 10.1124/pr.111.004614. [DOI] [PubMed] [Google Scholar]
- 9.Silverton L, Dean M, Moitra K. Variation and evolution of the ABC transporter genes ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8: implication for pharmacogenetics and disease. Drug Metabol Drug Interact. 2011;26(4):169–179. doi: 10.1515/DMDI.2011.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45(9):931–956. doi: 10.2165/00003088-200645090-00005. [DOI] [PubMed] [Google Scholar]
- 12.Parrott N, Davies B, Hoffmann G, Koerner A, Lave T, Prinssen E, et al. Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in neonates and infants. Clin Pharmacokinet. 2011;50(9):613–623. doi: 10.2165/11592640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452(1–2):3–7. doi: 10.1016/j.ijpharm.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 14.van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handb Exp Pharmacol. 2011;205:51–75. doi: 10.1007/978-3-642-20195-0_2. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, et al. Human ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther. 2015;98(3):266–287. doi: 10.1002/cpt.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Kalken CK, Giaccone G, van der Valk P, Kuiper CM, Hadisaputro MM, Bosma SA, et al. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol. 1992;141(5):1063–1072. [PMC free article] [PubMed] [Google Scholar]
- 17.Konieczna A, Erdosova B, Lichnovska R, Jandl M, Cizkova K, Ehrmann J. Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J Mol Histol. 2011;42(6):567–574. doi: 10.1007/s10735-011-9363-1. [DOI] [PubMed] [Google Scholar]
- 18.Fakhoury M, de Beaumais T, Guimiot F, Azougagh S, Elie V, Medard Y, et al. mRNA expression of MDR1 and major metabolising enzymes in human fetal tissues. Drug Metab Pharmacokinet. 2009;24(6):529–536. doi: 10.2133/dmpk.24.529. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Ellis EC, Gramignoli R, Dorko K, Tahan V, Hansel M, et al. Hepatobiliary disposition of 17-OHPC and taurocholate in fetal human hepatocytes: a comparison with adult human hepatocytes. Drug Metab Dispos. 2013;41(2):296–304. doi: 10.1124/dmd.112.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakhoury M, Litalien C, Medard Y, Cave H, Ezzahir N, Peuchmaur M, et al. Localization and mRNA expression of CYP3A and P-glycoprotein in human duodenum as a function of age. Drug Metab Dispos. 2005;33(11):1603–1607. doi: 10.1124/dmd.105.005611. [DOI] [PubMed] [Google Scholar]
- 21.Miki Y, Suzuki T, Tazawa C, Blumberg B, Sasano H. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol. 2005;231(1–2):75–85. doi: 10.1016/j.mce.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Mooij MG, Schwarz UI, De Koning BA, Leeder JS, Gaedigk R, Samsom JN, et al. Ontogeny of human hepatic and intestinal transporter expression during childhood: age matters. Drug Metab Dispos. 2014;42(8):1268–1274. doi: 10.1124/dmd.114.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HL, Chen HL, Liu YJ, Feng CH, Wu CY, Shyu MK, et al. Developmental expression of canalicular transporter genes in human liver. J Hepatol. 2005;43(3):472–477. doi: 10.1016/j.jhep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno T, Fukuda T, Masuda S, Uemoto S, Matsubara K, Inui KI, et al. Developmental trajectory of intestinal MDR1/ABCB1 mRNA expression in children. Br J Clin Pharmacol. 2014;77(5):910–912. doi: 10.1111/bcp.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad B, Evers R, Gupta A, Hop CE, Salphati L, Shukla S, et al. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42(1):78–88. doi: 10.1124/dmd.113.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad B, Lai Y, Lin Y, Unadkat JD. Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci. 2013;102(3):787–793. doi: 10.1002/jps.23436. [DOI] [PubMed] [Google Scholar]
- 27.Deo AK, Prasad B, Balogh L, Lai Y, Unadkat JD. Interindividual variability in hepatic expression of the multidrug resistance-associated protein 2 (MRP2/ABCC2): quantification by liquid chromatography/tandem mass spectrometry. Drug Metab Dispos. 2012;40(5):852–855. doi: 10.1124/dmd.111.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics. 2008;39(4):211–218. doi: 10.1055/s-0028-1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam J, Baello S, Igbal M, Kelly LE, Shannon PT, Chitayat D, Mattheyws SG, Koren G. The ontogeny of P-glycoprotein in the developing human blood-brain barrier: implication for opioid toxicity in neonates. Pediatr Res. 2015 doi: 10.1038/pr.2015.119. [DOI] [PubMed] [Google Scholar]
- 30.Thomson MMS, Krauel S, Hines RN, Scheutz EG, Meibohm B. Lack of effect of genetic variants on the age-associated protein expression of OATP1B1 and OATP1B3 in human pediatric liver [abstract]. 114th American Society for Clinical Pharmacology and Therapeutics Annual Meeting, Indianapolis, 5–9 Mar 2013.
