Abstract
We report a healthy patient with postpartum headache and neck stiffness which were diagnosed as symptoms of pseudoaneurysm of vertebral artery. She had received a Cesarean section under the spinal anesthesia, and complaint of headache and neck stiffness. Epidural blood patches were done twice, but symptoms persisted. Eight days later, she experienced sensory disturbance and emergent laminectomy was done. When persistent postpartum headache occurs after epidural blood patch, more precise differential diagnosis should be made and considering other possible pathologies.
Keywords: Epidural blood patch, Postdural puncture headache, Postpartum headache, Pseudoaneurysm, Spinal subdural hematoma
While spinal subdural hematoma (SSH) is not a common disease, the prognosis may be critical in the event of an outbreak. Its symptoms include sudden and localized back pain, sensorimotor deficit, and sphincter disturbance [1]. In previous reports, SSH has been attributed to coagulation disorder, anticoagulant therapy, spinal trauma, tumor, vascular malformation, and aneurysm [2,3].
This paper reports the case of a patient suspected of a postdural puncture headache (PDPH) who continued experiencing the headache even after epidural blood patches, and suffered SSH due to a vertebral pseudoaneurysm rupture.
Case Report
A healthy 33-year-old primipara underwent a cesarean section at 37 weeks of gestation. Upon arrival in the operation room, she received an electrocardiogram and was monitored for oxygen saturation and blood pressure. She was then placed in the lateral position and given a dural puncture between the 3rd and 4th lumbar vertebrae, using a 25-gauge Quinke needle (Hakko Co. Ltd., Chikuma, Japan). Afterwards, a CSF leak test was performed, and 8 mg of 0.5% hyperbaric bupivacaine were injected intrathecally. After the injection, the patient was placed in the supine position. Five minutes later, the height of the sensory block was confirmed to be at T4, and the operation was initiated.
During the operation, the patient's blood pressure ranged between 90/58 mmHg and 140/70 mmHg, the pulse between 80 and 90 per minute, and the peripheral capillary oxygen saturation between 98 and 100 percent. There was no abnormal neurologic sign. The operation ended 1 hour and 15 minutes after its initiation, and the patient was transferred to the recovery room. After 30 minutes in the recovery room, the patient complained neck stiffness and