Abstract
Objective
To evaluate the relationship between type 2 diabetes mellitus (DM) and oncological outcomes in early stage cervical cancer patients who underwent radical surgical resection.
Methods
Patients with early stage cervical cancer diagnosed between 2001 and 2014 were retrospectively enrolled. We assessed the outcomes of 402 non-DM and 42 DM patients with cervical cancer. We tested the prognostic value of DM via Cox proportional hazard modeling.
Results
Patients with DM were more likely to be older and overweight. In the DM group, 20 and 22 patients were and were not taking metformin, respectively. The 5-year recurrence-free survival (RFS) and 5-year overall survival (OS) rate for the whole study population were 88.49% and 96.34%, respectively. In the DM group, there was no evidence that metformin affected the RFS (p=0.553) or the OS (p=0.429). In multivariate analysis, age (p=0.007), histology (p=0.006), and deep stromal invasion (p=0.007) were independent adverse prognostic factors for RFS. There was a borderline significant association of increased RFS with DM (p=0.051). However, a time-varying-effect Cox model revealed that the DM was associated with a worse RFS (hazard ratio, 11.15; 95% CI, 2.00 to 62.08, p=0.022) after 5 years. DM (p=0.008), age (p=0.009), and node status (p=0.001) were the only 3 independent prognostic factors for OS.
Conclusion
Early stage cervical cancer patients with type 2 DM have a poorer oncological outcome than patients without DM.
Keywords: Diabetes Mellitus, Hysterectomy, Metformin, Prognosis, Uterine Cervical Neoplasms
INTRODUCTION
Diabetes mellitus (DM) primarily manifests as type 2 DM and is one of the most common metabolic diseases. It is characterized by an array of dysfunctions led by hyperglycemia resulting from the combination of resistance to insulin action, inadequate secretion of insulin, and inappropriate secretion of glucagon [1]. The World Health Organization (WHO) reported that the global prevalence of DM in 2000 was 171 million and predicted this would reach approximately 366 million by 2030 [2]. DM often causes or is associated with many other medical conditions or diseases such as neuropathy, cardiovascular and renal disease, and various cancers.
Although an association between DM and increased cancer risk has been recognized by clinicians for nearly 100 years [1,3], it is only in the last decade that significant epidemiological evidence has been amassed to firmly connect certain cancers-especially breast, colorectal, endometrial, hepatic, pancreatic, and kidney with type 2 DM [1,4,5]. The mechanisms of cancer development related with DM remain unclear [1,3]. A number of studies has been undertaken in recent years on the impact of type 2 DM and clinical outcomes of various cancers types including gynecologic cancers. These studies have demonstrated that type 2 DM has a negative impact on the outcome of these cancers [6,7,8,9,10,11,12].
Cervical cancer is a problem of increasing magnitude and is the leading cause of cancer death for women in developing countries including Thailand. The WHO estimates that yearly about 530,000 women worldwide are identified with cervical cancer and 275,000 women die from the disease [13].
Previous studies have shown that women with high serum glucose level or type 2 DM have an increased risk of cervical cancer [4,14,15,16]. In 2005, a large scale, population-based cohort study from Korea by Jee et al. [15] demonstrated that the risk for cervical cancer was approximately 2.2-fold higher in women with diabetes. They also found that cervical cancer patients with DM faced a worse outcome [15]. Since that study, there has been little published data on the relationship between high plasma glucose or type 2 DM and the clinical outcomes of cervical cancer [17,18,19]. Lee et al. [17] investigated the association between pretreatment random plasma glucose levels and cancer prognosis in 134 non-DM patients with locally advanced cervical cancer, and found that high glucose levels (≥102 mg/dL) were associated with a greater risk of recurrence and mortality in these patients. Similarly, Ahn et al. [18] studied the association between metabolic syndrome or metabolic components and the risk of recurrence of 127 patients with stage I–II cervical cancer who underwent radical surgery. They found that impaired fasting glucose (≥100 mg/dL) and hypertriglyceridemia were associated with higher risk of recurrence [18].
To the best of our knowledge, only one study has yet addressed the prognostic impact of type 2 DM on early stage cervical cancer [19]. Recently, a nationwide population-based study from Taiwan by Kuo et al. [19] demonstrated that early stage cervical cancer patients with type 2 DM had less favorable overall survival (OS) and cancer-specific survival after curative treatments. However, this study was based on the Taiwan Cancer Registry and National Health Insurance databases and included patients who were heterogeneous in terms of treatment modalities. Some clinicopathologic factors such as a lymphovascular space invasion (LVSI) and deep stromal invasion (DSI) that may affect the treatment outcome were not included. A nationwide population-based study could introduce surgical technique bias caused by gynecologic oncologists from different centers.