- 31.Yanin SB, Smith PB, Benjamin DK, Jr, Augustijns PF, Thakker DR, Annaert PP. Higher clearance of micafungin in neonates compared with adults: role of age-dependent micafungin serum binding. Biopharm Drug Dispos. 2011;32(4):222–232. doi: 10.1002/bdd.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 33.Wolking S, Schaeffeler E, Lerche H, Schwab M, Nies AT. Impact of genetic polymorphisms of ABCB1 (MDR1, P-glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin Pharmacokinet. 2015;54(7):709–735. doi: 10.1007/s40262-015-0267-1. [DOI] [PubMed] [Google Scholar]
- 34.Damme K, Nies AT, Schaeffeler E, Schwab M. Mammalian MATE (SLC47A) transport proteins: impact on efflux of endogenous substrates and xenobiotics. Drug Metab Rev. 2011;43(4):499–523. doi: 10.3109/03602532.2011.602687. [DOI] [PubMed] [Google Scholar]
- 35.Sumner DJ, Russell AJ. Digoxin pharmacokinetics: multicompartmental analysis and its clinical implications. Br J Clin Pharmacol. 1976;3(2):221–229. doi: 10.1111/j.1365-2125.1976.tb00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yukawa E, Akiyama K, Suematsu F, Yukawa M, Minemoto M. Population pharmacokinetic investigation of digoxin in Japanese neonates. J Clin Pharm Ther. 2007;32(4):381–386. doi: 10.1111/j.1365-2710.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 37.Yukawa M, Yukawa E, Suematsu F, Takiguchi T, Ikeda H, Aki H, et al. Population pharmacokinetic investigation of digoxin in Japanese infants and young children. J Clin Pharmacol. 2011;51(6):857–863. doi: 10.1177/0091270010374475. [DOI] [PubMed] [Google Scholar]
- 38.Hastreiter AR, van der Horst RL, Voda C, Chow-Tung E. Maintenance digoxin dosage and steady-state plasma concentration in infants and children. J Pediatr. 1985;107(1):140–146. doi: 10.1016/S0022-3476(85)80636-3. [DOI] [PubMed] [Google Scholar]
- 39.Ratnapalan S, Griffiths K, Costei AM, Benson L, Koren G. Digoxin-carvedilol interactions in children. J Pediatr. 2003;142(5):572–574. doi: 10.1067/mpd.2003.160. [DOI] [PubMed] [Google Scholar]
- 40.Pinto N, Halachmi N, Verjee Z, Woodland C, Klein J, Koren G. Ontogeny of renal P-glycoprotein expression in mice: correlation with digoxin renal clearance. Pediatr Res. 2005;58(6):1284–1289. doi: 10.1203/01.pdr.0000188697.99079.27. [DOI] [PubMed] [Google Scholar]
- 41.Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27(2–3):201–214. doi: 10.1016/S0169-409X(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 42.de Wildt SN, van Schaik RH, Soldin OP, Soldin SJ, Brojeni PY, van der Heiden IP, et al. The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol. 2011;67(12):1231–1241. doi: 10.1007/s00228-011-1083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, et al. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30(12):1352–1359. doi: 10.1016/j.healun.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyhlidal CA, Pearce RE, Gaedigk R, Calamia JC, Shuster DL, Thummel KE, et al. Variability in expression of CYP3A5 in human fetal liver. Drug Metab Dispos. 2015;43(8):1286–1293. doi: 10.1124/dmd.115.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53(2):123–139. doi: 10.1007/s40262-013-0120-3. [DOI] [PubMed] [Google Scholar]
- 46.Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC. Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol. 2009;68(3):413–421. doi: 10.1111/j.1365-2125.2009.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guy-Viterbo V, Baudet H, Elens L, Haufroid V, Lacaille F, Girard M, et al. Influence of donor-recipient CYP3A4/5 genotypes, age and fluconazole on tacrolimus pharmacokinetics in pediatric liver transplantation: a population approach. Pharmacogenomics. 2014;15(9):1207–1221. doi: 10.2217/pgs.14.75. [DOI] [PubMed] [Google Scholar]
