Abstract
Mitochondria are cellular organelles that produce energy in the form of ATP through a process termed oxidative phosphorylation (OXPHOS), which occurs via the protein complexes of the electron transport chain (ETC). In recent years it has become unequivocally clear that mitochondrial complexes of the ETC are not static entities in the inner mitochondrial membrane. These complexes are dynamic and in mammals they aggregate in different stoichiometric combinations to form supercomplexes (SCs) or respirasomes. It has been proposed that the net respiration is more efficient via SCs than via isolated complexes. However, it still needs to be determined whether the activity of a particular SC is associated with a disease etiology. Here we describe a simplified method to visualize and assess in-gel activity of SCs and the individual complexes with a good resolution on blue native polyacrylamide gel electrophoresis (BN-PAGE).
Keywords: Supercomplex, in-gel activity, mitochondria, oxidative phosphorylation, Cox7a2l, SCAFI
INTRODUCTION
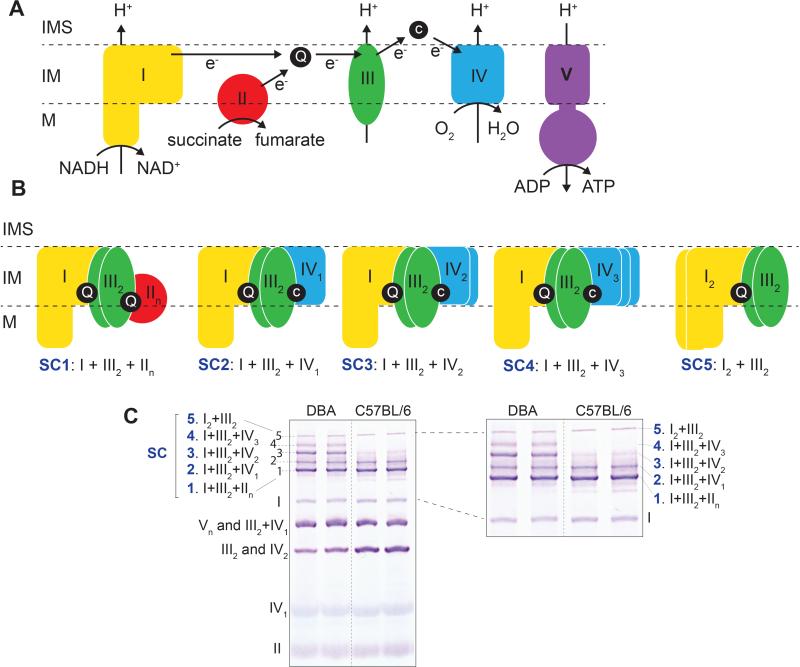
The structural and functional organization of mitochondrial complexes in supercomplexes (SC) has been a matter of fierce debate for more than 60 years (Barrientos and Ugalde, 2013; Lapuente-Brun et al., 2013; Mourier et al., 2014). Two models have been historically hypothesized (Schon and Dencher, 2009). According to the “fluid state” model, individual OXPHOS complexes diffuse freely in the mitochondrial inner membrane (Figure 1A) and electron transport occurs when the complexes randomly collide. Conversely, the “solid state” model proposes that OXPHOS complexes are organized in rigid higher-order assemblies known as SCs or respirasomes (Figure 1B). It is currently accepted that both organizations coexist (Figure 1C), giving rise to the “dynamic aggregate” or “plasticity” model (Schon and Dencher, 2009).
Figure 1. Schematic representation of the oxidative phosphorylation (OXPHOS) system showing its individual complexes, supercomplexes and its nomenclature.
(A) Mitochondrial electron transport chain (ETC) and ATP synthesis. In TCA cycle, NADH and succinate are electron donors, oxidized by NADH dehydrogenase (CI) and succinate dehydrogenase (CII), respectively. Ubiquinone (Q) is one of the electron acceptors which transfers the electrons to cytochrome c (c) by cytochrome c reductase (CIII). The electrons then pass to their terminal acceptor molecular oxygen (O2) to release water by cytochrome c oxidase (CIV). This electron transfer is coupled with the transfer of protons (H+) across mitochondrial inner membrane, except complex II. The electrochemical proton gradient generated drives the generation of chemical energy in the form of adenosine triphosphate (ATP) through ATP synthase (CV). Note that in this figure, for simplicity, all components are shown as monomers, however in reality their stoichiometry is more complex e.g. complex III exists as dimers (as shown in the figure). Dimers and multiple aggregation of CIV are also present (not shown). (B and C) Representative stoichiometry and nomenclature of mitochondrial supercomplexes shown as a cartoon (B), and in a BN-PAGE (C). I, II, III, IV, V: complexes I to V; SC, supercomplex; M, matrix; IM, inner membrane; IMS, inner membrane space.
In mammals, SCs are formed by different stoichiometric combinations of the four complexes in the ETC—Complexes I, II, III, and IV (Figure 1B, 1C)—although it is poorly understood how they are formed or how different SCs influence overall mitochondrial function (Acin-Perez et al., 2008; Lapuente-Brun et al., 2013; Moreno-Lastres et al., 2012; Mourier et al., 2014). Recently, Cox7a2l (SCAFI) was demonstrated to play a primary role in supercomplex formation (Lapuente-Brun et al., 2013). C57BL/6 mice—the most commonly used laboratory mouse strain—has a mutated version of the Cox7a2l protein and therefore they do not form SCs with multiple copies of CIV, in particular in the liver. In contrast, strains like DBA (PJ and JA, unpublished results), CBA, CD1, 129, and NZB have functional Cox7a2l and therefore can form SCs with multiple CIV subunits (Lapuente-Brun et al., 2013). The Cox7a2l positive DBA strain has five SCs in contrast to the Cox7a2l negative strain, which has only 3 of the 5 SCs (Figure 1B, 1C, 3, 4 and 5). SC 3 (I+III2+IV2) and 4 (I+III2+IV3) are not formed in Cox7a2l negative strains (see C57BL/6 lanes in Figure 1C, 3, 4 and 5). Additionally complex III2+IV1 is not formed in Cox7a2l negative strains (see C57BL/6 lanes in Figure 1C, 3, 4 and 5). However, the mechanism of assembly appears strongly tissue dependent: these SCs can be formed in the heart, even in the absence of Cox7a2l, though to a much lesser extent than in Cox7a2l proficient strains (PJ and JA, unpublished data).