Thus this study aimed to evaluate the relationship between type 2 DM and recurrence-free survival (RFS) and OS in a large cohort of early stage cervical cancer patients who underwent radical surgery at Songklanagarind Hospital―the largest tertiary care institute in Southern Thailand.
MATERIALS AND METHODS
This retrospective study was approved by the Ethics Committee of the Prince of Songkla University Faculty of Medicine (IRB number REC 58-249-12-3). All patients who presented with cervical cancer stages IA2–IB1 by the International Federation of Gynecology and Obstetrics (FIGO) 2009 and underwent radical hysterectomy with pelvic lymphadenectomy by staff doctors in the Division of Gynecologic Oncology at Songklanagarind Hospital between January 2001 and December 2014 were included. Patients who received neoadjuvant chemotherapy, radiotherapy, or concurrent chemoradiation before radical hysterectomy were excluded. This left 444 patients enrolled. All inpatient and outpatient medical records were reviewed.
All pertinent clinicopathologic data from the medical records (DM status, diabetic medication, age, body mass index [BMI], FIGO stage, tumor size, histology, LVSI, DSI, parametrial involvement [PI], node status, surgical margin, adjuvant therapy, and clinical outcome) were obtained and retrospectively reviewed. Clinical stage and histological classifications were based on the criteria established by the revised FIGO 2009 and WHO guidelines. Tumor size was determined by the attending gynecologic oncologist during a pelvic examination preceding surgery and was grouped into >2 or ≤2 cm. Tumors were classified according to cell type: squamous cell carcinoma, adenocarcinoma, adenosquamous cell carcinoma, and other cell types (such as small cell carcinoma and undifferentiated carcinoma). The depth of tumor invasion was measured in millimeters from the base of the surface epithelium to the deepest malignant cell and fractioned into thirds. The presence of LVSI was also recorded. The PI was defined as either a positive parametrial lymph node or malignant cells in the parametrial tissue by either a contiguous or discontiguous spread.
Surgical complications such as bowel injury, urinary tract injury and vascular injury were reviewed. We also reviewed postoperative morbidity such as febrile morbidity, wound infection and urinary tract infection. Febrile morbidity was defined as a temperature of 38°C or greater on two successive occasions 6 hours apart excluding the first 24 hours postoperatively as taken by oral measurement with a standard technique [20].
Adjuvant therapy after surgery was administered according to surgical risk factors. The time from radical surgery to initiation of adjuvant treatment was recorded. Follow-up after treatment was every 3 months in the first year, every 4 months in the second year, every 6 months in the third to fifth years, and annually thereafter.
The primary exposure of interest for the study was the presence of type 2, adult-onset DM at the time of cancer diagnosis. Patients diagnosed with DM at the time of preoperative work up were included as DM. Patients who developed DM after their diagnosis of cervical cancer were included in the control group. Patients who had a history of type 1 DM (n=0) and type 2 DM receiving insulin treatment due to uncontrolled blood glucose levels during the follow-up period (n=0) were excluded. Finally, patients were classified as non-DM (n=402) or DM (n=42). The BMI was calculated individually and was the body weight divided by the square of height (kg/m2) at the time of diagnosis. Patients were grouped into overweight (BMI ≥25 kg/m2) and non-overweight bins (BMI <25 kg/m2).
Prior to the study, it was expected that the records of about 440 eligible patients would be available of whom approximately 10% had been diagnosed with DM. With a 5-year OS of all patients of around 95%, this sample size had a power of 80% to detect a lower 5-year OS among DM patients of 85% as significant at a 2-sided α of 0.05. For a 5-year RFS of all patients of around 88%, a lower 5-year RFS among DM patients of 75% could be detected with a similar power and α.
The primary outcome measures were RFS and OS. RFS was calculated from the date of operation to the date of recurrence, and OS from the date of operation to the date of death or last follow-up. Patients who were lost to follow-up or did not experience an event during follow-up were censored at the latest date at which their status was known.
Data characteristics were summarized as frequency with a percentage. Univariate analysis was performed using the Kaplan-Meier method. Multivariate analysis was performed using Cox proportional hazards regression. Probability values of <0.05 were considered statistically significant. To satisfy the assumption of proportional hazards, the DM versus Non DM was fitted as a time-varying-effect variable in the RFS model. Data were analyzed using STATA ver. 10 (Stata Corp., College Station, TX, USA).
