Abstract
Chronic kidney disease (CKD) and cancer are connected in a number of ways in both directions: cancer can cause CKD either directly or indirectly through the adverse effects of therapies, CKD may conversely be a risk factor for cancer, and both may be associated because they share common risk factors, often toxins. In this review, we briefly address the issue of paraneoplastic nephropathies as well as that of toxin-related cancers and CKD, including analgesic and aristolochic acid nephropathies. We then focus on the links between the various stages of CKD and cancer incidence, and critically examine major epidemiologic surveys in the field. Compared with the general population, kidney transplant recipients have a three- to four-fold increase in overall cancer risk, and relative risks higher than three for about 20 specific tumors, most, but not all, of which known or suspected to be caused by viral agents. After dialysis, cancer risk increases 10 to 80% according to studies, with relative risks significantly higher than the general population for about ten cancer sites. There is emerging evidence for an excess risk of cancer in patients at early CKD stages.
Keywords: Chronic Disease, Humans, Kidney Diseases, Kidney Transplantation, Neoplasms, Renal Dialysis, Risk Factors
Since the publication of the definition and classification of chronic kidney disease (CKD) by the National Kidney Foundation Kidney Disease Outcome Quality Initiative (K/DOQI) in 2002,(1) its association with other diseases including cancer is increasingly recognized.(2) CKD and cancer are connected in a number of ways in both directions: cancer can cause CKD either directly or indirectly through the adverse effects of therapies, CKD may conversely be a risk factor for cancer, and both may be associated because they share common risk factors, most often toxins. (Figure) Paraneoplastic nephropathies are well-known complications of cancer,(3, 4) as well as CKD occuring after chemo- or radiotherapy, or after nephrectomy for kidney cancer.(5) It is also well-established that patients on renal replacement therapy for end-stage CKD, either dialysis or transplantation, (6, 7) are at high risk for cancer, but reliable quantification of the risk by cancer site is based on a few studies.(8–14) More recently, concern arose about the risk for cancer in patients at early CKD stages.(13, 15–17) In this review, we will briefly address the issue of cancer as a cause or a risk factor for CKD as well as that of toxin-related CKD and cancers, and will then focus on the links between the various CKD stages and cancer incidence.
Figure.
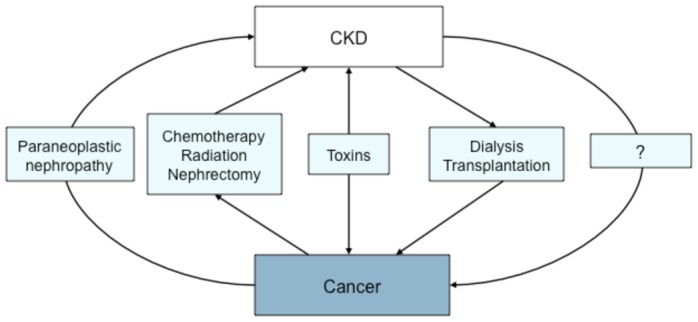
Various pathways linking CKD and cancer
Cancer as a cause or a risk factor for chronic kidney disease
The most frequent situation in which nephrologists have to face CKD in patients with cancer is that following the assessment of kidney function for dosage adjustment before chemotherapy. Because CKD, defined by an estimated glomerular filtration rate (eGFR) < 60 ml.min/1.73 m2, is common, with a prevalence of about 4% in the adult population aged 20 years and older, reaching 30% or more in the elderly, it is expected to be also common in patients with any type of cancer.(18) In the Renal Insufficiency and Anticancer Medications (IRMA) Study, Launay-Vacher at al showed that among 4,684 participants with cancer, aged an average of 58 yrs, 12% had an eGFR < 60 ml.min/1.73 m2 using the abbreviated MDRD equation, and 20% with the Cockcroft-Gault formula.(19) Association, however, does not mean causation, and the cure of most cancers is unlikely to improve the course of CKD. The issue here is that of the prevention of adverse drug effect from overdosage due to renal impairment.