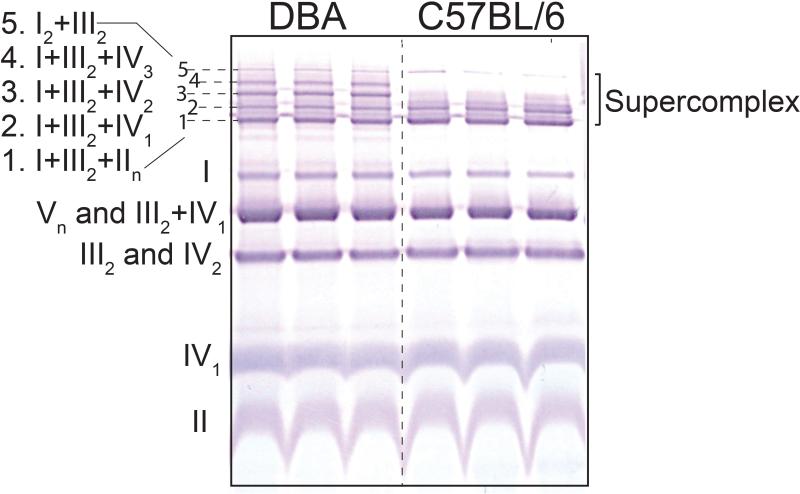
Figure 3. BN-PAGE showing mitochondrial complexes and supercomplexes.
Liver mitochondria were isolated form DBA and C57BL6/ mice. 50 μg of mitochondrial protein (8g/g digitonin to protein ratio) was used for BN-PAGE for a run time of 30 min at 150 V and 1 h at 250 V followed by immunoblotting with an OXPHOS antiboty cocktail. The five SC stoichiometric combinations are indicated as numbers 1 to 5. Note, in C57BL/6 mice only 3 SC bands are observed (1, 2 and 5) in contrast to the 5 bands present in DBA mice.
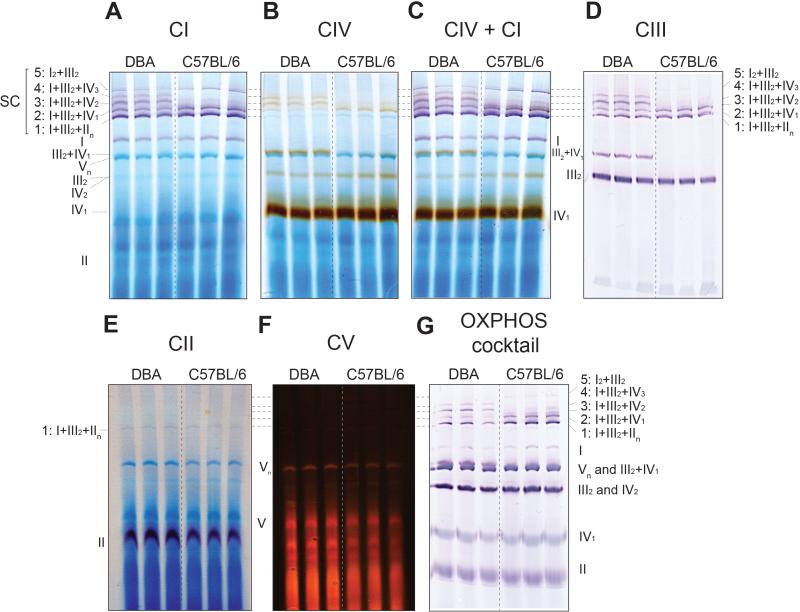
Figure 4. BN-PAGE, followed by in gel activity assay and immunoblotting to define the SC bands in DBA and C57BL/6 (representing the 2 typical patterns of SCs in rodents).
Liver mitochondria were isolated and 50 μg of mitochondrial protein (4g/g digitonin to protein ratio) was used for BN-PAGE for a run time of 30 min at 150 V and 150 min at 250 V. In-gel activity assays and western blot was performed thereafter. (A) CI activity is shown in violet (20 min incubation). (B) CIV in brown (40 min incubation). (C) CIV+CI in brown and violet, respectively (40 min incubation in CIV substrate followed by 20 min in CI substrate). (D) CIII is shown by immunoblotting against the UQCRC2 subunit of CIII. (E) CII activity is shown in violet (40 min incubation). (F) CV activity is shown in red (16 h incubation). (G) Western blot developed with an OXPHOS antibody cocktail. Note, SCs 3, 4 and III2+IV1 are absent in C57BL/6 mice.