RESULTS
The study included 444 patients, with a median age of 46 years. Of the study patients, 42 patients (9.46%) were categorized as DM and, 402 patients (90.54%) were categorized as non-DM. In the DM group, we found 20 patients taking metformin and 22 patients not taking metformin. The baseline characteristics of both DM and non-DM patients are given in Table 1. These characteristics show that there were no significant differences in FIGO stage, tumor size, histology, DSI, PI, node status, surgical margin or adjuvant treatment between the two groups. Not surprisingly, patients with DM were more likely to be older (p=0.039) and overweight (p=0.002). The patients with DM had lower LVSI (11.90% vs. 28.36%, p=0.022) compared with those without DM. Of the study patients, 96 (21.62%) received adjuvant treatment after radical hysterectomy. The overall median time to adjuvant treatment from radical surgery in our study was 30 days (25% quartile, 25 days; 75% quartile, 37 days). The median time to adjuvant treatment did not differ between patients with and without DM. It was 28 days (25% quartile, 26 days; 75% quartile, 37 days) and 30 days (25% quartile, 25 days; 75% quartile, 37 days; respectively; p=0.735). Table 2 compares surgical complications and postoperative morbidity between patients with DM and patients without DM. There were no significant differences in febrile morbidity between the two groups (p=0.555). The patients with DM had a higher rate of wound infection than those without DM (9.76% vs. 5.41%). However, there were no statistically significant differences between the two groups (p=0.208).
Table 1. Characteristics of DM and non-DM patients with early stage cervical cancer who received radical hysterectomy with pelvic node dissection.
| Variable | DM patients | Non-DM patients | p-value |
|---|---|---|---|
| Age (yr) | 0.039 | ||
| <40 | 4 (9.52) | 104 (25.87) | |
| 40-59 | 31 (73.81) | 259 (64.43) | |
| ≥60 | 7 (16.67) | 39 (9.70) | |
| Body mass index (kg/m2) | 0.002 | ||
| <25 | 16 (38.10) | 253 (62.94) | |
| ≥25 | 26 (61.90) | 149 (37.06) | |
| FIGO stage | 0.133 | ||
| 1A2 | 1 (2.38) | 37 (9.20) | |
| 1B1 | 41 (97.62) | 365 (90.80) | |
| Tumor size (cm) | 0.336 | ||
| ≤2 | 26 (61.90) | 278 (69.15) | |
| >2 | 16 (38.10) | 124 (30.85) | |
| Histology | 0.493 | ||
| Squamous cell carcinoma | 25 (59.52) | 237 (58.96) | |
| Adenocarcinoma | 16 (38.10) | 130 (32.34) | |
| Adenosquamous carcinoma | 1 (2.38) | 24 (5.97) | |
| Other | 0 | 11 (2.74) | |
| LVSI | 0.022 | ||
| No | 37 (88.10) | 288 (71.64) | |
| Yes | 5 (11.90) | 114 (28.36) | |
| Deep stromal invasion | 0.529 | ||
| No | 33 (78.57) | 298 (74.13) | |
| Yes | 9 (21.43) | 104 (25.87) | |
| Parametrial involvement | 0.807 | ||
| No | 40 (95.24) | 386 (96.02) | |
| Yes | 2 (4.76) | 16 (3.98) | |
| Node metastasis | 0.390 | ||
| No | 41 (97.62) | 380 (94.53) | |
| Yes | 1 (2.38) | 22 (5.47) | |
| Surgical margin | 0.335 | ||
| Free | 39 (92.86) | 386 (96.02) | |
| Not free | 3 (7.14) | 16 (3.98) | |
| Adjuvant therapy | 0.225 | ||
| No | 36 (85.71) | 312 (77.61) | |
| Yes | 6 (14.29) | 90 (22.39) | |
| Adjuvant therapy | 0.693 | ||
| No | 36 (85.72) | 312 (77.61) | |
| RT | 3 (7.14) | 56 (13.93) | |
| CMT | 0 | 5 (1.24) | |
| CCRT | 3 (7.14) | 29 (7.22) |
Values are presented as number (%).
CCRT, concurrent chemoradiation; CMT, chemotherapy; DM, diabetes mellitus; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; RT, radiation therapy.
Table 2. Surgical complication and postoperative morbidity.