- 48.Fukudo M, Yano I, Masuda S, Goto M, Uesugi M, Katsura T, et al. Population pharmacokinetic and pharmacogenomic analysis of tacrolimus in pediatric living-donor liver transplant recipients. Clin Pharmacol Ther. 2006;80(4):331–345. doi: 10.1016/j.clpt.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Lemaire S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Modulation of the cellular accumulation and intracellular activity of daptomycin towards phagocytized Staphylococcus aureus by the P-glycoprotein (MDR1) efflux transporter in human THP-1 macrophages and madin-darby canine kidney cells. Antimicrob Agents Chemother. 2007;51(8):2748–2757. doi: 10.1128/AAC.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baietto L, D’Avolio A, Cusato J, Pace S, Calcagno A, Motta I, et al. Effect of SNPs in human ABCB1 on daptomycin pharmacokinetics in Caucasian patients. J Antimicrob Chemother. 2015;70(1):307–308. doi: 10.1093/jac/dku368. [DOI] [PubMed] [Google Scholar]
- 51.Bradley JS, Benziger D, Bokesch P, Jacobs R. Single-dose pharmacokinetics of daptomycin in pediatric patients 3–24 months of age. Pediatr Infect Dis J. 2014;33(9):936–939. doi: 10.1097/INF.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 52.Woodworth JR, Nyhart EH, Jr, Brier GL, Wolny JD, Black HR. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob Agents Chemother. 1992;36(2):318–325. doi: 10.1128/AAC.36.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen-Wolkowiez M, Watt KM, Hornik CP, Benjamin DK, Jr, Smith PB. Pharmacokinetics and tolerability of single-dose daptomycin in young infants. Pediatr Infect Dis J. 2012;31(9):935–937. doi: 10.1097/INF.0b013e31825d2fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27(8):866–871. [PubMed] [Google Scholar]
- 55.Kusuhara H, Miura M, Yasui-Furukori N, Yoshida K, Akamine Y, Yokochi M, et al. Effect of coadministration of single and multiple doses of rifampicin on the pharmacokinetics of fexofenadine enantiomers in healthy subjects. Drug Metab Dispos. 2013;41(1):206–213. doi: 10.1124/dmd.112.048330. [DOI] [PubMed] [Google Scholar]
- 56.Akamine Y, Miura M, Sunagawa S, Kagaya H, Yasui-Furukori N, Uno T. Influence of drug-transporter polymorphisms on the pharmacokinetics of fexofenadine enantiomers. Xenobiotica. 2010;40(11):782–789. doi: 10.3109/00498254.2010.515318. [DOI] [PubMed] [Google Scholar]
- 57.Imanaga J, Kotegawa T, Imai H, Tsutsumi K, Yoshizato T, Ohyama T, et al. The effects of the SLCO2B1 c.1457C>T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics. 2011;21(2):84–93. doi: 10.1097/FPC.0b013e32834300cc. [DOI] [PubMed] [Google Scholar]
- 58.Martinez JM, Khier S, Morita S, Rauch C, Fabre D. Population pharmacokinetic analysis of fexofenadine in Japanese pediatric patients. J Pharmacokinet Pharmacodyn. 2014;41(2):187–195. doi: 10.1007/s10928-014-9356-2. [DOI] [PubMed] [Google Scholar]
- 59.Krishna R, Krishnaswami S, Kittner B, Sankoh AJ, Jensen BK. The utility of mixed-effects covariate analysis in rapid selection of doses in pediatric subjects: a case study with fexofenadine hydrochloride. Biopharm Drug Dispos. 2004;25(9):373–387. doi: 10.1002/bdd.425. [DOI] [PubMed] [Google Scholar]
- 60.Thorn CF, Klein TE, Altman RB. Codeine and morphine pathway. Pharmacogenet Genomics. 2009;19(7):556–558. doi: 10.1097/FPC.0b013e32832e0eac. [DOI] [PubMed] [Google Scholar]