In contrast, cancer-associated glomerulopathies are scarce, but more likely causally-related events. (3, 4, 20, 21) In a comprehensive review of paraneoplastic glomerulopathies, Ronco (3)pointed the heterogeneity of this entity and the diversity of both glomerular injuries and cancers that have been reported. Most often, associations are described as case reports or case series making risk assessment difficult. Clinical remission of the glomerulopathy after cancer removal or chemotherapy in many of the reported cases, however, provides indirect evidence for a causal link between these diseases. (3, 4, 21) Membranous nephropathy (MN) is by far the most frequent type of glomerulopathy associated with solid tumors, but minimal change disease, membranoproliferative glomerulonephritis, extracapillary glomerulonephritis and IgA nephropathy were also reported.(3, 20) Lefaucheur et al (21) showed that 24 patients out of a cohort of 240 with MN (10%) were diagnosed with cancer at the time of renal biopsy or within a year. The age-adjusted incidence of cancer in this cohort was about ten times higher than in the general population. Lung, prostate, and stomach cancers accounted for 13 out of 17 cases in men. Clinical presentation of the patients with cancer–associated MN did not differ from that of those with idiopathic MN, except that they were more often smokers. Interestingly, renal biopsies revealed 92% of patients with cancer-associated MN had more than eight inflammatory cells infiltrating the glomeruli as compared with only 25% of those with idiopathic MN. The determination of leukocyte number was proposed as a mean to identify MN patients who may need cancer screening. Among hemopathy-induced glomerulopathies, the association of Hodgkin’s disease with minimal change disease is the best known, followed by those of chronic lymphocytic leukemia and related B-cell lymphomas with MN and membranoproliferative glomerulonephritis.(3, 4) Regarding the pathophysiology of these paraneoplastic nephropathies, Ronco (3) clearly displayed that whereas it remains obscure for solid tumor-associated glomerulopathies, a molecular link, and even specific molecular abnormalities, can be demonstrated for lymphoplasmacytic disorders.
Finkel and Foringer recently reviewed several other nephropathies secondary to cancer including those occuring after chemo- or radiotherapy and multiple myeloma.(22) Based on a cohort of 1,027 patients with multiple myeloma, Kyle et al21 showed that half of them had serum creatinine > 1.3 mg/dL and 20% > 2 mg/dL at diagnosis. Ten percent of these patients would progress to end-stage renal disease (ESRD).(22–24) Although one of the most common hematological malignancies and despite a relative risk for cancer higher than 30 (13), multiple myeloma accounts for only 1,1% of incident ESRD patients starting renal replacement therapy in the USRDS registry and from 1.0 to 2.5% of those from the European countries contributing to the ERA-EDTA registry. (25, 26)
Toxin-related CKD and cancers
Toxins, such as analgesics and aristolochic acid, can induce both chronic interstitial nephritis and urothelial cancers. The association of analgesic nephropathy with the occurrence of urothelial carcinoma was first described in Sweden in 1965, and subsequently confirmed in several other countries. (27–32) Tumors were mainly transitional cell carcinomas located in the renal pelvis, the upper urinary tract and the bladder, but renal cell carcinomas were also described. (33) Phenacetin was recognized as the main culprit in both diseases and withdrawn from the market during the 1970s. In Australia, further evaluation of the effect of withdrawal of phenacetin-containing analgesics showed a significant decline in ESRD incidence due to analgesic nephropathy and a trend toward less renal pelvis and bladder cancers. (34, 35) Although the role of acetaminophen or other nonphenacetin analgesics in the occurrence of analgesic nephropathy is still debated, (36) neither of them has been convincingly linked with cancer. (37, 38)
Two other chronic interstitial nephritis are strongly correlated with the occurrence of urinary tract cancer : the aristolochic acid nephropathy (AAN) and the Balkan-endemic nephropathy (BEN). (39) AAN, initially called Chinese herb nephropathy, is a rapidly progressive interstitial nephritis leading to ESRD and urothelial cancer, which was first reported in Belgium in a hundred women treated with slimming pills containing Chinese herbs. (39, 40) The substitution of Stephania Tetandra by Aristolochia Fangji rich in aristolochic acid (AA), a toxic compound with nephrotoxic and carcinogenic properties, was established as the cause of this case cluster. Other cases were further reported in several countries. (41, 42) Sytematic removal of the native kidneys and ureters showed that 40–45% of the AAN patients on dialysis or transplanted displayed multifocal high-grade transitional carcinomas, mainly in the upper urinary tract. (43) Follow-up of those who received a kidney transplant showed a 40% cumulative incidence of bladder urothelial carcinoma. (44)
BEN is a chronic tubulointerstitial nephritis with slow progression to ESRD and an increased incidence of upper urinary tract urothelial carcinoma. (45–47) It is endemic in several rural areas of the Balkans. BEN and acid aristolochic nephropathy are very similar with respect to both clinical and morphological aspects. That exposure to AA through consumption of flour contaminated with seeds of Aristolochia clematitis may be responsible for BEN in endemic areas is an old hypothesis. (46) The detection of AA-specific DNA adducts in renal tissue of a number of BEN patients and individuals living in endemic areas recently provided new evidence for a causal association. (48, 49)
Chronic kidney disease as a risk factor for cancer
Cancers occurring after dialysis or transplantion have long been described, but risk assessment by cancer site has been available only recently. In contrast, little is known about the risk of cancer associated with CKD before end-stage.