| Variable | DM patients | Non-DM patients | p-value |
|---|---|---|---|
| Surgical complication | |||
| Hemorrhage ≥1,500 mL | 0.558 | ||
| Yes | 2 (5.13) | 24 (6.30) | |
| No | 37 (94.87) | 357 (93.70) | |
| Blood transfusion | 0.458 | ||
| Yes | 4 (10.26) | 33 (8.66) | |
| No | 35 (89.74) | 348 (91.34) | |
| Bowel injury | 0.740 | ||
| Yes | 0 | 3 (0.77) | |
| No | 41 (100) | 386 (99.23) | |
| Urinary tract injury | 0.546 | ||
| Yes | 0 | 6 (1.54) | |
| No | 41 (100) | 383 (98.46) | |
| Nerve injury | - | ||
| Yes | 0 | 0 | |
| No | 41 (100) | 389 (100) | |
| Vascular injury | 0.454 | ||
| Yes | 1 (2.44) | 5 (1.29) | |
| No | 40 (97.56) | 384 (98.71) | |
| Postoperative morbidity | |||
| Febrile morbidity | 0.555 | ||
| Yes | 10 (24.39) | 97 (24.94) | |
| No | 31 (75.61) | 292 (75.06) | |
| Fistula | 0.904 | ||
| Yes | 0 | 1 (0.26) | |
| No | 41 (100) | 387 (99.74) | |
| Pneumonia | - | ||
| Yes | 0 | 0 | |
| No | 41 (100) | 388 (100) | |
| Urinary tract infection | 0.229 | ||
| Yes | 4 (9.76) | 22 (5.67) | |
| No | 37 (90.24) | 366 (94.33) | |
| Vaginal stump infection | 0.592 | ||
| Yes | 2 (4.88) | 22 (5.67) | |
| No | 39 (95.12) | 366 (94.33) | |
| Wound infection | 0.208 | ||
| Yes | 4 (9.76) | 21 (5.41) | |
| No | 37 (90.24) | 367 (94.59) |
Values are presented as number (%).
DM, diabetes mellitus.
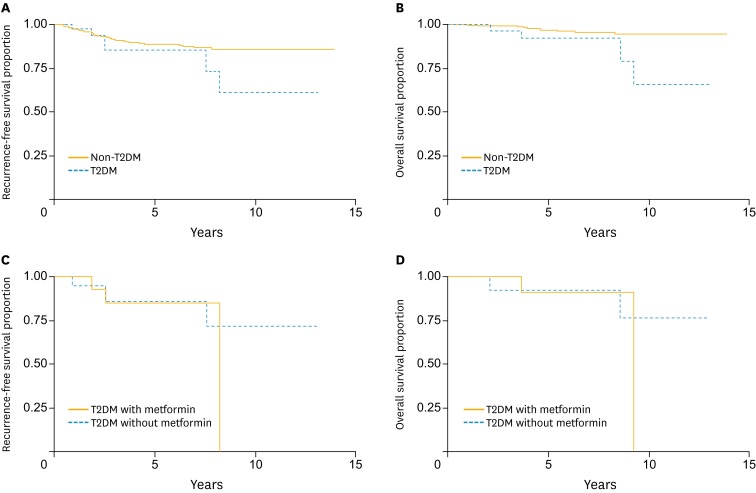
The median duration of follow-up was 4.02 years (25% quartile, 1.83 years; 75% quartile, 7.69 years). Forty-four of the 444 patients (6 patients with DM and 38 patients without DM) developed disease recurrence during their follow-up periods. The 5-year RFS and 5-year OS rate for the entire study population were 88.49% (95% CI, 84.42 to 91.55) and 96.34% (95% CI, 93.20 to 98.05), respectively. Univariate analysis found that age (p=0.06), histology (p=0.005), DSI (p=0.002), PI (p=0.013), and lymph node status (p=0.034) were prognostic factors for RFS, while age (p=0.02), DM (p=0.008), histology (p=0.02), PI (p=0.047), and node status (p=0.001) were associated with OS (Table 3). Further multivariate analysis found that DM (hazard ratio [HR], 2.68; 95% CI, 1.10 to 6.54; p=0.051 [borderline significance]) (Fig. 1A), age (HR, 2.82; 95% CI, 1.47 to 5.40; p=0.007), histology (HR, 6.61; 95% CI, 2.44 to 17.94; p=0.006), and DSI (HR, 2.53; 95% CI, 1.32 to 4.87; p=0.007) were independent adverse prognostic factors for RFS (Table 4). The statistically significant independent prognosis factors for OS were DM (HR, 6.53; 95% CI, 1.95 to 21.78; p=0.008) (Fig. 1B), age (HR, 5.39; 95% CI, 1.77 to 16.42; p=0.009) and node status (HR, 11.77; 95% CI, 3.45 to 40.13; p=0.001) (Table 5).
Table 3. Univariate analysis of 5-year recurrence-free survival and 5-year overall survival.