- 61.Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC, Brockmoller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem Pharmacol. 2013;86(5):666–678. doi: 10.1016/j.bcp.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clin Pharmacol Ther. 2003;74(6):543–554. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83(4):559–566. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- 64.Knibbe CA, Krekels EH, van den Anker JN, DeJongh J, Santen GW, van Dijk M, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet. 2009;48(6):371–385. doi: 10.2165/00003088-200948060-00003. [DOI] [PubMed] [Google Scholar]
- 65.Krekels EH, Tibboel D, de Wildt SN, Ceelie I, Dahan A, van Dijk M, et al. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014;53(6):553–563. doi: 10.1007/s40262-014-0135-4. [DOI] [PubMed] [Google Scholar]
- 66.Sadhasivam S, Chidambaran V, Zhang X, Meller J, Esslinger H, Zhang K, et al. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharmacogenomics J. 2015;15(2):119–126. doi: 10.1038/tpj.2014.56. [DOI] [PubMed] [Google Scholar]
- 67.Biesiada J, Chidambaran V, Wagner M, Zhang X, Martin LJ, Meller J, et al. Genetic risk signatures of opioid-induced respiratory depression following pediatric tonsillectomy. Pharmacogenomics. 2014;15(14):1749–1762. doi: 10.2217/pgs.14.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venkatasubramanian R, Fukuda T, Niu J, Mizuno T, Chidambaran V, Vinks AA, et al. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics. 2014;15(10):1297–1309. doi: 10.2217/pgs.14.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17(8):647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14(7):429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 72.Hedman M, Antikainen M, Holmberg C, Neuvonen M, Eichelbaum M, Kivisto KT, et al. Pharmacokinetics and response to pravastatin in paediatric patients with familial hypercholesterolaemia and in paediatric cardiac transplant recipients in relation to polymorphisms of the SLCO1B1 and ABCB1 genes. Br J Clin Pharmacol. 2006;61(6):706–715. doi: 10.1111/j.1365-2125.2006.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hedman M, Neuvonen PJ, Neuvonen M, Antikainen M. Pharmacokinetics and pharmacodynamics of pravastatin in children with familial hypercholesterolemia. Clin Pharmacol Ther. 2003;74(2):178–185. doi: 10.1016/S0009-9236(03)00153-X. [DOI] [PubMed] [Google Scholar]
- 74.Knebel W, Gastonguay MR, Malhotra B, El-Tahtawy A, Jen F, Gandelman K. Population pharmacokinetics of atorvastatin and its active metabolites in children and adolescents with heterozygous familial hypercholesterolemia: selective use of informative prior distributions from adults. J Clin Pharmacol. 2013;53(5):505–516. doi: 10.1002/jcph.66. [DOI] [PubMed] [Google Scholar]
- 75.Nies AT, Niemi M, Burk O, Winter S, Zanger UM, Stieger B, et al. Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. Genome Med. 2013;5(1):1. doi: 10.1186/gm405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Treiber A, Schneiter R, Hausler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007;35(8):1400–1407. doi: 10.1124/dmd.106.013615. [DOI] [PubMed] [Google Scholar]
- 77.Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, et al. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther. 2004;308(3):965–974. doi: 10.1124/jpet.103.060137. [DOI] [PubMed] [Google Scholar]