a mild headache. It was a new onset of neck stiffness and headache. The headache continued regardless of a change of position and the patient described that it felt "heavy in her head". It was not pulsatile, but tightening and consistent. The blood pressure fluctuated widely, between 130/65 mmHg and 170/98 mmHg. After 6 hours of observation in the recovery room, the patient was transferred to the ward.
The neck stiffness continued until the following day. The patient was suspected of PDPH and received an epidural blood patch (EBP) using an 18-gauge Tuohy needle following a dural puncture. The patient's autologous blood was injected in a 15 ml dose in the area in which the dural puncture had been performed, but the symptoms persisted and a second EBP was therefore performed the next day. The patient was discharged 6 days after the operation, with the headache still present although it had eased considerably. She was scheduled to receive follow-up monitoring.
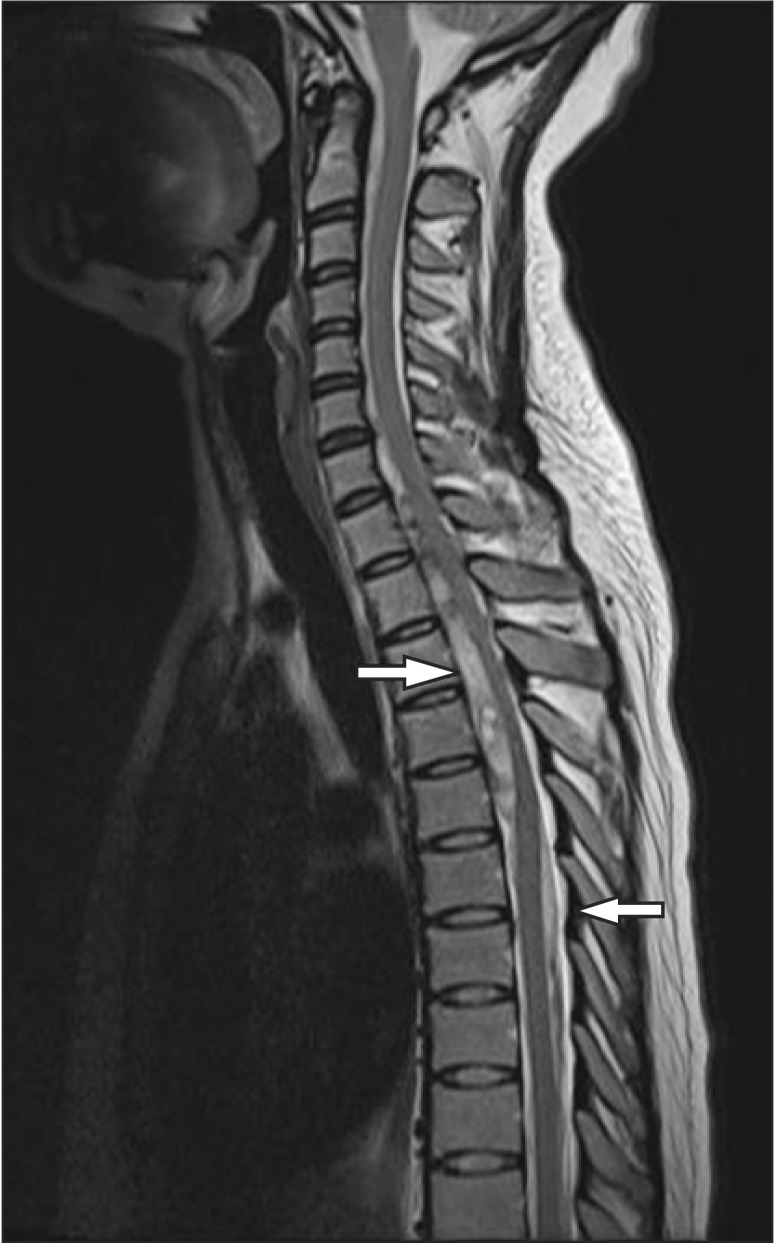
Two days after her discharge, the patient came to the emergency room for sudden dyspnea, paresthesia of the lower part of the body, and weakened muscle strength. The examination in the emergency room revealed that she was experiencing weakened muscle strength and sensory loss below T4. An emergency MRI scan revealed an anterior spinal subdural hematoma with spinal cord compression from C6 to T5, and an hematoma was also observed in the subdural space between T6 and T10 (Fig. 1).
Fig. 1. MRI scan shows anterior subdural hematoma (arrows) with spinal cord compression at C6 to T4 level. At T6 to T10, similar hematoma was seen in the posterior subdural space.
The patient underwent emergency decompressive laminectomy. There was no finding to presume of a bleeding tendency, as the tests before the anesthesia had reported the following: hemoglobin at 9.5 g/dl; platelet at 303000 /µl; PT at 12.7 seconds, PT INR at 1.0; and PTT at 36.9 seconds. The chest radiograph and electrocardiogram were normal. The vital signs recorded shortly before the operation showed a blood pressure of 140/65 mmHg and a pulse rate of 105 /min. There was no significant hemodynamic change during the operation.
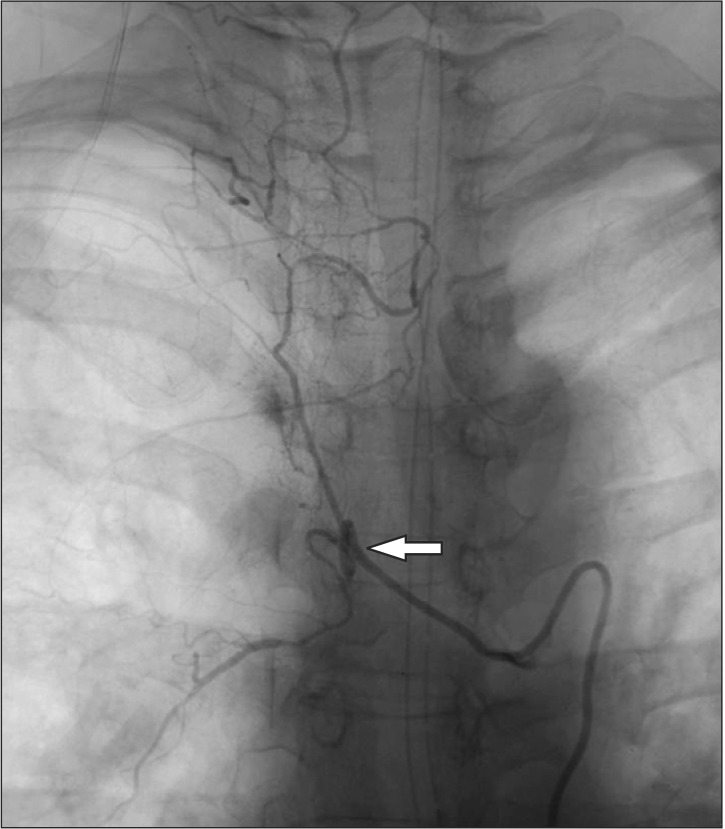
An arteriography conducted shortly after the operation revealed stasis of the contrast medium in the anterior spinal canal, between C5 and T4. Pseudoaneurysm rupture of the vertebral artery was diagnosed (Fig. 2).