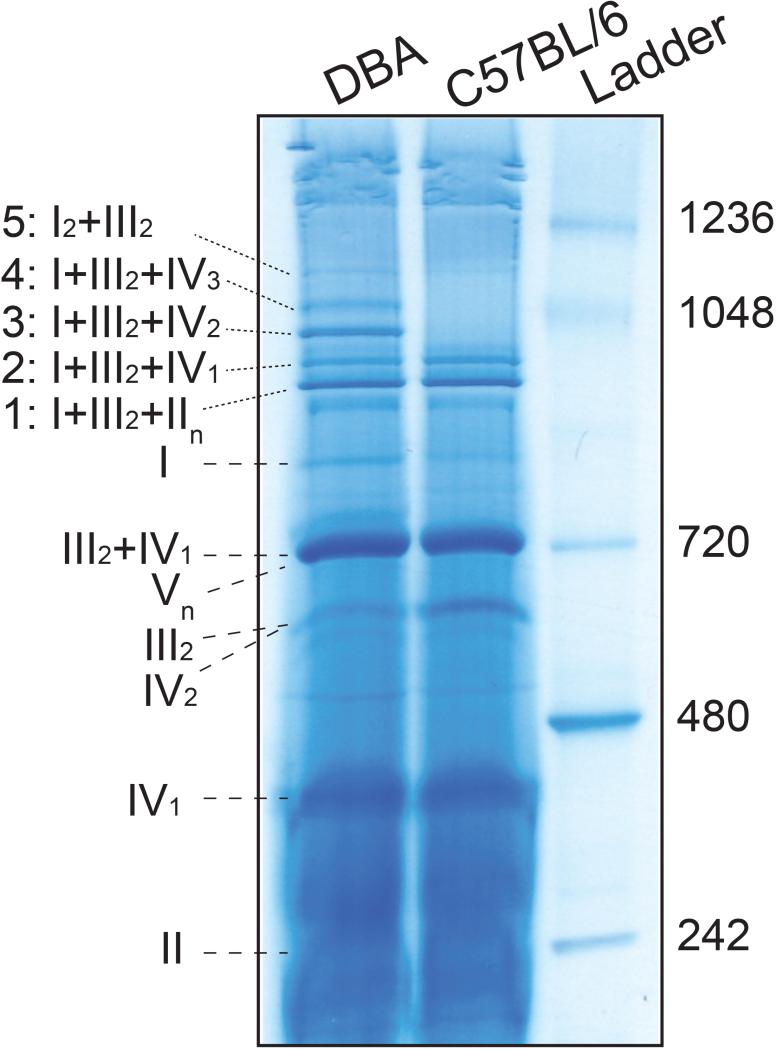
Figure 5. BN-PAGE, followed by Coomassie staining and destaining.
Liver mitochondria were isolated and 150 μg of mitochondrial protein (4g/g digitonin to protein ratio) was used for BN-PAGE for a run time of 30min at 150V and 150min at 250V. The gel was stained and then destained using the colloidal blue staining kit.
It has been suggested that association of mitochondrial complexes as SC may offer structural or functional advantages, such as the prevention of their destabilization and degradation, the enhancement of electron transport efficiency and substrate channeling, or the decrease of electron or proton leakages (Lenaz and Genova, 2010). As a consequence of their organization in SCs, a structural interdependence exists among the individual OXPHOS complexes (Chaban et al., 2014). This has major biological as well as biomedical implications, since mitochondrial complex enzyme assembly defects produce severe encephalomyopathies and neurodegenerative disorders in human and have been associated with the aging process (Gomez and Hagen, 2012; McKenzie et al., 2006). In human mitochondrial diseases, structural alterations primarily affecting one given complex often induce pleiotropic deleterious effects of the other complexes. For instance, pathogenic mutations in CIII subunits or assembly genes lead to pleiotropic deficiencies of CI and CIV in affected tissues (Lamantea et al., 2002), mutations in CIV subunits may lead to secondary CI deficiencies (D'Aurelio et al., 2006), and mutations affecting CI-specific genes can produce combined CI and CIII, or CI and CIV, deficiencies in patients (Budde et al., 2000; Saada et al., 2012). Given these findings, and the emerging role of SCs, it is quite tempting to speculate that these pleiotropic effects may be due to an impact on the formation of SCs, which stabilizes the individual complexes (Moreno-Lastres et al., 2012). Although some laboratory “wild-type” mouse inbred strains (e.g., C57BL/6, BALB/c) do lack particular SCs, this seems not to lead to evident phenotypical changes under basal conditions. However, it is very likely that lack of certain SC conformations may be rate-limiting under certain challenged conditions and contribute to major inter strain functional differences seen among the different inbred strains (Champy et al., 2008; Paigen et al., 1985). In this respect, linking a particular SC to a particular disease etiology/physiological status still needs to be uncovered. Importantly, careful thought should be given to the choice of the genetic background of the mouse model (all SCs present vs. absent) when studying metabolism in general and mitochondrial function in particular.
BASIC PROTOCOL
A high-resolution BN-PAGE followed by in-gel activity of the mitochondrial complexes is an efficient and easy approach to determine the stoichiometry of the SCs, as opposed to highly specialized mass spectrometric methods. In contrast to sodium dodecyl sulfate (SDS)-PAGE, BN-PAGE allows protein separation under native conditions and preserves protein-protein interactions, which is important for studying SCs. Following solubilization of mitochondria and centrifugation, the anionic dye Coomassie G-250 sample additive is added to the supernatant. This dye is sufficiently soluble in water, but it can also bind to membrane proteins because of its hydrophobic properties. Binding a large number of dye molecules imposes a charge shift on the proteins that causes even basic proteins to migrate to the anode at pH 7.5 during the electrophoresis step of BN-PAGE. Here, proteins are not separated according to the charge/mass ratio but according to size in acrylamide gradient gels. Protein migration gradually decelerates with running distance and with decreasing pore size of the gradient gel. Individual proteins stop migrating almost completely when they approach their size-dependent specific pore-size limit during BN-PAGE. Because negatively charged protein surfaces repel each other, the tendency of membrane proteins to aggregate is considerably reduced. Furthermore, membrane surfaces lose their hydrophobic character upon binding the dye, which converts membrane proteins into water-soluble proteins. This means that no detergent is required in the BN gels once Coomassie dye has occupied the protein surfaces. Therefore, the risk of denaturation of detergent-sensitive proteins is minimized during BN-PAGE (Wittig et al., 2007).