- 78.Treluyer JM, Gueret G, Cheron G, Sonnier M, Cresteil T. Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: in vivo/in vitro correlation and inducibility. Pharmacogenetics. 1997;7(6):441–452. doi: 10.1097/00008571-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Barst RJ, Ivy D, Dingemanse J, Widlitz A, Schmitt K, Doran A, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2003;73(4):372–382. doi: 10.1016/S0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 80.Beghetti M, Haworth SG, Bonnet D, Barst RJ, Acar P, Fraisse A, et al. Pharmacokinetic and clinical profile of a novel formulation of bosentan in children with pulmonary arterial hypertension: the FUTURE-1 study. Br J Clin Pharmacol. 2009;68(6):948–955. doi: 10.1111/j.1365-2125.2009.03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taguchi M, Ichida F, Hirono K, Miyawaki T, Yoshimura N, Nakamura T, et al. Pharmacokinetics of bosentan in routinely treated Japanese pediatric patients with pulmonary arterial hypertension. Drug Metab Pharmacokinet. 2011;26(3):280–287. doi: 10.2133/dmpk.DMPK-10-RG-113. [DOI] [PubMed] [Google Scholar]
- 82.Tzvetkov MV, Saadatmand AR, Bokelmann K, Meineke I, Kaiser R, Brockmoller J. Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT(3) antagonists tropisetron and ondansetron. Pharmacogenomics J. 2012;12(1):22–29. doi: 10.1038/tpj.2010.75. [DOI] [PubMed] [Google Scholar]
- 83.Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs) Toxicol Appl Pharmacol. 2013;273(1):100–109. doi: 10.1016/j.taap.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson TN, Tucker GT, Rostami-Hodjegan A. Development of CYP2D6 and CYP3A4 in the first year of life. Clin Pharmacol Ther. 2008;83(5):670–671. doi: 10.1038/sj.clpt.6100327. [DOI] [PubMed] [Google Scholar]
- 85.Stevens JC, Marsh SA, Zaya MJ, Regina KJ, Divakaran K, Le M, et al. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36(8):1587–1593. doi: 10.1124/dmd.108.021873. [DOI] [PubMed] [Google Scholar]
- 86.Allegaert K, Holford N, Anderson BJ, Holford S, Stuber F, Rochette A, et al. Tramadol and O-desmethyl tramadol clearance maturation and disposition in humans: a pooled pharmacokinetic study. Clin Pharmacokinet. 2015;54(2):167–178. doi: 10.1007/s40262-014-0191-9. [DOI] [PubMed] [Google Scholar]
- 87.O’Brien VP, Bokelmann K, Ramirez J, Jobst K, Ratain MJ, Brockmoller J, et al. Hepatocyte nuclear factor 1 regulates the expression of the organic cation transporter 1 via binding to an evolutionary conserved region in intron 1 of the OCT1 gene. J Pharmacol Exp Ther. 2013;347(1):181–192. doi: 10.1124/jpet.113.206359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goswami S, Yee SW, Stocker S, Mosley JD, Kubo M, Castro R, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2014;96(3):370–379. doi: 10.1038/clpt.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genomics. 2010;20(1):38–44. doi: 10.1097/FPC.0b013e328333bb11. [DOI] [PubMed] [Google Scholar]
- 90.Diaz M, Lopez-Bermejo A, Sanchez-Infantes D, Bassols J, de Zegher F, Ibanez L. Responsiveness to metformin in girls with androgen excess: collective influence of genetic polymorphisms. Fertil Steril. 2011;96(1):208–213.e2. doi: 10.1016/j.fertnstert.2011.04.075. [DOI] [PubMed] [Google Scholar]
- 91.Sanchez-Infantes D, Diaz M, Lopez-Bermejo A, Marcos MV, de Zegher F, Ibanez L. Pharmacokinetics of metformin in girls aged 9 years. Clin Pharmacokinet. 2011;50(11):735–738. doi: 10.2165/11593970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 92.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Somogyi A, Becker M, Gugler R. Cimetidine pharmacokinetics and dosage requirements in children. Eur J Pediatr. 1985;144(1):72–76. doi: 10.1007/BF00491931. [DOI] [PubMed] [Google Scholar]
- 94.Ziemniak JA, Wynn RJ, Aranda JV, Zarowitz BJ, Schentag JJ. The pharmacokinetics and metabolism of cimetidine in neonates. Dev Pharmacol Ther. 1984;7(1):30–38. doi: 10.1159/000457141. [DOI] [PubMed] [Google Scholar]