Fig. 2. Vertebral angiography. At T4 level, there was stagnation of contrast at the anterior spinal canal portion, pseudoaneurysm (arrow) was suspected.
Discussion
This paper reports the case of a patient with presumed PDPH following a cesarean section performed under spinal anesthesia, who continued to experience a headache even after EBPs and was diagnosed with pseudoaneurysm rupture of the vertebral artery.
Headache is one of the common puerperal complications. Differential diagnosis is challenging for this type of headache, as there are a number of possible causes. The causes of puerperal headaches can be categorized into primary and secondary causes [4]. Primary headaches include migraines and tension headaches, which can be commonly experienced by women of childbearing age. Around 50 to 75 percent of headaches are primary, and migraine is the most common kind [5]. On the other hand, secondary headaches are less frequent than primary headaches, they may bring about critical complications. Secondary headaches can be attributed to cardiovascular diseases such as strokes and high blood pressure, preeclampsia, and PDPH [4].
If a primary headache can be excluded from consideration, when diagnosing a headache that occurred after spinal anesthesia for a cesarean section, most anesthesiologists will suspect PDPH as the cause of the secondary headache. However, there have been case reports in which PDPH was wrongly diagnosed as the cause of the secondary headache, when it was in fact caused by other conditions such as cerebral venous thrombosis, cerebral vasoconstriction syndrome, encephalomeningitis, preeclampsia, or stroke [6,7,8].
According to the PDPH diagnosis guidelines of the International Headache Society [9], headache is worsened in a sitting or standing position, and is eased in a recumbent position. It must have one of the following four symptoms: neck stiffness, tinnitus, hypacusia, and photophobia. Most headache develops within 5 days after dural puncture and resolves spontaneously within 1 week, or within 48 hours after EBP.
In the present case, the patient's neck stiffness and headache were not pulsatile but consistent. Primary headache could easily be excluded from consideration as a basic analgesic did not ease the symptoms, and no nausea, vomiting, or photophobia was observed. Therefore, PDPH was diagnosed in view of the prevalence rate.
However, the symptoms did not improve even after two EBP. EBP is generally known to be successful in more than 90 percent of cases, although some studies have reported a success rate of 60 to 70 percent [10,11]. Therefore, some possibility of PDPH can always be present, even in the case of unsuccessful EBPs. In this case, however, the patient's headache was not eased despite the change of position, and there was no sign — such as tinnitus, hypacusia, and photophobia — indicating PDPH. Therefore, it is recommended that secondary headache be considered if the characteristics of the headache are different from those of typical PDPH, and if the headache continues after the EBP.
SSH is not common but can be critical when it occurs. Common causes of SSH include coagulation disorder, anticoagulant therapy, spinal trauma, tumor, vascular malformation such as AVM, and aneurysm [2,3]. SSH is often observed in the thoracic spine, and is characterized by sudden localized back pain, sensorimotor deficit, and sphincter disturbance [1]. MRI is the diagnostic method of choice for SSH. According to Ruppen et al. [12], the incidence of spinal epidural hematoma, spinal subdural hematoma, and intrathecal hematoma after central neuraxial blockade in the obstetric population has been estimated at 1 out of 168000. During pregnancy, fibrinogens and coagulation factors including factors VII, VIII, IX, X and XII are increased as a defense mechanism against blood loss. Over the full pregnancy term, the body experiences a hypercoagulable state. However, most cases have been reported in mothers with coagulation disorders or who had received anticoagulant therapy. Excessive or repeated EBP may bring about SSH or an epidural hematoma [13]. According to Beards et al. [14], the injection of 20 ml of blood during an EBP can induce a mass effect for 30 minutes to 3 hours, as it spreads 3 to 5 spinal segments within the epidural space the from the injected site. In contrast, there has been a case of SSH occurring 8 segments away from the EBP area as a result of the mass effect [15].