Cancer risk after kidney transplantion
From the beginning of transplantation, a high incidence of lymphoma and skin cancers, was observed in graft recipients. (7) The skin is by far the most frequent site of malignancies after transplantation. Nonmelanoma skin tumors, including squamous cell and basal cell carcinomas, account for 95% of these skin cancers, whereas rare tumors such as melanoma and Kaposi’s sarcoma account for the other 5%.(50, 51) However, as graft and patient survival have improved due to better immunosuppressive agents, the risk for various other types of cancer has also increased. Because the incidence of most cancers is low, it is difficult to assess from a single center. Moreover, reporting of cancers to transplant registries is often incomplete and may lead to underestimate the risk. In the past five years, nevertheless, a few large studies have examined site-specific cancer risk for a wide spectrum of tumors by linking national transplant registry database with those of either national cancer registry (10, 11, 13, 14) or Medicare billing claims. (12) Four of them which identified at least 500 cancer cases are described in Table 1. (14) Two studies (13, 14) did not include nonmelanoma skin cancers, and tended to show lower overall risk than the others. (10–12) In US kidney transplant patients, the risk (cumulative incidence) of skin cancer (excluding melanoma) was 7.4 % at three years and that of any nonskin cancer (but including melanoma), 7.5%.(12) Cancer risk (excluding nonmelanoma skin cancer) has been estimated at 20% after 10 yrs of immunosuppression in Australia and New Zealand, (7) but lower in Canada, 12% after 17 yrs. (14)
Table 1.
Description of main cohort studies investigating cancer incidence in patients with CKD before and after dialysis or transplantation for end-stage CKD
| Author, year [Ref] Country |
Population | Sample size | Follow- up (yrs) | n observed cancers | Age* (yrs) | Sex ratio | |
|---|---|---|---|---|---|---|---|
| Non end-stage CKD | Wong, 2009 [17] Australia |
Population-based cohort of predominantly white Australians | 3,049 | 10.1 | 711 | 49–97 | 0.74 |
| Jorgensen, 2008 [16] Norway |
Population-based cohort of Tromso inhabitants | 5,425 | 10.3 | 590 | 24–74 | 1.13 | |
| Vajdic, 2006 [13] Australia |
ANZDATA registry Patients with ESRD studied up to 5 yrs before starting RRT | 25,685 | 4.6 | 689a | 50 | 1.32 | |
| Dialysis | Maisonneuve, 1999 [9] Australia, New Zealand |
ANZDATA registry | 13,497 | 2.6 | 500 | 49 | 1.26 |
| Europe | ERA-EDTA registry | 296,903 | 2.9 | 6,849 | 52 | 1.40 | |
| USA | USRDS | 521,404 | 2.2 | 17,695 | 58 | 1.15 | |
| Vajdic, 2006 [13] Australia |
ANZDATA registry | 24,926 | 2.7 | 870a | 54 | 1.31 | |
| Kidney Transplant | Adami, 2003 [11] Sweden |
Transplant patients from in-patient registry | 5,004 | 7.4 | 639 | 46 | 1.53 |
| Kasiske, 2004 [12] USA |
Record linkage of USRDS with Medicare | 35,765 | 3.0 | 14.9%b | 46% > 50 |
1.50 | |
| Vajdic, 2006 [13] Australia |
ANZDATA registry | 10,180 | 8.5 | 1,236a | 41 | 1.41 | |
| Villeneuve, 2007 [14] Canada |
CORR registry | 11,033 | 7.3 | 778a | 30% > 50 |
1.72 |
CKD : chronic kidney disease; ANZDATA : Australian and New Zealand Dialysis and Transplant Registry; ERA-EDTA : European Renal Association and European Dialysis and Transplant Association Registry; USRDS : US Renal Data System; CORR : Canadian Organ Replacement register
Mean or median age or % of patients aged > 50 yrs or min-max in years
excluding nonmelanoma skin cancer
cumulative incidence at 3 yrs after transplantation (number of cancers not available)
All four studies showed that, compared with the general population, transplant recipients have a three- to four-fold overall increased risk of cancer. (Table 2) Standardized incidence ratios (SIR) or hazard ratios (HR) for common digestive cancers and lung cancer ranged from 1.4 to 3.6, and those for leukemia and liver cancers, from 1.8 to 7.9. SIR for cervix cancers ranged from 1.6 to 5.7, but those for vulvovaginal cancers were much higher, ranging from 5.5 to 24.5, as for penis cancer, higher than 15, in one study. (13) Cancers of the oral cavity, including the mouth, the lip and the tongue, were increased more than 10-fold compared with the general population. The risk for bladder cancer was increased about two- to fourfold, while kidney cancer was 5- to 15-fold more frequent. Thyroid cancer was also highly increased, about four- to 15-fold. Karposi’s sarcoma, non-Hodgkin’s lymphomas, and nonmelanoma skin cancers were 30- to more than a 100-fold increased compared to the general population. In contrast, the risks for prostate, breast and ovary cancers were not increased in three out of four studies. Vajdic et al (13) pointed that most of post-transplant cancers, including non-Hodgkin lymphoma, Kaposi sarcoma, and nonmelanoma skin, liver, genital and oral cavity cancers, were known or suspected to be caused by viral agents, such as Epstein-Barr virus, human T-cell lymphotrophic virus type I, human papillomavirus and herpes virus, hepatitis B and C viruses. Although the increased cancer risk in transplant recipients is primarily due to impaired immunity resulting from immunosuppressive drugs, other risk factors may also play a role : older age, male gender, White and non-Hispanic ethnicity, diabetes-related ESRD. (12, 52) More than five years of dialysis before transplantation was also associated with an increase in cancer risk. (13) These findings raise concern about cancer prevention, screening strategies and cost-effectiveness, and management in this high risk population. (53–55) Several guidelines, as reviewed by Webster et al, (53) strongly recommend cancer screening in the transplant population despite the lack of randomized clinical trials establishing benefits in this setting.