- 95.Lloyd CW, Martin WJ, Taylor BD, Hauser AR. Pharmacokinetics and pharmacodynamics of cimetidine and metabolites in critically ill children. J Pediatr. 1985;107(2):295–300. doi: 10.1016/S0022-3476(85)80155-4. [DOI] [PubMed] [Google Scholar]
- 96.Ohta KY, Inoue K, Yasujima T, Ishimaru M, Yuasa H. Functional characteristics of two human MATE transporters: kinetics of cimetidine transport and profiles of inhibition by various compounds. J Pharm Sci. 2009;12(3):388–396. doi: 10.18433/j3r59x. [DOI] [PubMed] [Google Scholar]
- 97.Burckhardt BC, Brai S, Wallis S, Krick W, Wolff NA, Burckhardt G. Transport of cimetidine by flounder and human renal organic anion transporter 1. Am J Physiol Renal Physiol. 2003;284(3):F503–F509. doi: 10.1152/ajprenal.00290.2002. [DOI] [PubMed] [Google Scholar]
- 98.Sprowl JA, van Doorn L, Hu S, van Gerven L, de Bruijn P, Li L, et al. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther. 2013;94(5):585–592. doi: 10.1038/clpt.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurata T, Muraki Y, Mizutani H, Iwamoto T, Okuda M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab Pharmacokinet. 2010;25(4):328–334. doi: 10.2133/dmpk.DMPK-10-RG-004. [DOI] [PubMed] [Google Scholar]
- 100.Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374–380. doi: 10.1097/FPC.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzvetkov MV, Saadatmand AR, Lotsch J, Tegeder I, Stingl JC, Brockmoller J. Genetically polymorphic OCT1: another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin Pharmacol Ther. 2011;90(1):143–150. doi: 10.1038/clpt.2011.56. [DOI] [PubMed] [Google Scholar]
- 102.Allegaert K, Anderson BJ, Verbesselt R, Debeer A, de Hoon J, Devlieger H, et al. Tramadol disposition in the very young: an attempt to assess in vivo cytochrome P-450 2D6 activity. Br J Anaesth. 2005;95(2):231–239. doi: 10.1093/bja/aei170. [DOI] [PubMed] [Google Scholar]
- 103.Garrido MJ, Habre W, Rombout F, Troconiz IF. Population pharmacokinetic/pharmacodynamic modelling of the analgesic effects of tramadol in pediatrics. Pharm Res. 2006;23(9):2014–2023. doi: 10.1007/s11095-006-9049-7. [DOI] [PubMed] [Google Scholar]
- 104.Mikkelsen TS, Thorn CF, Yang JJ, Ulrich CM, French D, Zaza G, et al. PharmGKB summary: methotrexate pathway. Pharmacogenet Genomics. 2011;21(10):679–686. doi: 10.1097/FPC.0b013e328343dd93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopez-Lopez E, Gutierrez-Camino A, Bilbao-Aldaiturriaga N, Pombar-Gomez M, Martin-Guerrero I, Garcia-Orad A. Pharmacogenetics of childhood acute lymphoblastic leukemia. Pharmacogenomics. 2014;15(10):1383–1398. doi: 10.2217/pgs.14.106. [DOI] [PubMed] [Google Scholar]
- 106.Thompson PA, Murry DJ, Rosner GL, Lunagomez S, Blaney SM, Berg SL, et al. Methotrexate pharmacokinetics in infants with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;59(6):847–853. doi: 10.1007/s00280-006-0388-1. [DOI] [PubMed] [Google Scholar]
- 107.Radtke S, Zolk O, Renner B, Paulides M, Zimmermann M, Moricke A, et al. Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood. 2013;121(26):5145–5153. doi: 10.1182/blood-2013-01-480335. [DOI] [PubMed] [Google Scholar]