| Variable | 5-Year recurrence-free survival | 5-Year overall survival | ||
|---|---|---|---|---|
| 95% CI | p-value | 95% CI | p-value | |
| Age (yr) | 0.006 | 0.02 | ||
| <40 | 83.66 (73.78-90.06) | 93.17 (84.08-97.16) | ||
| 40-59 | 91.13 (86.33-94.31) | 97.06 (92.94-98.79) | ||
| ≥60 | 81.61 (57.87-92.73) | 100 | ||
| Diabetes mellitus | 0.158 | 0.008 | ||
| No | 88.75 (84.50-91.89) | 96.77 (93.51-98.40) | ||
| Yes | 85.45 (65.40-94.34) | 91.78 (70.85-97.89) | ||
| Body mass index (kg/m2) | 0.174 | 0.278 | ||
| <25 | 86.90 (81.35-90.89) | 95.59 (91.24-97.80) | ||
| ≥25 | 91.16 (84.41-95.07) | 97.54 (90.53-99.38) | ||
| FIGO stage | 0.385 | 0.25 | ||
| 1A2 | 92.38 (72.34-98.08) | 100 | ||
| 1B1 | 88.15 (83.84-91.37) | 96.04 (92.65-97.88) | ||
| Tumor size (cm) | 0.162 | 0.093 | ||
| ≤2 | 90.26 (85.47-93.53) | 97.51 (93.48-99.06) | ||
| >2 | 84.33 (75.37-90.24) | 93.66 (86.15-97.17) | ||
| Histology | <0.005 | 0.02 | ||
| Squamous cell carcinoma | 90.02 (84.84-93.50) | 96.64 (92.06-98.60) | ||
| Adenocarcinoma | 91.93 (84.90-95.77) | 97.82 (91.09-99.48) | ||
| Adenosquamous carcinoma | 69.46 (39.87-86.56) | 94.44 (66.64-99.20) | ||
| Other | 53.03 (17.04-79.67) | 77.14 (34.49-93.87) | ||
| LVSI | 0.064 | 0.397 | ||
| No | 90.62 (86.13-93.71) | 96.76 (92.79-98.56) | ||
| Yes | 83.09 (73.24-89.57) | 95.17 (87.41-98.19) | ||
| Deep stromal invasion | 0.002 | 0.458 | ||
| No | 92.28 (88.22-94.98) | 96.55 (92.77-98.37) | ||
| Yes | 76.22 (64.31-84.62) | 95.78 (87.31-98.64) | ||
| Parametrial involvement | 0.013 | 0.047 | ||
| No | 89.52 (85.49-92.48) | 96.81 (93.61-98.42) | ||
| Yes | 64.48 (30.40-85.07) | 87.50 (58.60-96.72) | ||
| Node metastasis | 0.034 | <0.001 | ||
| No | 89.43 (85.34-92.42) | 97.70 (94.84-98.98) | ||
| Yes | 70.59 (42.25-86.86) | 72.12 (40.85-88.75) | ||
| Surgical margin | 0.110 | 0.574 | ||
| Free | 89.22 (85.15-92.23) | 96.50 (93.27-98.20) | ||
| Not free | 73.07 (40.42-89.70) | 93.33 (61.26-99.03) | ||
| Adjuvant therapy | 0.098 | 0.510 | ||
| No | 90.59 (86.30-93.58) | 97.19 (93.71-98.76) | ||
| Yes | 81.39 (69.66-88.93) | 93.50 (83.18-97.58) | ||
FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion.
Fig. 1.
(A) Recurrence-free survival of early stage cervical cancer patients by diabetes mellitus status. (B) Overall survival of early stage cervical cancer patients by diabetes mellitus status. (C) Recurrence-free survival among diabetic cervical cancer patients who used metformin versus those who did not. (D) Overall survival among diabetic cervical cancer patients who used metformin versus those who did not. T2DM, type 2 diabetes mellitus
Table 4. Multivariate analysis of recurrence-free survival.
| Variable | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Diabetes mellitus | 0.051 | ||
| No | 1 | - | |
| Yes | 2.68 | 1.10-6.54 | |
| Age (yr) | 0.007 | ||
| <40 | 2.82 | 1.47-5.40 | |
| 40-59 | 1 | - | |
| ≥60 | 1.87 | 0.72-4.85 | |
| Histology | 0.006 | ||
| Squamous cell carcinoma | 1 | - | |
| Adenocarcinoma | 0.85 | 0.41-1.75 | |
| Adenosquamous carcinoma | 2.23 | 0.83-6.02 | |
| Other | 6.61 | 2.44-17.94 | |
| Deep stromal invasion | 0.007 | ||
| No | 1 | - | |
| Yes | 2.53 | 1.32-4.87 | |
| Diabetes mellitus (time-varying-effect Cox model) | |||
| In first 5 years | 0.276 | ||
| No | 1 | - | |
| Yes | 1.89 | 0.65-5.50 | |
| After 5 years | 0.022 | ||
| No | 1 | - | |
| Yes | 11.15 | 2.00-62.08 | |
| Age (yr) | 0.009 | ||
| <40 | 2.74 | 1.43-5.25 | |
| 40-59 | 1 | - | |
| ≥60 | 1.96 | 0.76-5.10 | |
| Histology | 0.007 | ||
| Squamous cell carcinoma | 1 | - | |
| Adenocarcinoma | 0.86 | 0.41-1.78 | |
| Adenosquamous carcinoma | 2.21 | 0.82-5.97 | |
| Other | 6.53 | 2.41-17.71 | |
| Deep stromal invasion | 0.007 | ||
| No | 1 | - | |
| Yes | 2.56 | 1.32-4.94 |
Table 5. Multivariate analysis of overall survival.