Table 2.
Relative risk of cancer in patients with non end-stage CKD and in those on dialysis or with a kidney transplant for end-stage CKD
| Cancer site | Non end-stage CKD
|
Dialysis
|
Kidney Transplant
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| [Ref] | n | RR or HR (95%CI)a,b or SIR (95%CI)c | [Ref] | n | SIR (95%CI) | [Ref] | n | SIR (95%CI) or RR (95%CI)d | |
| All cancer | [17] | M : 245 | 1.3 (1.1,1.5)a | [9] ANZ | 500 | 1.8 (1.7,2.0) | [11] | 639 | 3.9 (3.6,4.2) |
| W : 237 | 1.0 (0.9,1.2)a | [9] Euro | 6,849 | 1.1 (1.0,1.1) | [12]d | - | - | ||
| [16] | 590 | 1.2 (p<0.001)b | [9] USA | 17,695 | 1.2 (1.2,1.2) | [14] | 778 | 2.5 (2.3,2.7)e | |
| [13] | 689 | 1.2 (1.1,1.3)c | [13] | 870 | 1.4 (1.3,1.5) | [13] | 1,236 | 3.3 (3.1,3.5)e | |
|
| |||||||||
| Genitourinary | |||||||||
|
| |||||||||
| Kidney | [16] | 17 | 1.4 (1.0,2.1)b | [9] ANZ | 70 | 9.8 (7.7,12.3) | [11] | 28 | 5.2 (3.4,7.5) |
| [13] | 193 | 13.2 (11.4,15.1)c | [9] Euro | 680 | 3.3 (3.1,3.6) | [12]d | - | 14.6 | |
| [9] USA | 1,303 | 3.7 (3.5,3.9) | [14] | 71 | 7.3 (5.7,9.2) | ||||
| [13] | 86 | 5.6 (4.5,7.0) | [13] | 70 | 7.3 (5.7,9.2) | ||||
| Bladder (B) or urinary tract (UT) | [17] | M : 16 (UT) | 5.0 (p<0.04)a† | [9] ANZ | 53 | 4.8 (3.6,6.2) | [11] | - | - |
| [16] | 35 (B) | 1.8 (1.5,2.3)b | [9] Euro | 660 | 1.5 (1.4,1.7) | [12]d | - | 3.6 | |
| [13] | 85 (B) | 2.7 (2.2,3.4)c | [9] USA | 933 | 1.4 (1.3,1.5) | [14] | 24 | 2.0 (1.3,3.0) | |
| [13] | 63 | 2.2 (1.7,2.9) | [13] | 42 | 3.3 (2.4,4.5) | ||||
| Uterus cervix | [13] | 11 | 1.6 (0.8,2.9)c | [9] ANZ | 15 | 4.0 (2.4,6.6) | [11] | - | |
| [9] Euro | 146 | 1.6 (1.3,1.8) | [12]d | - | 5.7 | ||||
| [9] USA | 290 | 2.5 (2.2,2.8) | [14] | 6 | 1.6 (0.6,3.4) | ||||
| [13] | 13 | 2.6 (1.4,4.4) | [13] | 13 | 2.5 (1.3,4.3) | ||||
| Prostate | [17] | 62 | NS | [9] ANZ | 36 | 1.2 (0.9,1.7) | [11] | 20 | 1.1 (0.7,1.7) |
| [16] | 110 | 1.0 (0.9,1,3) | [9] Euro | 422 | 0.9 (0.8,1.0) | [12]d | - | 1.6 | |
| [13] | 153 | 1.2 (1.0, 1.4) | [9] USA | 2,018 | 0.7 (0.6,0.7) | [14] | 37 | 0.9 (0.6,1.3) | |
| [13] | 74 | 0.7 (0.5,0.8) | [13] | 41 | 1.0 (0.7,1.3) | ||||
|
| |||||||||
| Hematopoietic | |||||||||
|
| |||||||||
| Non-Hodgkin lymphoma | [13] | 35 | 1.5 (1.1,2.1) | [9] ANZ | 11 | 1.4 (0.8,2.6) | [11] | 27 | 3.8 (2.5,5.6) |
| [9] Euro | 102 | 0.6 (0.5,0.8) | [12]d | - | 29.1 | ||||
| [9] USA | 736 | 1.7 (1.5,1.8) | [14] | 155 | 9.9 (8.4–11.5) | ||||
| [13] | 35 | 1.5 (1.1,2.1) | [13] | 125 | 8.8 (7.4,10.5) | ||||
| Leukemia | [13] | 16 | 0.9 (0.5,1.4) | [9] ANZ | 11 | 1.4 (0.8,2.6) | [11] | - | - |
| [9] Euro | 102 | 0.6 (0.5,0.8) | [12]d | - | 7.9 | ||||
| [9] USA | 736 | 1.7 (1.5,1.8) | [14] | 17 | 2.3 (1.3,3.6) | ||||
| [13] | 35 | 1.5 (1.1,2.1) | [13] | 24 | 2.5 (1.7,3.7) | ||||
|
| |||||||||
| Oral cavity | |||||||||
|
| |||||||||
| Lip (L) | [13] | 22 (L) | 1.9 (1.2,2.8)c | [9] ANZ | 3 (T) | 1.9 (0.6–5.9) | [11] | 39 (L) | 54.8 (39.0,74.9) |
| Tongue (T) or Mouth (M) | [9] Euro | 90(T) | 2.0 (1.6–2.5) | [12]d | - (M) | 11.1 | |||
| [9] USA | 144(T) | 1.8 (1.5–2.1) | [14] | 54 (L) | 31.3 (23.5,40.8) | ||||
| [13] | 29 (L) | 3.7 (2.5–5.3) | [13] | 283 (L) | 47.1 (42.8,52.3) | ||||
|
| |||||||||
| Digestive | |||||||||
|
| |||||||||
| Colon | [17] | M :35 | NS | [9] ANZ | 49 | 1.1 (0.9,1.5) | [11] | 25 | 2.4 (1.5,3.5) |