- 108.Ramsey LB, Panetta JC, Smith C, Yang W, Fan Y, Winick NJ, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121(6):898–904. doi: 10.1182/blood-2012-08-452839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramsey LB, Bruun GH, Yang W, Trevino LR, Vattathil S, Scheet P, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22(1):1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trevino LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27(35):5972–5978. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zgheib NK, Akra-Ismail M, Aridi C, Mahfouz R, Abboud MR, Solh H, et al. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2014;24(8):387–396. doi: 10.1097/FPC.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 112.Lopez-Lopez E, Ballesteros J, Pinan MA, Sanchez de Toledo J, Garcia de Andoin N, Garcia-Miguel P, et al. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Genomics. 2013;23(2):53–61. doi: 10.1097/FPC.0b013e32835c3b24. [DOI] [PubMed] [Google Scholar]
- 113.Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE. PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics. 2014;24(1):73–79. doi: 10.1097/FPC.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fukuda T, Goebel J, Cox S, Maseck D, Zhang K, Sherbotie JR, et al. UGT1A9, UGT2B7, and MRP2 genotypes can predict mycophenolic acid pharmacokinetic variability in pediatric kidney transplant recipients. Ther Drug Monit. 2012;34(6):671–679. doi: 10.1097/FTD.0b013e3182708f84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Landowski CP, Sun D, Foster DR, Menon SS, Barnett JL, Welage LS, et al. Gene expression in the human intestine and correlation with oral valacyclovir pharmacokinetic parameters. J Pharmacol Exp Ther. 2003;306(2):778–786. doi: 10.1124/jpet.103.051011. [DOI] [PubMed] [Google Scholar]
- 116.Ye J, Liu Q, Wang C, Meng Q, Sun H, Peng J, et al. Benzylpenicillin inhibits the renal excretion of acyclovir by OAT1 and OAT3. Pharmacol Rep. 2013;65(2):505–512. doi: 10.1016/S1734-1140(13)71026-0. [DOI] [PubMed] [Google Scholar]
- 117.Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem. 2011;286(1):243–251. doi: 10.1074/jbc.M110.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sampson MR, Bloom BT, Lenfestey RW, Harper B, Kashuba AD, Anand R, et al. Population pharmacokinetics of intravenous acyclovir in preterm and term infants. Pediatr Infect Dis J. 2014;33(1):42–49. doi: 10.1097/01.inf.0000435509.75114.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimberlin DW, Jacobs RF, Weller S, van der Walt JS, Heitman CK, Man CY, et al. Pharmacokinetics and safety of extemporaneously compounded valacyclovir oral suspension in pediatric patients from 1 month through 11 years of age. Clin Infect Dis. 2010;50(2):221–228. doi: 10.1086/649212. [DOI] [PubMed] [Google Scholar]
- 120.Maeda K, Tian Y, Fujita T, Ikeda Y, Kumagai Y, Kondo T, et al. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur J Pharm Sci. 2014;1(59):94–103. doi: 10.1016/j.ejps.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 121.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71(2):619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- 122.Sokal EM, Kelly D, Wirth S, Mizerski J, Dhawan A, Frederick D. The pharmacokinetics and safety of adefovir dipivoxil in children and adolescents with chronic hepatitis B virus infection. J Clin Pharmacol. 2008;48(4):512–517. doi: 10.1177/0091270007313325. [DOI] [PubMed] [Google Scholar]