| Variable | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Diabetes mellitus | 0.008 | ||
| No | 1 | - | |
| Yes | 6.53 | 1.95-21.78 | |
| Age (yr) | 0.009 | ||
| <40 | 5.39 | 1.77-16.42 | |
| 40-59 | 1 | - | |
| ≥60 | 1.44 | 0.17-12.53 | |
| Node metastasis | 0.001 | ||
| No | 1 | - | |
| Yes | 11.77 | 3.45-40.13 |
The RFS profile did not differ significantly between patients with DM and patients without DM (85.45% [95% CI, 65.40 to 94.34] and 88.75% [95% CI, 84.50 to 91.89], respectively; p=0.158) (Fig. 1A). However, DM may have negative impacts on the long term RFS. This was confirmed in the time-varying-effect Cox model in which DM was associated with a worse RFS after first 5 years (HR, 11.15; 95% CI, 2.00 to 62.08; p=0.022) (Table 4).
In addition, within the DM group, metformin use did not affect the 5-year RFS (use vs. non-use, 85.12% [95% CI, 52.34 to 96.07] vs. 85.86% [95% CI, 52.91 to 96.41], p=0.553) nor 5-year OS (use vs. non-use, 90.91% [95% CI, 50.81 to 98.67] vs. 92.31% [95% CI, 56.64 to 98.88], p=0.429). The results of the correlation analysis are presented in Fig. 1C, D. Further comparisons within this group were limited due to the small sample size.
DISCUSSION
A prior population-based cohort study from Korea reported that having cervical cancer with DM was associated with an HR of 2.50 for the risk of all-causes mortality compared to those without DM [15]. However, a review of the literature indicates that the impact of type 2 DM on clinicopathological factors and the prognosis of patients with early stage cervical cancer has not been sufficiently studied to draw anything more than tentative associations. In the current study, we found that of the 444 patients, the prevalence of type 2 DM within our surgical early stage cervical cancer population was 9.5%. We also found that there was no significant difference between patients with and without type 2 DM in FIGO stage, tumor size, histology, DSI, PI, node status, surgical margin or adjuvant treatment. However, our study did find that patients with type 2 DM were more likely to be older and overweight, which is comparable to previous studies [7,9,19]. These findings may be explained by the fact that the majority of patients with type 2 DM are obese. One unexpected finding was that patients with type 2 DM had a lower LVSI. The reason for this is unknown.
Concerning oncologic outcome, this study found that type 2 DM is a meaningful prognostic factor. It shows a borderline independent association with RFS (HR, 2.68; 95% CI, 1.10 to 6.54; p=0.051). However, a time-varying-effect Cox model revealed that type 2 DM was associated with a worse RFS after first 5 years (HR, 11.15; 95% CI, 2.00 to 62.08; p=0.022). Furthermore, we found that early stage cervical cancer patients with type 2 DM had a decreased OS after controlling for comorbid conditions such as obesity and other clinicopathological factors. These results concur with an earlier study [15,19]. Similarly, Shah et al. [8] evaluated the impact of DM on progression-free survival (PFS) and OS in 367 women with epithelial ovarian cancer. They found that patients with DM had poorer survival (PFS and OS) than patients without DM; this association is independent of obesity [8]. In addition, a study by Ko et al. [10] assessed 1,411 endometrial cancer patients and found that DM was associated with worse RFS and OS in type I (endometriod) endometrial cancer. Neither DM nor BMI was associated with outcomes in type II (serous and clear cell) or high grade endometrial cancer [10].
The mechanisms underlying the influence of DM on cancer incidence and progression are still largely unknown [1,3,9]. However, there are several possible explanations for the association of DM and cancer. First, patients with type 2 DM have reduced insulin sensitivity with compensatory hyperinsulinemia with an increased level of circulating insulin-like growth factor-1 (IGF-1). IGF-1 is well known to stimulate cell proliferation in many organs including the cervix, and it plays an important role in cancer development and metastasis [1,3,4,21]. Second, hyperglycemia in patients with type 2 DM may play a significant role in tumor progression-this promotes DNA damage and activates different signaling pathways significantly associated with tumorigenesis and metastasis [9,22]. Hyperglycemia may be responsible for the excess glucose supply to cancer cells, resistance to apoptosis, and tumor cell resistance to therapy [1].