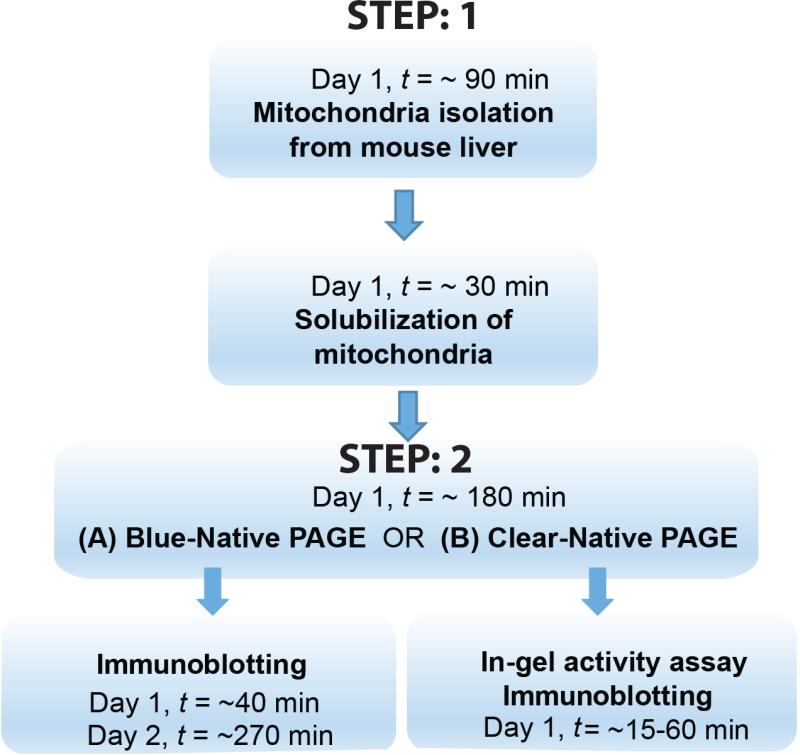
Here, we describe a high-throughput method for resolving SCs and assessing their activity. This protocol will guide researchers through the two broad steps involved in mitochondrial SC visualization/analysis (see Figure 2 for a scheme).
Figure 2. Schematic representation of the workflow.
STEP 1 involves isolation and solubalization of mitochondria. In STEP 2, one can choose to either do BN-PAGE followed by immunoblotting (STEP 2(A)) or CN-PAGE (minor modification of BN-PAGE) followed by in-gel activity of the supercomplexes and complexes, with or without additional immunoblotting of a separate gel, run simultaneously (STEP 2(B)).
STEP 1. Mitochondria isolation from mouse liver (applies for others organs as well)
MATERIALS
Mouse tissue ~30 mg liver. ~ 15 mg is sufficient for organs with high mitochondrial content (e.g. brain and heart).
Note: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
Sucrose (Amresco, cat. no. 0335)
EGTA (Sigma, cat. no. E3889)
1 M HEPES solution (Gibco, cat. no. 15630-056)
Tris (hydroxymethyl) aminomethane (Euromedex)
Protease inhibitor cocktail (Roche, cat. no. 11697498001)
0.1 M EGTA/Tris (see reagents and solutions)
Isolation buffer (see reagents and solutions)
Bovine serum albumin (BSA, Sigma cat. no. A7030)
Bradford protein assay (Bio-Rad, cat. no. 500-0006)
PX-SR 50 E stirrer (POLYMIX)
Wheaton Glass 2-mL Potter-Elvehjem Tissue Grinder Set (Wheaton, cat. no. 358029)
15-ml polypropylene Falcon tubes, 2-ml centrifuge tubes, 1.5-ml centrifuge tubes
METHOD
Sacrifice a mouse under anesthesia (e.g. 3-5% isoflurane (Adams and Pacharinsak, 2015)) and excise the liver, rinse the liver briefly with ice-cold IB, excise and put about 30 mg of liver tissue into an ice-cold Wheaton Glass tube (appropriate for 2-ml homogenization volume).
Note 1: Snap frozen tissues that are appropriately stored can also be used with this protocol. Freezing does not affect the activity of the SCs.
-
2.
Add 2 ml of ice-cold IB into the Wheaton glass tube, homogenize the liver at 1500 rpm (or max. speed). For liver, 20 strokes are required. Increase the number of strokes if the tissue is not homogenized well. For cells (primary or cultured), ~40 strokes will be required.
Note 2: Keep the homogenizer probe under the surface of IB to avoid foaming.
-
3.
Transfer 2 ml of the homogenate into a 2-ml Eppendorf tube and centrifuge at 600 × g for 10 min at 4°C. This step will separate the cell debris from the mitochondrial extract.
-
4.
Transfer the supernatant (about 1.5 ml, leave the supernatant which is close to the pellet to avoid contamination) to a 1.5 ml tube and centrifuge at 7,000 × g for 10 min at 4°C. Discard the supernatant and resuspend the pellet (which is the mitochondria) in 2ml of cold IB by tapping the tube. Centrifuge again at 7,000 × g for 10 min at 4°C.
-
5.
Decant the supernatant and resuspend the pellet in ~0.25 ml of cold IB. This volume can be changed depending on the pellet size. Care should be taken not to dissolve the pellet in too much IB, which would impact the protein determination.
ALTERNATIVE PROTOCOL
The pellet (mitochondria) from step 5 can be stored at −80°C after the removal of the supernatant. Frozen mitochondria can be stored at −80°C for yrs. Alternatively, the resuspended mitochondria from step 5 can also be frozen and stored at −80°C for several months for later use. Although 1-2 cycles of freezing-thawing of the resuspended mitochondrial extract does not affect the quality of the BN-PAGE and immunoblotting, it is always better to make aliquots of the extract before storage.
-
6.
Measure the mitochondrial protein concentration from the above resuspended pellet (to determine the mitochondrial protein content) using the Bradford method (or other alternative protein determination method of choice), using BSA as a standard.