- 123.Hughes WT, Shenep JL, Rodman JH, Fridland A, Willoughby R, Blanchard S, et al. Single-dose pharmacokinetics and safety of the oral antiviral compound adefovir dipivoxil in children infected with human immunodeficiency virus type 1. The Pediatrics AIDS Clinical Trials Group. Antimicrob Agents Chemother. 2000;44(4):1041–1046. doi: 10.1128/AAC.44.4.1041-1046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ince I, Knibbe CA, Danhof M, de Wildt SN. Developmental changes in the expression and function of cytochrome P450 3A isoforms: evidence from in vitro and in vivo investigations. Clin Pharmacokinet. 2013;52(5):333–345. doi: 10.1007/s40262-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 125.De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, et al. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet. 2012;51(2):105–117. doi: 10.2165/11595640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 126.Kacevska M, Ivanov M, Wyss A, Kasela S, Milani L, Rane A, et al. DNA methylation dynamics in the hepatic CYP3A4 gene promoter. Biochimie. 2012;94(11):2338–2344. doi: 10.1016/j.biochi.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 127.Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E. Human hepatic CYP2B6 developmental expression: the impact of age and genotype. Biochem Pharmacol. 2009;78(2):184–190. doi: 10.1016/j.bcp.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 128.Joseph S, Nicolson TJ, Hammons G, Word B, Green-Knox B, Lyn-Cook B. Expression of drug transporters in human kidney: impact of sex, age, and ethnicity. Biol Sex Differ. 2015;6:4. doi: 10.1186/s13293-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tay-Sontheimer J, Shireman LM, Beyer RP, Senn T, Witten D, Pearce RE, et al. Detection of an endogenous urinary biomarker associated with CYP2D6 activity using global metabolomics. Pharmacogenomics. 2014;15(16):1947–1962. doi: 10.2217/pgs.14.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Admiraal R, van Kesteren C, Boelens JJ, Bredius RG, Tibboel D, Knibbe CA. Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child. 2014;99(3):267–272. doi: 10.1136/archdischild-2013-303721. [DOI] [PubMed] [Google Scholar]
- 131.Knibbe CA, Krekels EH, Danhof M. Advances in paediatric pharmacokinetics. Expert Opin Drug Metab Toxicol. 2011;7(1):1–8. doi: 10.1517/17425255.2011.539201. [DOI] [PubMed] [Google Scholar]
- 132.Ince I, de Wildt SN, Tibboel D, Danhof M, Knibbe CA. Tailor-made drug treatment for children: creation of an infrastructure for data-sharing and population PK-PD modeling. Drug Discov Today. 2009;14(5–6):316–320. doi: 10.1016/j.drudis.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Krekels EH, Neely M, Panoilia E, Tibboel D, Capparelli E, Danhof M, et al. From pediatric covariate model to semiphysiological function for maturation: part I-extrapolation of a covariate model from morphine to Zidovudine. CPT Pharmacomet Syst Pharmacol. 2012;1:e9. doi: 10.1038/psp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahmood I. Dosing in children: a critical review of the pharmacokinetic allometric scaling and modelling approaches in paediatric drug development and clinical settings. Clin Pharmacokinet. 2014;53(4):327–346. doi: 10.1007/s40262-014-0134-5. [DOI] [PubMed] [Google Scholar]
- 135.De Cock RF, Allegaert K, Brussee JM, Sherwin CM, Mulla H, de Hoog M, et al. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration. Pharm Res. 2014;31(10):2643–2654. doi: 10.1007/s11095-014-1361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther. 2009;86(6):634–643. doi: 10.1038/clpt.2009.151. [DOI] [PubMed] [Google Scholar]
- 137.Meyer UA, Zanger UM, Schwab M. Omics and drug response. Annu Rev Pharmacol Toxicol. 2013;53:475–502. doi: 10.1146/annurev-pharmtox-010510-100502. [DOI] [PubMed] [Google Scholar]
- 138.Emami-Riedmaier A, Schaeffeler E, Nies AT, Morike K, Schwab M. Stratified medicine for the use of antidiabetic medication in treatment of type II diabetes and cancer: where do we go from here? J Intern Med. 2015;277(2):235–247. doi: 10.1111/joim.12330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.