Third, patients with type 2 DM had a significantly higher BMI than the patients without type 2 DM. This reflects a larger amount of visceral adipose tissue. Adipose tissue produces many cytokines-mostly interleukin 6. This plays a causative role in regulating mitogenic activity. Adipose tissue produces free fatty acids, monocyte chemoattractant protein, tumor necrotic factor α and plasminogen activator inhibitor 1. Each of these factors might play an etiologic role in regulating malignant transformation or cancer progression [1]. Two studies have found that high BMI (obesity) was associated with increased cervical cancer risk [23,24]. In addition, a large population-based study found that a high BMI was associated with increased death rates for cervical cancer [25]. However, in our study we found no association between high BMI and oncological outcomes of cervical cancer as was shown in a previous study [18]. This discrepancy could be explained by the fact that all patients in our study and the Ahn et al. [18] study had early stage disease in which prognosis is generally good after radical surgery. In contrast, the Calle at al. [25] study included all stages of cervical cancer with accompanying poorer prognosis. Moreover, the number of patients in our study and the Ahn et al. [18] study may not have been sufficient to detect small effects of high BMI on survival outcomes in early stage cervical cancer. These above explanations and discoveries support our finding that type 2 DM is associated with poor cervical cancer prognosis.
Metformin is a commonly used oral hypoglycemic drug for type 2 DM that reduces both insulin and glucose levels. Epidemiological studies and preclinical studies suggest that metformin may reduce cancer risk in type 2 DM patients [1,3]. Recent studies have suggested that metformin use was associated with longer PFS and OS in cancer patients [26,27,28]. In cervical cancer, preclinical studies have shown that metformin can inhibit cervical cancer cell growth by induced AMP-activated protein kinase [1,29]. To the best of our knowledge, no previous studies have investigated any possible association of metformin with survival outcome in cervical cancer. With only 42 DM patients, our study could not detect an effect of metformin on oncological outcomes. However, the preliminary evidence suggests that further studies with larger sample sizes are needed.
Of the various clinicopathological variables of early stage cervical cancer that have been reported as prognostic factors, i.e., tumor size, histopathology, LVSI, DSI PI and lymph node metastasis [30,31], we only found age, histology and DSI to be significant adverse indicators for DFS. We also found that age and node status were independent adverse prognostic factors for OS.
The major strengths of this study include the relatively large number of early stage cervical cancer patients in one center and the relatively long follow-up. All patients were treated uniformly at a single institution with uniform surgical techniques. However, this study was limited by its retrospective nature, and there may have been unmeasured confounding variables such as comorbidities associated with type 2 DM including hypertensive disorder or hyperlipidemia-these clearly could increase the risk of death in some patients. Another potential confounder was that blood glucose and insulin levels were not considered in the analysis, which could have influenced our results. Finally, we did not have data on disease-specific mortality.
In conclusion, our study suggests that type 2 DM may be an important prognostic factor for early stage cervical cancer. Type 2 DM should be considered when evaluating early stage cervical cancer patients. Further high-quality studies with more focus on glucose levels and certain treatments of type 2 DM such as metformin with a larger number of patients are needed.
ACKNOWLEDGMENTS
We would like to thank Dr. Alan Geater, Epidemiology Unit, Prince of Songkla University Faculty of Medicine for assistance with statistical analysis and valuable comments.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Funding: This research received a full grant from the Prince of Songkla University Faculty of Medicine.
References
- 1.Szablewski L. Diabetes mellitus: influences on cancer risk. Diabetes Metab Res Rev. 2014;30:543–596. doi: 10.1002/dmrr.2573. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1100. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Joung KH, Jeong JW, Ku BJ. The association between type 2 diabetes mellitus and women cancer: the epidemiological evidences and putative mechanisms. Biomed Res Int. 2015;2015:920618. doi: 10.1155/2015/920618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan YS, Feng L, Tang SH, Li WG, Xu M, Liu TF, et al. Glucose metabolism disorders in cancer patients in a Chinese population. Med Oncol. 2010;27:177–261. doi: 10.1007/s12032-009-9189-9. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013–1021. doi: 10.1007/s00125-011-2051-6. [DOI] [PubMed] [Google Scholar]
- 6.Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539–582. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhru A, Buckanovich RJ, Griggs JJ. The impact of diabetes on survival in women with ovarian cancer. Gynecol Oncol. 2011;121:106–117. doi: 10.1016/j.ygyno.2010.12.329. [DOI] [PubMed] [Google Scholar]