-
7.
Aliquot 50 μg of mitochondrial protein into another 1.5-ml Eppendorf tube, centrifuge at 7,000 × g for 10 min at 4°C. Discard the supernatant and keep the pellet for BN-PAGE (STEP 2).
STEP 2(A). BN-PAGE and Immunoblotting (BASIC PROTOCOL 1)
MATERIALS
5% digitonin (Novex, cat. no. BN2006)
NativePAGE Sample Buffer (4×; Novex, cat. no. BN20032)
NativePAGE 5% Coomassie G-250 sample additive (Novex, cat. no. BN2004)
NativePAGE Running Buffer (20×; Novex, cat. no. BN2001)
Coomassie Brilliant Blue G-250 (SERVA, cat. no. 17524)
NativePAGE 3-12% Bis-Tris Protein Gels 1.0mm × 15 well (Novex, cat. no. BN2012BX10) or NativePAGE 3-12% Bis-Tris Protein Gels 1.0mm × 10 well (Novex, cat. no. BN2011BX10)
Colloidal blue staining kit (Novex, cat. no. LC6025)
XCell SureLock Mini-Cell (Novex, cat. no. EI0001)
Prot/Elec Tips (Bio-Rad, cat. no. 223-9915)
NuPAGE Transfer Buffer (20×; Novex, cat. no. NP0006-1)
iBlot Gel Transfer Device (Invitrogen, cat. no. IB1001EU)
iBlot Gel Transfer Stacks, PVDF, mini (Novex, cat. no. IB4010-02)
Methanol (Fisher Scientific, cat. no. M/4000/17)
Western Breeze Chromogenic Immunodetection System (Novex, cat. no. WB7103) This kit can be used only for mouse. A different kit is available for rabbit (Novex, cat. no. WB7105) and goat (Novex, cat. no. WB7107), where only the secondary antibody is different. All these kits contain Blocker/Diluent part A, Blocker/Diluent part B, 16× Antibody wash solution, secondary antibody solution, chromogenic substrate. Blocking solution, Primary antibody solution and Antibody wash solution should be prepared. fresh from the above stock according to the manufacture's instructions.
Native PAGE anode buffer (see reagents and solutions)
Dark blue cathode buffer (see reagents and solutions)
Light blue cathode buffer (see reagents and solutions)
Blocking solution (see reagents and solutions)
Anti-OxPhos Complex Kit (Life technologies, cat. no. 457999)
Anti-MTCO1 antibody (Abcam, cat. no. ab14705)
Other antibodies of choice can be used for any other immunoblotting.
Primary antibody solution contains anti-OxPhos antibody cocktail and anti-MTCO1 antibody (see reagents and solutions)
Note 3: Anti-MTCO1 antibody is used to enhance the intensity of complex IV in ETC, which is weaker than the other ETC complexes when using only anti-OxPhos antibody cocktail.
Antibody wash solution (see reagents and solutions)
METHOD
-
8.
Sample buffer cocktail preparation: To make 20 μl of sample buffer for 50 μg protein, mix 5 μl of NativePAGE sample buffer (4×), 8 μl of 5% digitonin, and 7 μl of water.
Refer to the calculation below for sample buffer cocktail preparation for 50 μg protein for two different detergent/protein ratio.
| Protein | Digitonin/Protein ratio | Sample Buffer (4x) | Digitonin (5%) | Water | Final volume | Coomassie G-250 sample additive (5%)* |
|---|---|---|---|---|---|---|
| 50 μg | 8 g/g | 5 μl | 8 μl | 7 μl | 20 μl | 2 μl |
| 50 μg | 4 g/g | 5 μl | 4 μl | 11 μl | 20 μl | 1 μl |
Add Coomassie G-250 sample additive just before loading the samples into the gel
Note 4: The digitonin/protein ratio is very critical for solubilizing the membrane proteins for BN-PAGE.
The above sample buffer preparation is for 50 μg of proteins, and the digitonin/protein ratio is 8 g/g. The digitonin concentration should be adjusted depending on the protein concentration, such that the digitonin/protein ratio is kept between 4 g/g-8 g/g. For tissues with high mitochondrial content (e.g. heart), 8 g/g digitonin gives a better resolution than 4 g/g digitonin.
Note 5: heat the digitonin stock at 95°C for 3 min, mix and cool on ice, before making the master mix.
-
9.
Add 20 μl of sample buffer cocktail to 50 μg mitochondrial protein (obtained from point 7 of step 1). Solubilize the pellet by gently mixing it with a 20p pipette. Care should be taken to avoid the formation of bubbles. Incubate the solubilized mitochondria on ice for 20 min.
-
10.
Centrifuge at 20,000 × g for 10 min at 4°C and then collect 15 μl of the supernatant into new tubes.
-
11.
Add 2 μl of Coomassie G-250 sample additive to the above supernatant. The G-250 volume should be such that the final G-250 concentration is 1/4th the detergent concentration. This step should be done just before loading the samples into the gel.
-
12.
Set up the electrophoresis system (XCell SureLock Mini-Cell): Remove the white tape on the bottom of a NativePAGE 3-12% gradient gel and place it in the apparatus.
-
13.
Wash the wells of the gel with 1 ml of dark blue cathode buffer. Put 20 ml of dark blue cathode buffer in the inner chamber and check for leakage.
-
14.
Load 15 μl of sample into the gel by using Prot/Elec Tips. Fill the inner chamber with about 180 ml of dark blue cathode buffer, and then fill the outside chamber with about 600 ml of running buffer.
Note 6: Carefully add the dark blue cathode buffer without disturbing the samples in wells.
-
15.
