Abstract
Background
The optimal combination of anesthetic agent and technique may have an influence on long-term outcomes in cancer surgery. In vitro and in vivo studies suggest that propofol independently reduces migration of cancer cells and metastasis. Thus, the authors retrospectively examined the link between propofol-based total intravenous anesthesia (TIVA) and recurrence or overall survival in patients undergoing modified radical mastectomy (MRM).
Methods
A retrospective analysis of the electronic database of all patients undergoing MRM for breast cancer between January 2007 and December 2008 was undertaken. Patients received either propofol-based TIVA (propofol group) or sevoflurane-based anesthesia (sevoflurane group). We analyzed prognostic factors of breast cancer and perioperative factors and compared recurrence-free survival and overall survival between propofol and sevoflurane groups.
Results
A total of 363 MRMs were carried out during the period of the trial; 325 cases were suitable for analysis (173 cases of propofol group, and 152 cases of sevoflurane group). There were insignificant differences between the groups in age, weight, height, histopathologic results, surgical time, or postoperative treatment (chemotherapy, hormonal therapy, and radiotherapy). The use of opioids during the perioperative period was greater in propofol group than in sevoflurane group. Overall survival was no difference between the two groups. Propofol group showed a lower rate of cancer recurrence (P = 0.037), with an estimated hazard ratio of 0.550 (95% CI 0.311–0.973).
Conclusions
This retrospective study provides the possibility that propofol-based TIVA for breast cancer surgery can reduce the risk of recurrence during the initial 5 years after MRM.
Keywords: Breast neoplasms, Propofol, Recurrence, Sevoflurane
Introduction
The resection of the tumor is the most crucial treatment of solid tumors including breast cancer. Paradoxically, curative resection may create a period of vulnerability during which tumor cells disseminated in the process of manipulation of the tumor mass can surpass the defenses function of host and end up as metastasis [1]. Despites the likelihood of dispersing cancer cells during surgery, however, only a small minority of patients develops clinical metastases, a phenomenon which may depend on the host defenses, including cell-mediated immunity and in particular, natural killer (NK) cell function. The perioperative immune function of the host, therefore, may be a crucial component in tumor recurrence and overall survival following surgery in cancer patients.
Several factors during the perioperative period can lead to deterioration of the host's immune system, thereby promoting metastasis. These include the surgery itself, most anesthetics, opioid analgesics, postoperative pain, and stress [2,3,4,5]. Most anesthetics suppress a number of immune systems, including NK cells, which play central roles in preventing tumor dissemination and establishment in vitro [6].
It has recently been demonstrated that propofol exerts anti-tumor properties through variable mechanisms, including suppression of the survival capability, dispersion, and invasion of cancer cells [7]. However, the effect of propofol-based total intravenous anesthesia on the outcome of breast cancer surgery had not been previously evaluated in the clinical setting. Therefore, we conducted a retrospective analysis of electronic records to make a comparison with overall survival and the recurrence-free survival after modified radical mastectomy (MRM) in patients with breast cancer who underwent propofol-based total intravenous anesthesia or sevoflurane-based anesthesia.
Materials and Methods
After obtaining approval from our Institutional Review Board (approval number: K-1411-002-033) and the registration in the national clinical trial (http://cris.nih.go.kr. Ref: KCT0001464), we reviewed the electronic medical records of 363 patients who underwent MRM for invasive ductal carcinoma of the breast between January 2007 and December 2008. We excluded patients who had bilateral breast cancer, previous breast cancer surgery, metastatic breast cancer, breast cancers other than invasive ductal carcinoma (e.g. lymphoma, apocrine, or mucinous cell carcinoma), and other cancer.