| F :41 | NS | [9] Euro | 831 | 0.9 (0.9,1.0) | [12]d | - | 2.8 | ||
| [16] | 90 | 1.1 (1.0,1.4)b | [9] USA | 2,343 | 1.2 (1.1,1.2) | [14] | 51 | 1.4 (1.0,1.8) | |
| [13] | 83 | 1.3 (1.1,1.7)c | [13] | 78 | 1.2 (0.9,1.5) | [13] | 82 | 2.4 (1.9,2.9) | |
| Liver | [13] | 4 | 2.9 (0.8,7.3)c | [9] ANZ | 3 | 1.5 (0.5,4.6) | [11] | - | - |
| [9] Euro | 162 | 1.2 (1.0,1.4) | [12]d | - | 4.5 | ||||
| [9] USA | 192 | 1.5 (1.3,1.7) | [14] | 5 | 1.8 (0.6,4.3) | ||||
| [13] | 14 | 2.3 (1.3,3.8) | [13] | 10 | 3.2 (1.5,5.9) | ||||
|
| |||||||||
| Skin | |||||||||
|
| |||||||||
| Melanoma (MA) | 79 (MA) | 1.0 (0.8,1.3) | [13] | 57 (MA) | 1.0 (0.8,1.4) | [11] | 267 (NMA) | 57.7 (51.0,65.1) | |
| Nonmelanoma skin (NMA) | [13] | 3 (K) | 19.6 (4.1, 57.4) | 6 (K) | 58.9 (21.2,126.0) | [12]d | - (NMA) | 92.3 | |
| Kaposi sarcoma (K) | [14] | 20 (MA) | 1.9 (1.2,3.0) | ||||||
| [13] | 111 (MA) | 2.5 (2.1,3.1) | |||||||
|
| |||||||||
| Other | |||||||||
|
| |||||||||
| Lung | [17] | M :27 | 9.0 (2.0, 30.0)a† | [9] ANZ | 57 | 1.4 (1.1,1.8) | [11] | 24 | 1.7 (1.1,2.5) |
| F :18 | NS | [9] Euro | 978 | 0.9 (0.8,0.9) | [12]d | - | 3.6 | ||
| [16] | 77 | 1.2 (1.0,1.5)b | [9] USA | 2,901 | 1.1 (1.1,1.2) | [14] | 108 | 2.1 (1.7,2.5) | |
| [13] | 34 | 1.1 (0.7,1.5) | [13] | 135 | 1.6 (1.3,1.9) | [13] | 102 | 1.7 (1.1,2.5) | |
| Thyroid | [13] | 15 | 2.6 (1.4,4.2) | [9] ANZ | 11 | 5.9 (3.3,10.7) | [11] | 6 | 3.8 (1.4,8.2) |
| [9] Euro | 87 | 1.9 (1.5,2.3) | [12]d | - | 14.7 | ||||
| [9] USA | 216 | 2.4 (2.1,2.8) | [14] | 31 | 6.9 (4.7,9.8) | ||||
| [13] | 38 | 9.2 (6.5,12.7) | [13] | 23 | 5.0 (3.1,7.4) | ||||
N : number of observed cancers; SIR : standardized incidence ratio; RR : relative risk or rate ratio; HR : hazard ratio; 95% CI : 95% confidence interval; NS : non significant; NA : not available because of insufficient number of cancer cases; M : men; W : women
eGFR : estimated GFR with the abbreviated MDRD equation in ml/min/1.73 m2;
Per 10 ml/min/1.73 m2 decrease in eGFR;
HR for an eGFR < 40 as compared to > 40 ml/min/1.73 m2, adjusted for age, smoking, sun-related skin damage and blood pressure
HR of cancer per 1-SD increase of log ACR,
SIR of cancer up to 5 yrs before RRT as compared to the general population
Age-adjusted rate ratio (n cases and CI not available) for cancer in women (except for prostate) 3 years after kidney transplantation
Excluding nonmelanoma skin cancer
Cancer risk after dialysis
Two major studies examined site-specific cancer risk associated with dialysis. (Table 1) (9, 13) Although the largest of the two, that of Maisonneuve et al (9) should be interpreted with cautious, particularly regarding data from Europe, where both coverage of dialysis patients and cancer follow-up are likely to be incomplete, and from the US, where cancer follow-up may not be complete as well. Cancer risk may therefore be underestimated for these two populations, in contrast with Australia and New Zealand where both coverage and follow-up are the most complete. Compared to the general population, and after adjusting for age and gender, cancer risk of end-stage CKD patients was indeed increased 10% and 20% in Europe and the US, respectively, whereas, in Australia and New Zealand, it was 40% (9) to 80%. (Table 2) (13) Cancers most strongly associated with dialysis included Kaposi sarcoma and tumors of the oral cavity, kidney, bladder, stomach, liver, lung, cervix