Turn on the power supply and run the gel at 150 V for 30 min. Remove the dark blue buffer with a 10-ml size pipette or suction tube and fill the inner chamber with 200 ml of light blue buffer. Run the gel at 250 V for 60 min. If better separation of the bands is desired then the gel can be run for maximum of 150 min at 250 V. A longer run time than 150 min has minimal impact on the separation and resolution of bands.
Note 7: If In-gel activity has to be performed then this step should be modified, as explained in STEP 2 (B).
-
16.
Transfer of the BN-PAGE gel: Take the gel out of the cassette and wash it with water. Then incubate the gel in transfer buffer for 15 min with gentle shaking.
Note 8: To remove the gradient gel, take it from the bottom part, which is stronger and therefore chances of breakage are minimized.
-
17.
Prepare the iBlot Gel Transfer Device: Attach the sponge to the lid of the transfer apparatus. At the bottom of the apparatus, place the anode stack, which has the PVDF membrane on the top. Carefully place the gel onto the center of the membrane. Remove the bubbles (if any) with the roller provided with the apparatus. Pre-wet one filter paper in the transfer buffer and place it onto the gel. Place the cathode stack on the filter paper with the rough part facing up. Close the lid and select program 3 (P3: 7 min) to start transfer.
-
18.
After finishing the transfer, carefully remove the membrane from the apparatus and wash it with 8% acetic acid to fix the proteins for 5 min with gentle shaking. Wash twice with water for 5 min with shaking and thereafter air-dry the membrane by clipping it by one side.
Immunoblotting
-
19.
Incubate the membrane with 100% methanol for 5min. Repeat this step 2 additional times with gentle shaking to remove the Coomassie blue. Wash the membrane 3 times with water for 5min.
-
20.
Incubate with 10ml of blocking solution for 30 min at 21°C with gentle shaking. During this time, prepare the primary antibody solution. Wash the membrane twice with water for 5 min.
-
21.
Incubate with 10ml of primary antibody solution for 90 min at 21°C with gentle shaking. During this time, prepare the antibody wash solution.
-
22.
Wash twice with 10 ml of antibody wash solution for 5min, with gentle shaking. Wash twice with water for 5 min.
-
23.
Incubate with 10 ml of secondary antibody solution for 45 min at 21°C with gentle shaking.
-
24.
Wash twice with 10 ml of antibody wash solution for 5 min with gentle shaking. Wash twice with water for 5 min.
-
25.
Incubate with chromogenic substrate solution with gentle shaking. When the expected bands are clear enough, as shown in Figure 3 and 4G, the reaction can be stopped by replacing the solution with water. Wash twice with water for 5 min.
Note 9: Normally it takes ~ 3-10 min depending on the sample type and antibody quality. Maximum incubation time is 60 min. If the signal is not seen after 60 min then the antibody should be replaced and/or protein quantity should be increased.
STEP 2 (B). Clear-Native (CN)-PAGE and In-gel activity of Complex I, IV, IV+I, II, V and III (BASIC PROTOCOL 2)
Refer to REAGENTS AND SOLUTIONS for making the substrate for Complex I, II, IV and V activity
METHOD
For in-gel supercomplex/complex activity, electrophoresis should be done on ice. It is recommended to run two gels simultaneously wherein one gel can be used for immunobloting and the other for in-gel activity. Alternatively, if samples are less, the gel can be cut into half after the run and used for immunobloting and in-gel activity.
Perform CN (clear native)-PAGE: This is basically STEP 2 (A) (BN-PAGE) with modifications. The modified steps are in italics below.
-
8.
Prepare Sample buffer cocktail as explained in point 8 of STEP 2 (A)
-
9.
Add 20 μl of sample buffer to 50 μg mitochondrial protein (obtained from point 7 of STEP 1). Solubilize the pellet by gently mixing it with a 20p pipette. Incubate the solubilized mitochondria on ice for 20 min.
-
10.
Centrifuge at 20,000 × g for 10 min at 4°C and then collect 15 μl of the supernatant into new tubes.
-
11.
Add 2 μl of Coomassie G-250 sample additive to the above supernatant. The G-250 volume should be such that the final G-250 concentration is 1/4th the detergent concentration.
-
12.
Set up the electrophoresis system (XCell SureLock Mini-Cell): Remove the white tape on the bottom of a NativePAGE 3-12% gradient gel and place it in the apparatus.
-
13.
Dissolve 0.022 g Coomassie Brilliant Blue G-250 in 200 ml of Native PAGE anode buffer, mix well. This is the light blue cathode buffer.
-
14.
Wash the wells of the gel with 1 ml of light blue cathode buffer. Put 20 ml of light blue cathode buffer in the inner chamber and check for leakage.
-
15.
Load 15 μl of sample into the gel by using Prot/Elec Tips. Fill the inner chamber with about 180 ml of light blue cathode buffer, and then fill the outside chamber with about 600 ml of running buffer.
-
16.
Run the CN-PAGE, at 150 V for 30 min. After 30 min the light blue cathode buffer is changed to the clear cathode/running buffer, to avoid excesssive blue color of the Coomassie dye on the gel which interferes with the color of the activity. The gel is run for additional 150 min at 250 V for better separation of the SC bands. Immunoblotting can also be performed with this BN-PAGE method also by continuing from point 8 of STEP 2 and then following STEP 3. If the activity assay and immunoblotting has to be compared in parallel then the BN-PAGE should be performed as explained above (continuing from point 8 of STEP 2 and then following STEP 3).
-
17.
After completion of the run, the gel should be carefully removed and placed in ice cold water.
-
18.
Complex I activity: Incubate the gel in 20 ml of the Complex I substrate solution. Appearance of violet bands is indicative of Complex I activity. The time for activity depends on the samples, protein amount and the experimental conditions. For 50 μg liver ~15-20 min is generally sufficient. Stop the reaction with 10% of acetic acid. Wash the gel with water and scan. An example is shown in Figure 4A.
-
19.