All patients received intramuscular glycopyrrolate 0.2 mg with, or without midazolam 0.05 mg/kg, 30 min before surgery. In the operating room, hemodynamic monitoring and bispectral index monitoring (BIS) were performed. General anesthesia was induced with thiopental sodium, rocuronium, and sevoflurane with or without opioid in sevoflurane-based anesthesia group (sevoflurane group), whereas it was induced with a target effect-site concentration (Ce) of propofol of 4–5 µg/ml and rocuronium with or without opioid in propofol-based total intravenous anesthesia group (propofol group). After endotracheal intubation, anesthesia was adjusted to maintain BIS values of 40–60 with 1.5–2.0 vol% sevoflurane in the sevoflurane group, or 1.5–4.0 µg/ml (Ce) of propofol in the propofol group, with or without opioids in N2O 2 L/min and O2 2 L/min. In the post-anesthesia care unit and ward, the patients received synthetic opioids or nonsteroidal anti-inflammatory drugs (NSAIDs) for analgesia, if they were necessary.
We obtained the following patient data from the electronic medical records: age, weight, height, American Society of Anesthesiology physical status, tumor size grade, invasion grade of axillary lymph nodes, histologic grade, estrogen receptor (ER) status, progesterone receptor (PR) status, epidermal growth factor receptor type 2 (HER2) expression, and whether postoperative adjuvant hormonal therapy, chemotherapy or radiotherapy was used. We also obtained information on the type of anesthetics used, the use of perioperative opioids and NSAIDs, the duration of surgery, and the site of first metastasis.
The main outcomes were the recurrence-free survival and overall survival during the initial 5 years after surgery. Recurrence-free survival was defined from the date of surgery to the date of first recurrence, which was clarified as locoregional recurrence or distant metastases confirmed by clinical evidence or radiological examination. Overall survival was defined from the date of surgery to the date of death. Patients, who were lost to follow-up during initial 5 years after surgery, were statistically censored at the date of the last follow-up.
Statistical analysis
Our sample size was fixed on the basis of the available patients during a given period because our study was naturally retrospective. Using SPSS sample power 3.0 software, we conducted a power analysis prior to data collection in order to estimate the minimal effect size, in which we could detect a two-fold difference in the risk of recurrence between the groups, with a power greater than 80% and a significance level of 0.05. We determined the study interval for getting the enough enrolled patients on the basis of minimal sample size and the rate of excluded cases. We used SPSS statistical software (version 22.0, IBM Corp., Armonk, NY, USA) for statistical analysis. Normally distributed continuous variables were compared with the independent samples t-test, and variables that were not normally distributed were compared with the Mann-Whitney U test. Categorical variables were analyzed using the chi-square test or Fisher's exact test. There were some data missing for tumor size grade, histologic grade, and invasion of axillary lymph node, because some patients underwent previous excisional tumor biopsy in a different hospital. Patients with these missing data were excluded from the analysis related to the respective variables. Recurrence-free survival and overall survival were estimated by a Kaplan Meier log-rank test. Cox proportional hazards regression was used for univariate and multivariate analysis of perioperative and clinicopathologic variables influencing the recurrence-free survival. Only the meaningful variables (P < 0.25) from the univariate analysis were included in the multivariate ananlysis. Associations with P < 0.05 were considered statistically significant.
Results
The data from 363 consecutive patients who underwent MRM were reviewed. Patients were excluded due to missing electronic medical records (2), metastatic breast cancer (8), other breast cancer (mucinous 3, apocrine 4, lymphoma 1, medullary 1), bilateral breast cancer or previous breast cancer (13), other cancer (5), or refusal of postoperative treatment (1). The data from the 325 remaining patients (173 in the propofol group, 152 in the sevoflurane group) were analyzed.