- 8.Shah MM, Erickson BK, Matin T, McGwin G, Jr, Martin JY, Daily LB, et al. Diabetes mellitus and ovarian cancer: more complex than just increasing risk. Gynecol Oncol. 2014;135:273–280. doi: 10.1016/j.ygyno.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vavallo A, Simone S, Lucarelli G, Rutigliano M, Galleggiante V, Grandaliano G, et al. Pre-existing type 2 diabetes mellitus is an independent risk factor for mortality and progression in patients with renal cell carcinoma. Medicine (Baltimore) 2014;93:e183. doi: 10.1097/MD.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko EM, Walter P, Clark L, Jackson A, Franasiak J, Bolac C, et al. The complex triad of obesity, diabetes and race in Type I and II endometrial cancers: prevalence and prognostic significance. Gynecol Oncol. 2014;133:28–32. doi: 10.1016/j.ygyno.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Li H, Gu L, Ma X, Li X, Gao Y, et al. The Impact of Diabetes Mellitus on Renal Cell Carcinoma Prognosis: A Meta-Analysis of Cohort Studies. Medicine (Baltimore) 2015;94:e1055. doi: 10.1097/MD.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantiello F, Cicione A, Salonia A, Autorino R, De Nunzio C, Briganti A, et al. Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: a systematic review. Int J Urol. 2015;22:22–32. doi: 10.1111/iju.12644. [DOI] [PubMed] [Google Scholar]
- 13.International Collaboration of Epidemiological Studies of Cervical Cancer. Appleby P, Beral V, Berrington de González A, Colin D, Franceschi S, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–1630. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Hernandez D. Type 2 diabetes mellitus and habits lifestyle increases the risk of cervical cancer: a cross-sectional population-based study. Austin J Obstet Gynecol. 2014;1:7. [Google Scholar]
- 15.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Stocks T, Rapp K, Bjørge T, Manjer J, Ulmer H, Selmer R, et al. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS Med. 2009;6:e1000201. doi: 10.1371/journal.pmed.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YY, Choi CH, Kim CJ, Song TJ, Kim MK, Kim TJ, et al. Glucose as a prognostic factor in non-diabetic women with locally advanced cervical cancer (IIB-IVA) Gynecol Oncol. 2010;116:459–522. doi: 10.1016/j.ygyno.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Ahn HK, Shin JW, Ahn HY, Park CY, Lee NW, Lee JK, et al. Metabolic components and recurrence in early-stage cervical cancer. Tumour Biol. 2015;36:2201–2208. doi: 10.1007/s13277-014-2831-y. [DOI] [PubMed] [Google Scholar]
- 19.Kuo HY, Lin ZZ, Kuo R, Shau WY, Lai CL, Yang YY, et al. The prognostic impact of type 2 diabetes mellitus on early cervical cancer in Asia. Oncologist. 2015;20:1051–1058. doi: 10.1634/theoncologist.2015-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirdchim W, Hanprasertpong J, Prasartwanakit V, Geater A. Risk factors for febrile morbidity after abdominal hysterectomy in a university hospital in Thailand. Gynecol Obstet Invest. 2008;66:34–43. doi: 10.1159/000115843. [DOI] [PubMed] [Google Scholar]
- 21.Shen MR, Hsu YM, Hsu KF, Chen YF, Tang MJ, Chou CY. Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation that is modulated by alphavbeta3 integrin signaling. Carcinogenesis. 2006;27:962–1033. doi: 10.1093/carcin/bgi336. [DOI] [PubMed] [Google Scholar]
- 22.Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626–2662. doi: 10.2337/db07-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacey JV, Jr, Swanson CA, Brinton LA, Altekruse SF, Barnes WA, Gravitt PE, et al. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer. 2003;98:814–835. doi: 10.1002/cncr.11567. [DOI] [PubMed] [Google Scholar]
- 24.Ulmer H, Bjørge T, Concin H, Lukanova A, Manjer J, Hallmans G, et al. Metabolic risk factors and cervical cancer in the metabolic syndrome and cancer project (Me-Can) Gynecol Oncol. 2012;125:330–335. doi: 10.1016/j.ygyno.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 25.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1663. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 26.Romero IL, McCormick A, McEwen KA, Park S, Karrison T, Yamada SD, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol. 2012;119:61–68. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:707–717. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 28.Choi Y, Kim TY, Oh DY, Lee KH, Han SW, Im SA, et al. The impact of diabetes mellitus and metformin treatment on survival of patients with advanced pancreatic cancer undergoing chemotherapy. Cancer Res Treat. 2016;48:171–179. doi: 10.4143/crt.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yung MM, Chan DW, Liu VW, Yao KM, Ngan HY. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 2013;13:327. doi: 10.1186/1471-2407-13-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chittithaworn S, Hanprasertpong J, Tungsinmunkong K, Geater A. Association between prognostic factors and disease-free survival of cervical cancer stage IB1 patients undergoing radical hysterectomy. Asian Pac J Cancer Prev. 2007;8:530–534. [PubMed] [Google Scholar]
- 31.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy according to tumor size divided by 2-cm interval in patients with early cervical cancer. Ann Oncol. 2011;22:59–67. doi: 10.1093/annonc/mdq321. [DOI] [PubMed] [Google Scholar]