It is suspected that the patient in this case experienced SSH due to the rupture of a pseudoaneurysm in the vertebral artery. The patient did not present an abnormal medical history, experience of anticoagulant therapy, or a finding indicating a bleeding tendency. Moreover, the EBP was performed 10 segments away from the suspected location of the pseudoaneurysm rupture. Therefore, the possibility that the rupture was caused by a mass effect is low. Consequently, it is suspected that the pseudoaneurysm was formed in the vertebral artery due to an injury or other causes unrecognized by the patient. The headache and neck stiffness experienced by the patient seem to have arisen as the pseudoaneurysm was developing. The rupture can be attributed to the lack of adequate testing and timely intervention while the patient showed a systolic blood pressure as high as 170 mmHg, headache, and neck stiffness.
In light of the prevalence, the headaches experienced by mothers who deliver under spinal anesthesia are often presumed to be PDPHs or migraines. However, this method of diagnosis paradoxically increases the risk of misdiagnosis, as it excludes other possible causes. Therefore, if the headache continues without demonstrating typical characteristics of the aforementioned causes, secondary causes must be considered. In certain cases, a thorough neurologic examination and an early radiologic examination should be performed along with other meticulous examinations in order to allow for prompt diagnosis and intervention.
References
- 1.Payer M, Agosti R. Spontaneous acute spinal subdural hematoma: spontaneous recovery from severe paraparesis--case report and review. Acta Neurochir (Wien) 2010;152:1981–1984. doi: 10.1007/s00701-010-0758-7. [DOI] [PubMed] [Google Scholar]
- 2.Morandi X, Riffaud L, Chabert E, Brassier G. Acute nontraumatic spinal subdural hematomas in three patients. Spine (Phila Pa 1976) 2001;26:E547–E551. doi: 10.1097/00007632-200112010-00022. [DOI] [PubMed] [Google Scholar]
- 3.Boukobza M, Haddar D, Boissonet M, Merland JJ. Spinal subdural haematoma: a study of three cases. Clin Radiol. 2001;56:475–480. doi: 10.1053/crad.2000.0466. [DOI] [PubMed] [Google Scholar]
- 4.Klein AM, Loder E. Postpartum headache. Int J Obstet Anesth. 2010;19:422–430. doi: 10.1016/j.ijoa.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: a prospective cohort study. Can J Anaesth. 2005;52:971–977. doi: 10.1007/BF03022061. [DOI] [PubMed] [Google Scholar]
- 6.Karci A, Boyaci F, Yaka E, Cakmur R, Men S, Elar Z. Cerebral venous thrombosis initially considered as a complication of spinal-epidural anaesthesia. J Int Med Res. 2005;33:711–714. doi: 10.1177/147323000503300615. [DOI] [PubMed] [Google Scholar]
- 7.Ghatge S, Uppugonduri S, Kamarzaman Z. Cerebral venous sinus thrombosis following accidental dural puncture and epidural blood patch. Int J Obstet Anesth. 2008;17:267–270. doi: 10.1016/j.ijoa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Lee JJ, Parry H. Bacterial meningitis following spinal anaesthesia for caesarean section. Br J Anaesth. 1991;66:383–386. doi: 10.1093/bja/66.3.383. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Abouleish E, Vega S, Blendinger I, Tio TO. Long-term follow-up of epidural blood patch. Anesth Analg. 1975;54:459–463. doi: 10.1213/00000539-197554040-00012. [DOI] [PubMed] [Google Scholar]
- 11.Berger CW, Crosby ET, Grodecki W. North American survey of the management of dural puncture occurring during labour epidural analgesia. Can J Anaesth. 1998;45:110–114. doi: 10.1007/BF03013247. [DOI] [PubMed] [Google Scholar]
- 12.Ruppen W, Derry S, McQuay H, Moore RA. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399. doi: 10.1097/00000542-200608000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Diaz JH. Permanent paraparesis and cauda equina syndrome after epidural blood patch for postdural puncture headache. Anesthesiology. 2002;96:1515–1517. doi: 10.1097/00000542-200206000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Beards SC, Jackson A, Griffiths AG, Horsman EL. Magnetic resonance imaging of extradural blood patches: appearances from 30 min to 18 h. Br J Anaesth. 1993;71:182–188. doi: 10.1093/bja/71.2.182. [DOI] [PubMed] [Google Scholar]
- 15.Verduzco LA, Atlas SW, Riley ET. Subdural hematoma after an epidural blood patch. Int J Obstet Anesth. 2012;21:189–192. doi: 10.1016/j.ijoa.2011.10.003. [DOI] [PubMed] [Google Scholar]