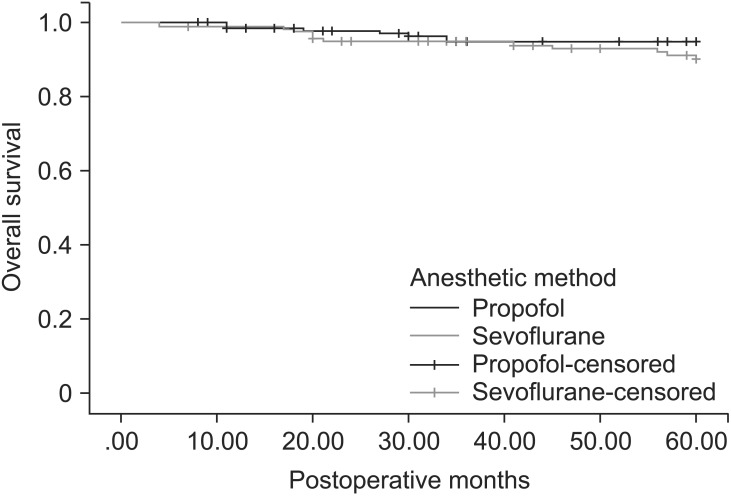
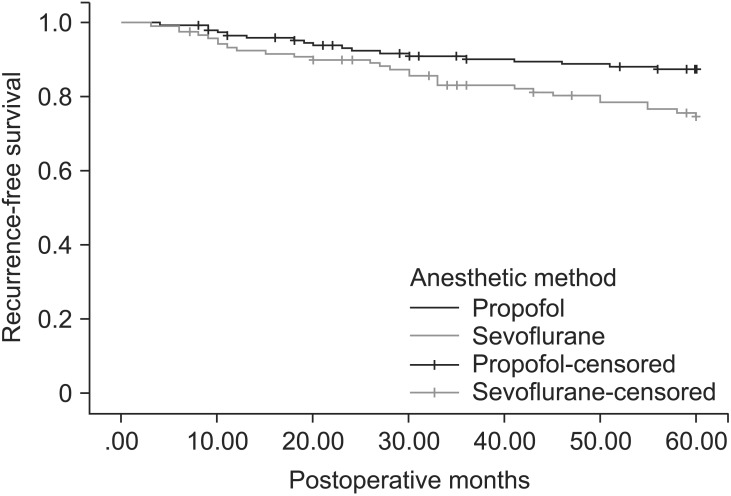
Patient demographic data and prognostic factors of breast cancer are shown in Table 1. Patient characteristics and prognostic factors of breast cancer were similar in the propofol group and the sevoflurane group. There were insignificant differences between the propofol group and the sevoflurane group in the perioperative factors, except that there was a greater use of intraoperative and postoperative opioids in the propofol group (Table 2). The opioids used during the operation included alfentanil 0.5 mg, fentanyl 50–150 µg, or remifentanil 1–3 ng/ml/min (Ce). All perioperative analgesics are listed in Table 2. The patients received fentanyl 25–50 µg, meperidine 25–50 mg, ketorolac 30 mg, or diclofenac 90 mg at a time and most of them were administered more than two kind of analgesics during postoperative 3 days. Recurrences were documented in 20 of 173 patients (11.6%) in the propofol group, and in 29 of 152 patients (19.1%) in the sevoflurane group. Deaths during the first 5 years after surgery were observed in 9 of 173 patients (5.2%) in the propofol group, and 11 of 153 patients (7.2%) in the sevoflurane group. In a Kaplan-Meier analysis with adjustment for the variable follow-up period of each patient, the propofol group had a longer recurrence-free survival (P = 0.037) than the sevoflurane group, but overall survival was not significantly different (P = 0.383) (Figs. 1 and 2).
Table 1. Patient Characteristics and Prognostic Factors of Breast Cancer.