Complex IV activity: Incubate the gel in 20ml of complex IV substrate. Appearance of brown bands is indicative of complex IV activity. For 50 μg liver ~30-40 min is sufficient. Stop the reaction with 10% of acetic acid. Wash the gel with water and scan. An example is shown in Figure 4B.
-
20.
Complex IV + I activity can be performed on the same gel. In this case first the gel is incubated in Complex IV substrate. Once the appropriate brown signal is observed (Figure 3B), the gel is washed with water for ~1 min and incubated in complex I substrate. Following the appearance of the appropriate blue signal the reaction is stopped with 10% of acetic acid. Wash the gel with water and scan (see Figure 4C for an example).
-
21.
Complex II activity: Incubate the gel in 10ml of complex II substrate. Appearance of violet bands is indicative of complex II activity. For 50 μg liver ~40 min is sufficient. Stop the reaction with 10% of acetic acid. Wash the gel with water and scan. An example is shown in Figure 4E.
-
22.
Complex V activity: Incubate the gel in 10ml of complex V substrate overnight (~ 16 h). Appearance of transparent/silver bands is indicative of complex V activity. Stop the reaction with 50% of methanol. Wash the gel with water and scan. Since the bands are transparent, the gel should be inverted (to black background) after scanning to visualize the activity clearly. An example is shown in Figure 4F.
-
23.
Complex III and OXPHOS: Immunoblot can be performed to detect CIII (Figure 4D). For the alignment of the bands, an OXPHOS immunoblotting should be performed in parallel (see Figure 3G).
Alternate protocol for SC analysis
After running the BN-PAGE (STEP: 2), instead of immunoblotting, the complexes and SCs can be visualized by a simple Coomassie staining and destaining method. However, the protein concentration should be higher (~100 μg) in order to visualize the SC bands (Figure 5).
REAGENTS AND SOLUTIONS
Use Milli-Q-purified water or equivalent in all recipes and protocol steps.
EGTA/Tris, 0.1 M
Dissolve 3.8 g of EGTA in 50 ml of water, adjust pH to 7.4 using Tris powder, add more water to bring 100 ml and store at 4°C for up to 1 yr.
Isolation buffer (IB)
Dissolve 6.846 g of sucrose, 0.121 g of Tris, 1 ml of 0.1 M EGTA/Tris, in 80 ml of water. Adjust pH to 7.4 with 1 M HEPES and bring the volume to 100 ml with water. The buffer aliquots can be stored at −20°C for 6 months. Add 1× of the protease inhibitor cocktail fresh, prior to mitochondrial isolation.
Native PAGE anode buffer
To make 1 L anode buffer, add 50 ml of 20× Native PAGE anode buffer to 950 ml of water.
Dark blue cathode buffer
Dissolve 0.044 g Coomassie Brilliant Blue G-250 in 220 ml of Native PAGE anode buffer, mix well.
Light blue cathode buffer
Add 20 ml of dark blue cathode buffer into 180ml of Native PAGE anode buffer, mix well. The buffer can only be used once.
Blocking solution
To make 10 ml of blocking solution, add 2 ml of Blocker/Diluent part A and 3 ml of Blocker/Diluent part B into 5 ml of water.
Primary antibody solution
To make 10 ml of solution, add 2 ml of Blocker/Diluent part A and 1 ml of Blocker/Diluent part B, 10 μl of anti-OxPhos antibody cocktail, 5 μl of anti-MTCO1 antibody into 7 ml of water.
Note 3: Anti-MTCO1 antibody is used to enhance the intensity of complex IV in ETC, which is weaker than the other ETC complexes when using only anti-OxPhos antibody cocktail.
Antibody wash solution
Dilute 5 ml of 16× antibody wash solution with 75 ml of water.
Complex I activity substrate
This buffer should be prepared fresh each time from the stock solutions. In 20 ml of water add (i) 2 mM Tris-HCl (40 μl from 1 M Tris-HCl stock) pH 7,4 (ii) 0,1 mg/ml NADH (2 mg) (iii) 2,5 mg/ml Nitrotetrazolium Blue chloride (NTB 50 mg).
Complex IV activity substrate
This buffer can be prepared as a stock solution and stored at −20°C for months. For 100 ml of substrate add (i) 50 mg Diaminobenzidine (DAB) (ii) 100 mg cytochrome c and (iii) 90 ml of 50 mM phosphate buffer pH 7.4 to 10 ml water. Make 10/15 ml aliquots and store at −20°C.
Complex II activity substrate
This buffer should be prepared fresh each time. For 10 ml substrate, add (i) 200 μl of 1 M Sodium Succinate (ii) 25 mg NTB (iii) 8 μl of 250 mM Phenazine Methosulphate (prepare 250 mM stock in DMSO) and (iii) 5mM Tris-HCl (50 μl from 1 M Tris-HCl) to 9.742 ml water.
Complex V activity substrate
This buffer should be prepared fresh each time from the stock solutions. For 10 ml substrate, add (i) 35 mM Tris (350 μl from 1 M Tris) (ii) 270mM glycine (2.7 ml from 1 M glycine) (iii) 14 mM MgSO4 (140 μl from 1 M MgSO4) (IV) 10 mM ATP (100 μl from 1 M ATP) and (V) 0.2% Pb(NO3)2 (20 mg) to 6.710 ml water.
COMMENTARY
Background Information
BN-PAGE was developed for the separation of mitochondrial membrane proteins and complexes in the mass range of 10 kDa to 10 MDa (Schagger and von Jagow, 1991). It can also be used to determine native protein masses and oligomeric states and to identify physiological protein–protein interactions. This protocol can be used to analyze the presence or absence of a particular SC in different mouse models or to assess the SC activity under different environmental and physiological states. The advantage of this protocol over the other protocols used for BN-PAGE is that it allows clear resolution and sharp complex and SC bands. This was achieved by the combination of NativePAGE 3-12% Bis-Tris Protein Gels, optimization of digitonin to protein concentration and of the running time. This protocol does not describe the method for in-gel activity of CIII. Due to the overlapping substrates of CIII and CIV it is not easy to detect CIII, although it has been shown earlier at higher protein concentration from bovine heart mitochondria (Wittig et al., 2007).