and thyroid. Relative risk for Kaposi sarcoma was higher than 50, that for kidney ranged from three to ten and for thyroid from two to nine, whereas increases for the other sites were about two-fold. As for transplantation, more than half of these cancers have viral etiology and may have been enhanced either by dialysis-induced immune dysfunction or by immunosuppressive or cytotoxic therapy for various glomerulonephritis or vasculitides. (6) Other mechanisms of carcinogenesis in uremia, as reviewed by Mandayam et al, (6) are related to the underlying kidney disease, such as acquired cystic kidney disease for renal cell carcinoma, as well as to increased DNA damage as a result of impaired DNA repair or reduced antioxidant capacity.
Cancer risk in patients with early stage CKD
In contrast with cancer risk associated with dialysis and transplantation, the potential of early stage CKD as a risk factor for cancer is not established. Concern arose recently, however, from a few large cohorts which studied cancer mortality or incidence in individuals with and without CKD, defined by their baseline estimated glomerular filtration rate (eGFR) or albuminuria level. (15–17) Fried et al (15) were the first to display an increase in cancer mortality associated with decreasing renal function in the elderly population from the Cardiovascular Health Study. Excess in mortality risk was limited to the highest vs lowest quartile for cystatin C (>1.22 vs <0.93 mg/L) as well as to the lowest vs highest quartile for MDRD eGFR (<60 vs > 81 ml/min/1.73 m2) with adjusted hazard ratio (HR) of 1.79 (95% confidence interval: 1.33;2.42) and 1.30 (0.97;1.74), respectively. Because of the relatively small number of events, however, the authors were unable to adjust for other factors than age, gender, and race. These findings were confirmed and further investigated by Wong et al in the Blue Mountains Eye Study. (Table 1) (17) In this cohort, the authors observed an excess risk of cancer associated with moderate CKD, although it was limited to men.(Table 2) The excess risk began at an eGFR of 55 mL/min/1.73 m2, consistent with Fried et al’s findings, (15) and increased linearly as eGFR declined. Every 10 mL mL/min/1.73 m2 was associated with a 29% increase of cancer risk independent of age and smoking, with the greatest risk at an eGFR < 40 mL/min/1.73 m2. The association seemed to be site specific for lung and urinary tract cancers. With a larger but younger cohort, Jorgensen also observed a 20% higher risk of all cancer with each standard deviation increase of albuminuria (log of albumin to creatinine ratio), and significant higher risks for lung, colon, kidney, and bladder cancers. (Tables 1 and 2) Vajdic et al (13) examined the risk of cancer up to five years before starting renal replacement therapy in a cohort end-stage CKD patients. (Table 1) As expected, these patients were at higher risk for cancers known to cause ESRD such as kidney and bladder cancers and multiple myeloma, but they also showed excess risk for Kaposi sarcoma, non-Hodgkin lymphoma, and for cancers of the oral cavity, colon and thyroid. (Table 2) Immunologic alterations associated with CKD would be a likely cause for the excess of virus-related cancers, but explanations are lacking for the excess of thyroid cancer.