| Propofol (N = 173) |
Sevoflurane (N = 152) |
P value | |
|---|---|---|---|
| Age (yr) | 50.2 (9.7) | 50.5 (10.9) | 0.799 |
| Weight (kg) | 59.1 (8.5) | 57.7 (7.8) | 0.137 |
| Height (cm) | 157.0 (5.7) | 155.8 (5.3) | 0.254 |
| ASA | 0.097 | ||
| I | 5 (2.9) | 0 (0) | |
| II | 159 (91.9) | 142 (93.4) | |
| III | 9 (5.2) | 10 (6.6) | |
| Grade of tumor size | 0.854 | ||
| 1 | 73 (42.7) | 65 (43.0) | |
| 2 | 84 (49.1) | 75 (49.7) | |
| 3 | 11 (6.41) | 10 (6.6) | |
| 4 | 3 (1.8) | 1 (0.7) | |
| Lymph node invasion | 0.810 | ||
| 1 (none) | 69 (39.9) | 65 (43.3) | |
| 2 (1–3 positive) | 60 (34.7) | 48 (32.0) | |
| 3 (>3 positive) | 44 ( 25.4) | 37 (24.7) | |
| Histologic grade | |||
| 1 | 28 (17.2) | 24 (16.6) | |
| 2 | 77 (47.2) | 77 (53.1) | |
| 3 | 58 (35.6) | 44 (30.3) | |
| Hormonal receptor status | |||
| Estrogen positive | 93 (53.8) | 93 (61.7) | 0.177 |
| Progesteron positive | 89 (51.4) | 74 (48.7) | 0.619 |
| HER-2 expression | 83 (48.0) | 70 (46.1) | 0.729 |
| Hormonal therapy | 118 (68.2) | 110 (72.4) | 0.413 |
| Chemotherapy | 164 (94.8) | 137 (90.1) | 0.109 |
| Radiotherapy | 50 (29.5) | 31 (20.4) | 0.060 |
Values are mean (SD) or number of patients (%). ASA: physical status of American Society of Anesthesiology, Grade of tumor size: 1; ≤ 20 nm, 2; > 20 nm, but ≤ 50 nm, 3; > 50 nm, 4; any size with direct extension to the chest wall and/or to the skin, Histologic grade: 1 = least aggressive tumor appearance, 2 = intermediate appearance, 3 = most aggressive appearance. HER-2: epidermal growth factor receptor type 2 expression.
Table 2. Perioperative Factors and Site of Recurrence.
| Propofol (N = 173) |
Sevoflurane (N = 152) |
P value | |
|---|---|---|---|
| Time of surgery (min) | 102.0 (30.3) | 102.1 (28.4) | 0.988 |
| Intraoperative opioid | 0.001 | ||
| None | 1 (0.6) | 85 (55.9) | |
| Remifentanil | 116 (67.1) | 0 (0) | |
| Fentanyl | 55 (31.8) | 56 (36.8) | |
| Alfentanil | 1 (8.3) | 11 (7.2) | |
| Postoperative opioid | 0.001 | ||
| None | 63 (36.4) | 96 (63.2) | |
| Fentanyl | 19 (11.0) | 22 (14.5) | |
| Meperidine | 91 (52.6) | 34 (22.4) | |
| Postoperative NSAID | 0.203 | ||
| None | 81 (46.8) | 67 (44.1) | |
| Ketolorac | 14 (8.1) | 6 (3.9) | |
| Diclofenac | 78 (45.1) | 79 (52.0) | |
| Perioperative transfusion | 5 (2.9) | 3 (2.0) | 0.728 |
| Site of recurrence | 0.137 | ||
| None | 154 (89.0) | 124 (81.6) | |
| Bone | 1 (0.6) | 9 (5.9) | |
| Brain | 1 (0.6) | 1 (0.7) | |
| Lung | 9 (5.2) | 10 (6.6) | |
| Chest wall | 4 (2.3) | 4 (2.6) | |
| Liver | 4 (2.3) | 4 (2.6) |
Values are mean (SD) or number of patients (%). NSAID: non-steroidal antiinflammatory drug.
Fig. 1. Kaplan-Meier overall survival estimated for 325 patients receiving propofol-based total intravenous anesthesia (173) and sevoflurane-based anesthesia (152). Univariate analysis by log-rank test (P = 0.383).
Fig. 2. Kaplan-Meier recurrence-free survival estimated for 325 patients receiving propofol-based total intravenous anesthesia (173) and sevoflurane-based anesthesia (152). Univariate analysis by log-rank test (P = 0.037).
Risk factors related to recurrence of breast cancer were compared in Table 3. The significant risk factors in univariate analysis were considered as the grade of tumor size (P = 0.001, hazard ratio [HR] = 4.321, 95% confidence interval [CI] 2.034–9.264), invasion grade of lymph nodes (P = 0.002, HR = 3.144, 95% CI 1.525–6.479), histologic grade (P = 0.031, HR = 3.616, 95% CI 1.230–11.641), radiotherapy (P = 0.001, HR = 2.886, 95% CI 1.648–5.055), type of anesthetics (P = 0.038, HR = 0.550, 95% CI 0.311–0.973). Blood transfusion, endocrine therapy, and chemotherapy were found not to be associated with recurrence. In multivariate analysis of meaningful factors such as tumor size grade, invasion grade of lymph nodes, histologic grade, endocrine therapy, chemotherapy, radiotherapy, and anesthetics, the anesthetic technique remained a significant risk factor for recurrence after surgery (P = 0.014, HR = 0.478, 95% CI = 0.265–0.862) (Table 3).