Critical Parameters
Always wear gloves, protective eyewear, and a laboratory coat while handling the detergents.
All steps for mitochondrial isolation must be performed as quickly as possible at 4°C to inactivate proteases and phospholipases. The protocol can be scaled up according to the amount of mitochondria required. Instead of mouse liver samples, this protocol is also applicable for other mouse tissues such as heart muscle, quadriceps muscle, or brown adipose tissue.
If the sample is in a SDS-PAGE sample buffer, prepare a fresh lysate without SDS using digitonin. Do not use SDS-PAGE samples for native gel electrophoresis.
Do not heat the samples for native gel electrophoresis.
If Coomassie G-250 sample additive is not added to the samples prior to loading into the gel, then the bands will appear diffused. They will not appear sharp and well resolved. Similarly, a self-made gradient gel may not give reproducible results and sharp bands.
Troubleshooting
Streaking of bands may indicate that either the mitochondrial extract is contaminated with cell lysate or the detergent concentration is suboptimal for the amount of protein used. In this situation, centrifuge the samples at 20,000 × g for 10 min at 4°C and increase the digitonin to protein ratio to 8g/g.
If no bands appear on the gel after incubating it in the substrate solutions for CI, CII, and CIV for more than 1 hr, then increase the amount of protein load. Use an accurate and sensitive protein estimation method.
Anticipated Results
With this brief protocol, one can easily assess how many SCs are present in tissues of a particular mouse strain. Typical examples are shown in Figures 1c, 3, 4 and 5. A mouse strain, which lacks Cox7a2l/SCAFI will have the SC pattern of C57BL/6 and the strain which has functional cox7a2l will show a SC pattern similar to that of DBA mice (Figures 1c, 3, 4 and 5). SC activity is also altered by fasting and feeding (Lapuente-Brun et al., 2013).
Time Considerations
The initial isolation and solubilization of mitochondria will take ~60 min for up to 4 samples. If the sample number is more than 10, then it is advisable to do extraction and solubilization on day 1 and to continue with BN-PAGE on day 2. BN-PAGE will take ~180 min and immunoblotting followed by in-gel activity will take ~6 h. In total execution of this protocol will take 2-3 days for completion.
ACKNOWLEDGEMENTS
J.A. is the Nestlé Chair in Energy Metabolism and the research in his laboratory is supported by the Ecole Polytechnique Fédérale de Lausanne (EPFL), the National Institutes of Health (NIH; R01AG043930), Krebsforschung Schweiz (Swiss Cancer League; KFS-3082-02-2013), SystemsX.ch (SySX.ch 2013/153), and the Swiss National Science Foundation (SNSF; 31003 A-140780).
Footnotes
CONFLICTS OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Molecular cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Adams S, Pacharinsak C. Mouse anesthesia and analgesia. Current protocols in mouse biology. 2015;5:51–63. doi: 10.1002/9780470942390.mo140179. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Ugalde C. I function, therefore I am: overcoming skepticism about mitochondrial supercomplexes. Cell Metab. 2013;18:147–149. doi: 10.1016/j.cmet.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, Van Coster R, Baethmann M, Voit T, Trijbels JM, Smeitink JA. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochemical and biophysical research communications. 2000;275:63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- Chaban Y, Boekema EJ, Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochimica et biophysica acta. 2014;1837:418–426. doi: 10.1016/j.bbabio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome. 2008;19:318–331. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- D'Aurelio M, Gajewski CD, Lenaz G, Manfredi G. Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Human molecular genetics. 2006;15:2157–2169. doi: 10.1093/hmg/ddl141. [DOI] [PubMed] [Google Scholar]
- Gomez LA, Hagen TM. Age-related decline in mitochondrial bioenergetics: does supercomplex destabilization determine lower oxidative capacity and higher superoxide production? Seminars in cell & developmental biology. 2012;23:758–767. doi: 10.1016/j.semcdb.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamantea E, Carrara F, Mariotti C, Morandi L, Tiranti V, Zeviani M. A novel nonsense mutation (Q352X) in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscular disorders : NMD. 2002;12:49–52. doi: 10.1016/s0960-8966(01)00244-9. [DOI] [PubMed] [Google Scholar]
- Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, Navas P, Cruz R, Carracedo A, Lopez-Otin C, Perez-Martos A, Fernandez-Silva P, Fernandez-Vizarra E, Enriquez JA. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxidants & redox signaling. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. Journal of molecular biology. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A, Ugalde C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012;15:324–335. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourier A, Matic S, Ruzzenente B, Larsson NG, Milenkovic D. The respiratory chain supercomplex organization is independent of COX7a2l isoforms. Cell Metab. 2014;20:1069–1075. doi: 10.1016/j.cmet.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Saada A, Edvardson S, Shaag A, Chung WK, Segel R, Miller C, Jalas C, Elpeleg O. Combined OXPHOS complex I and IV defect, due to mutated complex I assembly factor C20ORF7. Journal of inherited metabolic disease. 2012;35:125–131. doi: 10.1007/s10545-011-9348-y. [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Analytical biochemistry. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- Schon EA, Dencher NA. Heavy breathing: energy conversion by mitochondrial respiratory supercomplexes. Cell Metab. 2009;9:1–3. doi: 10.1016/j.cmet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Wittig I, Karas M, Schagger H. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Molecular & cellular proteomics : MCP. 2007;6:1215–1225. doi: 10.1074/mcp.M700076-MCP200. [DOI] [PubMed] [Google Scholar]