Conclusion
Whereas paraneoplastic nephropathies and toxin-related cancers and CKD are both scarce and specific, a wide variety of cancers can occur at many sites in patients with CKD before or after renal replacement therapy. Kidney transplant recipients are at very high risk of cancers, most, but not all, of which with likely viral etiology. Patients on dialysis and individuals with early stage CKD also experience, but to a lower extent, an excess risk for a number of tumors. These epidemiologic findings should prompt clinicians and health authorities to assess strategies for cancer screening in the high risk population of CKD patients, particularly after transplantation.
Acknowledgments
Financial support : none
Footnotes
Conflict of interest : none
References
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–266. [PubMed] [Google Scholar]
- 2.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 3.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355–377. doi: 10.1046/j.1523-1755.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 4.Audard V, Larousserie F, Grimbert P, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–2260. doi: 10.1038/sj.ki.5000341. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandayam S, Shahinian VB. Are chronic dialysis patients at increased risk for cancer? J Nephrol. 2008;21:166–174. [PubMed] [Google Scholar]
- 7.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(Suppl):S254–264. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 8.Iseki K, Osawa A, Fukiyama K. Evidence for increased cancer deaths in chronic dialysis patients. Am J Kidney Dis. 1993;22:308–313. doi: 10.1016/s0272-6386(12)70323-2. [DOI] [PubMed] [Google Scholar]
- 9.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 10.Birkeland SA, Lokkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355:1886–1887. doi: 10.1016/s0140-6736(00)02298-4. [DOI] [PubMed] [Google Scholar]
- 11.Adami J, Gabel H, Lindelof B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 13.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. Jama. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 14.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 15.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen L, Heuch I, Jenssen T, Jacobsen BK. Association of albuminuria and cancer incidence. J Am Soc Nephrol. 2008;19:992–998. doi: 10.1681/ASN.2007060712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong G, Hayen A, Chapman JR, et al. Association of CKD and Cancer Risk in Older People. J Am Soc Nephrol. 2009;20:1341–1350. doi: 10.1681/ASN.2008090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 19.Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 20.Birkeland SA, Storm HH. Glomerulonephritis and malignancy: a population-based analysis. Kidney Int. 2003;63:716–721. doi: 10.1046/j.1523-1755.2003.00771.x. [DOI] [PubMed] [Google Scholar]
- 21.Lefaucheur C, Stengel B, Nochy D, et al. Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006;70:1510–1517. doi: 10.1038/sj.ki.5001790. [DOI] [PubMed] [Google Scholar]
- 22.Finkel KW, Foringer JR. Renal disease in patients with cancer. Nat Clin Pract Nephrol. 2007;3:669–678. doi: 10.1038/ncpneph0622. [DOI] [PubMed] [Google Scholar]
- 23.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Woolfson R. Paraproteins and the kidney. In: Cohen EP, editor. Cancer and the Kidney. New York: Oxford University Press; 2005. [Google Scholar]
- 25.USRDS 2008 Annual Data Report : Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 26.Tsakiris DJ, Stel V, Finne P, et al. Incidence and outcome of patients starting renal replacement therapy for multiple myeloma : an ERA-EDTA registry study. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp679. submitted. [DOI] [PubMed] [Google Scholar]
- 27.Hultengren N, Lagergren C, Ljungqvist A. Carcinoma of the renal pelvis in renal papillary necrosis. Acta Chir Scand. 1965;130:314–320. [PubMed] [Google Scholar]
- 28.Taylor JS. Carcinoma of the urinary tract and analgesic abuse. Med J Aust. 1972;1:407–409. doi: 10.5694/j.1326-5377.1972.tb106534.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahony JF, Storey BG, Ibanez RC, Stewart JH. Analgesic abuse, renal parenchymal disease and carcinoma of the kidney or ureter. Aust N Z J Med. 1977;7:463–469. doi: 10.1111/j.1445-5994.1977.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 30.Lornoy W, Morelle V, Becaus I, Fonteyne E. Development of malignant uroepithelial neoplasms in the upper urinary tracts in nephropathies caused by analgesic abuse: clinical study based on 16 cases. J Urol Nephrol (Paris) 1979;85:589–592. [PubMed] [Google Scholar]