Table 3. Univariate and Multivariate Associations with Cancer Recurrence: Cox Regression Model.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Tumor size grade (1 vs. 2,3,4) | 4.321 | 2.034 | 9.264 | 0.001 | 2.868 | 1.282 | 6.414 | 0.010 |
| LN grade (1 vs. 2,3) | 3.144 | 1.525 | 6.479 | 0.002 | 1.594 | 0.689 | 3.687 | 0.276 |
| Histologic grade (1 vs. 2,3) | 3.616 | 1.230 | 11.641 | 0.031 | 1.946 | 0.571 | 6.634 | 0.287 |
| ER (pos vs. neg) | 0.898 | 0.511 | 1.576 | 0.707 | ||||
| PR (pos vs. neg) | 1.070 | 0.611 | 1.874 | 0.813 | ||||
| HER (pos vs. neg) | 0.997 | 0.569 | 1.747 | 0.991 | ||||
| Endocrine therapy (no. vs. yes) | 1.883 | 0.883 | 4.016 | 0.102 | 1.459 | 0.655 | 3.252 | 0.355 |
| Chemotherapy (no. vs. yes) | 3.582 | 0.494 | 25.953 | 0.207 | 1.112 | 0.141 | 8.788 | 0.920 |
| Radiotherapy (no. vs. yes) | 2.886 | 1.648 | 5.055 | 0.001 | 1.806 | 0.959 | 3.400 | 0.067 |
| Anesthetics (sevoflurane vs. propofol) | 0.550 | 0.311 | 0.973 | 0.038 | 0.478 | 0.265 | 0.862 | 0.014 |
| Intraoperatvie use of opioid (no vs. yes) | 0.871 | 0.469 | 1.619 | 0.662 | ||||
| Postoperative use of opioid (no vs. yes) | 1.169 | 0.667 | 2.048 | 0.586 | ||||
| Postoperative use of NSAID (no vs. yes) | 1.164 | 0.658 | 2.058 | 0.602 | ||||
| Transfusion (no vs. yes) | 2.282 | 0.554 | 9.395 | 0.253 | ||||
HR: hazard ratio, 95% CI: 95% confidence interval, LN: lymph node, ER: estrogen receptor, PR: progesterone receptor, HER: epidermal growth factor receptor, NSAID: non-steroidal anti-inflammatory drugs.
Discussion
The anesthetic technique may have an influence on postoperative long-term outcome of patients. Our study showed that propofol-based total intravenous anesthesia for MRM reduced the risk of recurrence of breast cancer during the initial 5 years of follow-up, compared with sevoflurane-based anesthesia.
The influences of anesthetics on host's anti-tumor defenses have been studied in vitro, in vivo, and in some human studies. Ketamine and thiopental have been shown to increase the number of viable tumor cells in lung tissue, and suppress NK cell activity, whereas propofol did not lead to suppression of NK cell activity [3].
It has been recognized that opioids inhibit both cellular and humoral immune functions, and morphine exhibit a dose-dependent immunosuppression [4]. However, recent study suggested that morphine may reduce negative impact of pain on the immune system and have the beneficial impact on particular cancer disease through µ-opioid receptor [8].
Synthetic opioids including fentanyl [9] and remifentanil [10] suppressed some immune response, but did not suppress immune resistance in low doses [11]. It was demonstrated that this opioid-induced immunosuppression and the promotion of tumor growth were naloxone-sensitive or preventable by coadministration of cyclooxygase-2 (COX-2) inhibitor [12]. In our study, fentanyl, remifentanil, or alfentanil was intraoperatively administered to most of patients in the propofol group, in contrast to the sevoflurane group. The result may be explained as the conventional method of total intravenous anesthesia (TIVA) consisting of propofol, opioids, and nitrous oxide. Furthermore, there was a high incidence of the need of opioids in the propofol group during the postoperative period, and this phenomenon may be explained either by opioid-induced hyperalgesia or by rapid awakening after propofol-based total intravenous anesthesia. Opioid-induced hyperalgesia has been reported after remifentanil-propofol anesthesia in humans, although it may be reduced in propofol-remifentanil anesthesia more than in sevoflurane-remifentanil anesthesia [13,14]. Contrary to some results suggesting the suppression of cancer-related immunity by opioids, we could not find any deleterious effect of perioperative opioids on the recurrence of cancer. The large sized, population-based study showed no clinically significant association with uses of opioids and the recurrence of breast cancer [15]. It may be explained by relatively small dose used in our patients, compared with those in other studies [11], or more patients of advanced stages, which were less vulnerable to the effect of opioid on cancer recurrence [16].
NSAIDs and COX inhibitors have been shown to have anti-tumor and anti-angiogenic properties in a rat model [17]. Forget et al. [18] showed that NSAIDs, particularly given shortly before the surgery decreases the risk of recurrence of breast cancer, compared with other analgesics. They also suggested that the ideal period for administering a drug that interacts with NK cells may be right before surgery. In our study, postoperative administration of NSAIDs had no effect on the outcome of recurrence.
Nitrous oxide may not influence on cancer recurrence, whereas it does impair immune systems, including important component for confronting cancer [19]. We routinely used 50% nitrous oxide and 50% oxygen in both anesthetic groups in the current study.
Volatile anesthetics have been shown in various studies to suppress elements of immune function including NK cell and lymphocytes. Volatile anesthetics increased hypoxia-inducible factors, which function as the protector of tissues and lead to tumorigenesis and metastasis [20]. Both isoflurane and sevoflurane, commonly used volatile anesthetics, exhibit immunosuppression and tumorigenesis through a number of mechanisms including suppression of NK cell activity and lymphocyte function, proliferation, apoptosis, and invasion of cancer cells [21,22].
In contrast to most anesthetics, propofol has been suggested in some studies to have anti-tumor properties and maintain anti-tumor immunity in vitro and in vivo [3,7,23]. Propofol, in contrast to isoflurane, stimulated the activation and differenciation of T helper lymphocytes, a key step in anti-infective and anti-tumor immune responses [23]. However, despite the body of evidences and studies highlighting the anti-tumoral aspects of propofol, there have been few clinical trials conducted to explore the hypothesis that propofol-based total intravenous anesthesia may be the most ideal agent for primary surgery of the cancer. Only one study showed that there was greater inhibition of the proliferation of ER-negative breast cancer cells in the serum of patients receiving propofol/paravertebral anesthesia-analgesia for breast cancer than in that of patients receiving sevoflurane/opioid anesthesia-analgesia [24]. However, this result demonstrated the combined effect of propofol and regional anesthetic techniques because regional anesthetic techniques such as paravertebral analgesia can also take a role in reducing the risk of recurrence of breast cancer during the initial year of follow-up after surgery [25].
Inada et al. [26] suggested that the reported anti-tumor function of propofol could be associated with COX inhibition. They postulated that the maximal therapeutic effect of COX-2 inhibitors might be shown if these were given perioperatively because tumor cells may be more easily microdisseminated during the manipulation of tumor.
NSAIDs and COX-2 inhibitors are generally considered "cancer prophylaxis", as demonstrated in human studies [27]. In co-culture of macrophages and NK cells, propofol dramatically increased NK cell interferon-γproduction, and the actions of propofol were similar to those of the selective COX-2 inhibitor [28]. Although there is no remarkable finding yet that the COX inhibitory function by propofol is relevant to its anti-tumor porperties, one mechanism of the anti-tumor effect of propofol may be associated with its similar action to COX-2 inhibitors. The results in our study may support this theory: propofol-based total intravenous anesthesia reduced the risk of recurrence during the 5-year follow-up period after breast cancer surgery, even if it was administered more frequently with opioids. We may be able to explain why opioids, used in most of the patients undergoing propofol-based total intravenous anesthesia, did not result in an increased risk of recurrence of breast cancer, because COX-2 inhibitors have been deemed to have a preventive ability against opioid-induced immunosuppression.