- 31.Dubach UC, Rosner B, Sturmer T. Epidemiologic study of analgesic abuse: mortality study in 7275 working women (1968–1987) Kidney Int. 1991;40:728–733. doi: 10.1038/ki.1991.267. [DOI] [PubMed] [Google Scholar]
- 32.Dubach UC, Rosner B, Sturmer T. An epidemiologic study of abuse of analgesic drugs. Effects of phenacetin and salicylate on mortality and cardiovascular morbidity (1968 to 1987) N Engl J Med. 1991;324:155–160. doi: 10.1056/NEJM199101173240304. [DOI] [PubMed] [Google Scholar]
- 33.Lornoy W, Becaus S, de Vleeschouwer M, et al. Renal cell carcinoma, a new complication of analgesic nephropathy. Lancet. 1986;327:1271–1272. doi: 10.1016/s0140-6736(86)91407-8. [DOI] [PubMed] [Google Scholar]
- 34.McCredie M, Stewart J, Smith D, Supramaniam R, Williams S. Observations on the effect of abolishing analgesic abuse and reducing smoking on cancers of the kidney and bladder in New South Wales, Australia, 1972–1995. Cancer Causes Control. 1999;10:303–311. doi: 10.1023/a:1008900319043. [DOI] [PubMed] [Google Scholar]
- 35.McCredie M, Stewart JH, Mathew TH, Disney AP, Ford JM. The effect of withdrawal of phenacetin-containing analgesics on the incidence of kidney and urothelial cancer and renal failure. Clin Nephrol. 1989;31:35–39. [PubMed] [Google Scholar]
- 36.Feinstein AR, Heinemann LA, Curhan GC, et al. Relationship between nonphenacetin combined analgesics and nephropathy: a review. Ad Hoc Committee of the International Study Group on Analgesics and Nephropathy. Kidney Int. 2000;58:2259–2264. doi: 10.1046/j.1523-1755.2000.00410.x. [DOI] [PubMed] [Google Scholar]
- 37.Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353–2358. doi: 10.1016/j.juro.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 38.Bergman K, Muller L, Teigen SW. Series: current issues in mutagenesis and carcinogenesis, No. 65. The genotoxicity and carcinogenicity of paracetamol: a regulatory (re)view. Mutat Res. 1996;349:263–288. doi: 10.1016/0027-5107(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 39.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 40.Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 41.Lord GM, Tagore R, Cook T, Gower P, Pusey CD. Nephropathy caused by Chinese herbs in the UK. Lancet. 1999;354:481–482. doi: 10.1016/S0140-6736(99)03380-2. [DOI] [PubMed] [Google Scholar]
- 42.Stengel B, Jones E. End-stage renal insufficiency associated with Chinese herbal consumption in France. Nephrologie. 1998;19:15–20. [PubMed] [Google Scholar]
- 43.Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 44.Lemy A, Wissing KM, Rorive S, et al. Late onset of bladder urothelial carcinoma after kidney transplantation for end-stage aristolochic acid nephropathy: a case series with 15-year follow-up. Am J Kidney Dis. 2008;51:471–477. doi: 10.1053/j.ajkd.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Djukanovic LRZ. Balkan endemic nephropathy. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. [Google Scholar]
- 46.Grollman AP, Jelakovic B. Role of environmental toxins in endemic (Balkan) nephropathy. October 2006, Zagreb, Croatia. Am Soc Nephrol. 2007;18:2817–2823. doi: 10.1681/ASN.2007050537. [DOI] [PubMed] [Google Scholar]
- 47.Stefanovic V, Radovanovic Z. Balkan endemic nephropathy and associated urothelial cancer. Nat Clin Pract Urol. 2008;5:105–112. doi: 10.1038/ncpuro1019. [DOI] [PubMed] [Google Scholar]
- 48.Arlt VM, Stiborova M, vom Brocke J, et al. Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis. 2007;28:2253–2261. doi: 10.1093/carcin/bgm082. [DOI] [PubMed] [Google Scholar]
- 49.Stiborova M, Frei E, Arlt VM, Schmeiser HH. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat Res. 2008;658:55–67. doi: 10.1016/j.mrrev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 51.Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients--where do we stand today? Am J Transplant. 2008;8:2192–2198. doi: 10.1111/j.1600-6143.2008.02386.x. [DOI] [PubMed] [Google Scholar]
- 52.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant. 2007;7:2140–2151. doi: 10.1111/j.1600-6143.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 53.Webster AC, Wong G, Craig JC, Chapman JR. Managing cancer risk and decision making after kidney transplantation. Am J Transplant. 2008;8:2185–2191. doi: 10.1111/j.1600-6143.2008.02385.x. [DOI] [PubMed] [Google Scholar]
- 54.Wong G, Howard K, Craig JC, Chapman JR. Cost-effectiveness of colorectal cancer screening in renal transplant recipients. Transplantation. 2008;85:532–541. doi: 10.1097/TP.0b013e3181639d35. [DOI] [PubMed] [Google Scholar]
- 55.Wong G, Webster AC, Chapman JR, Craig JC. Reported cancer screening practices of nephrologists: results from a national survey. Nephrol Dial Transplant. 2009;24:2136–2143. doi: 10.1093/ndt/gfp009. [DOI] [PubMed] [Google Scholar]