An additional benefit of propofol-based total intravenous anesthesia for cancer surgery may be found in a different mechanism. The stress response to surgery is attenuated by propofolbased total intravenous anesthesia more than by inhalational anesthesia. Ke et al. [29] suggested that TIVA using propofol and remifentanil reduced the inflammatory response to surgical stress-induced inflammatory response more than isoflurane-based anesthesia did. They also showed a significantly lower release of proinflammatory cytokines in propofol-remifentanil group than in isoflurane-based anesthesia group. In our study, there was no attempt for the evaluation of anti-inflammatory or pro-inflammatory responses in the patients, because this was designed in the retrospective study.
Numerous studies have been conducted to search risk factors for recurrence following cancer surgery. The treatment and prognosis for invasive ductal carcinoma of breast were demonstrated to be dependent on factors, such as the size of tumor, histological grade, lymph node grade, expression of ER, PR, HER-2, and the Nottingham Prognostic Index (NPI) [30]. In this study, we excluded the NPI in the light of the multicollinearity problem between the risk factors. On top of sevoflurane, we also confirmed risk factors such as tumor size grade, lymph node invasion, histologic grade, chemotherapy, and radiotherapy, which were consistent with those of the other trials. There were no principles regarding anesthetic techniques for cancer patients, whereas those for surgical techniques have been available for various cancers. This represented the first trial to include anesthetic technique among the risk factors for recurrence after cancer surgery. However, in this study, anesthetics had insignificant effects on the overall survival of breast cancer patients.
In our study, there is an important limitation inherent in its retrospective, observational nature. Retrospective studies may have uncontrolled and unrecognized biases, such as more uses of various opioids in propofol-based total intravenous anesthesia group depending on the nature of TIVA. Clinical care was not standardized, and the involved patients were not randomized; thus, the effects of unpredicted confounding factors and selection bias may be included. For instance, more anesthesiologists preferred propofol-based total intravenous anesthesia for breast cancer surgery, because of its over-anesthetic properties such as antiemetic function. Therefore, more patients underwent propofol-based total intravenous anesthesia, although this phenomenon did not lead to a statistical significance. To confirm our study and advance a hypothesis, prospective and multicenter studies are necessary. We undertook this trial in a single institution, and we do not know whether our model can be generalized to other hospital because the surgical and medical treatments may affect on the recurrence of breast cancer. Ultimately, a prospective study may be too complicated, costly, and time-consuming to confirm definitive answers to these assumptions.
In conclusions, we observed that propofol-based total intravenous anesthesia substantially reduced tumor recurrence after breast cancer surgery, and prospective and multicenter studies may be necessary to provide the validity of our findings.
Acknowledgments
We would like to thank Woo Chul Noh, M.D., Ph. D. (Breast Cancer Center, Korean Cancer Center Hospital) for providing us with data on patients undergoing modified radical mastectomy.
References
- 1.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–1828. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–555. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 3.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 4.Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. 1993;265:1071–1078. [PubMed] [Google Scholar]
- 5.Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199. doi: 10.1016/s0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 6.Snyder GL, Greenberg S. Effect of anesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 7.Ecimovic P, Murray D, Doran P, Buggy DJ. Propofol and bupivacaine in breast cancer cell function in vitro-role of the NET1 gene. Anticancer Res. 2014;34:1321–1331. [PubMed] [Google Scholar]
- 8.Juneja R. Opioids and cancer recurrence. Curr Opin Support Palliat Care. 2014;8:91–101. doi: 10.1097/SPC.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 9.Shavit Y, Ben-Eliyahu S, Zeidel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11:255–260. doi: 10.1159/000078444. [DOI] [PubMed] [Google Scholar]
- 10.Sacerdote P, Gaspani L, Rossoni G, Panerai AE, Bianchi M. Effect of the opioid remifentanil on cellular immune response in the rat. Int Immunopharmacol. 2001;1:713–719. doi: 10.1016/s1567-5769(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 11.Yardeni IZ, Beilin B, Mayburd E, Alcalay Y, Bessler H. Relationship between fentanyl dosage and immune function in the postoperative period. J Opioid Manag. 2008;4:27–33. doi: 10.5055/jom.2008.0005. [DOI] [PubMed] [Google Scholar]
- 12.Farooqui M, Li Y, Rogers T, Poonawala T, Griffin RJ, Song CW, et al. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumor growth, metastasis, and mortality, without compromising analgesia. Br J Cancer. 2007;97:1523–1531. doi: 10.1038/sj.bjc.6604057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echevarría G, Elgueta F, Fierro C, Bugedo D, Faba G, Iñiguez-Cuadra R, et al. Nitrous oxide (N2O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anesthesia in humans. Br J Anaesth. 2011;107:959–965. doi: 10.1093/bja/aer323. [DOI] [PubMed] [Google Scholar]
- 14.Shin SW, Cho AR, Lee HJ, Kim HJ, Byeon GJ, Yoon JW, et al. Maintenance anaesthetics during remifentanil-based anaesthesia might affect postoperative pain control after breast cancer surgery. Br J Anaesth. 2010;105:661–667. doi: 10.1093/bja/aeq257. [DOI] [PubMed] [Google Scholar]
- 15.Cronin-Fenton DP, Heide-Jørgensen U, Ahern TP, Lash TL, Christiansen PM, Ejlertsen B, et al. Opioids and breast cancer recurrence: a Danish population-based cohort study. Cancer. 2015;121:3507–3514. doi: 10.1002/cncr.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cata JP, Keerty M, Keerty D, Feng L, Norman PH, Gottumukkala V, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–908. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leahy KM, Ornverg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 18.Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110:1630–1635. doi: 10.1213/ANE.0b013e3181d2ad07. [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann E, Marschalek C, Schlemitz K, Dalton JE, Gruenberger T, Herbst F, et al. Nitrous oxide may not increase the risk of cancer recurrence after colorectal surgery: a follow-up of a randomized controlled trial. BMC Anesthesiol. 2009;9:1. doi: 10.1186/1471-2253-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, et al. Isoflurane, a commonly used volatile anesthetic causes renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119:593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 21.Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78:700–706. doi: 10.1097/00000542-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Fan Y, Liu K, Wang Y. Effects of different general anesthetic techniques on immune responses in patients undergoing surgery for tongue cancer. Anaesth Intensive Care. 2014;42:220–227. doi: 10.1177/0310057X1404200209. [DOI] [PubMed] [Google Scholar]
- 23.Ren XF, Li WZ, Meng FY, Lin CF. Differential effects of propofol and isoflurane on the activation of T-helper cells in lung cancer patients. Anaesthesia. 2010;65:478–482. doi: 10.1111/j.1365-2044.2010.06304.x. [DOI] [PubMed] [Google Scholar]
- 24.Deegan CA, Murray D, Doran P, Ecimovic P, Moriarty DC, Buggy DJ. Effect of anesthetic technique on oestrogen receptor-negative breast cancer cell function in vitro. Br J Anaesth. 2009;103:685–690. doi: 10.1093/bja/aep261. [DOI] [PubMed] [Google Scholar]
- 25.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inada T, Kubo K, Shingu K. Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J Anesth. 2011;25:569–575. doi: 10.1007/s00540-011-1163-y. [DOI] [PubMed] [Google Scholar]
- 27.Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blinded, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inada T, Kubo K, Shingu K. Promotion of interferon-gamma production by natural killer cells via suppression of murine peritoneal macrophage prostaglandin E2 production using intravenous anesthetic propofol. Int Immunopharmacol. 2010;10:1200–1208. doi: 10.1016/j.intimp.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anesthesia with propofol and remifentanil and inhalational anesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36:74–78. doi: 10.1177/0310057X0803600113. [DOI] [PubMed] [Google Scholar]
- 30.Kurshumliu F, Gashi-Luci L, Kadare S, Alimehmeti M, Gozalan U. Classification of patients with breast cancer according to Nottingham Prognostic Index highlights significant differences in immunohistochemical marker expression. World J Surg Oncol. 2014;12:243. doi: 10.1186/1477-7819-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]