Abstract
Objectives To evaluate whether patients who experience cardiac arrest in hospital receive epinephrine (adrenaline) within the two minutes after the first defibrillation (contrary to American Heart Association guidelines) and to evaluate the association between early administration of epinephrine and outcomes in this population.
Design Prospective observational cohort study.
Setting Analysis of data from the Get With The Guidelines-Resuscitation registry, which includes data from more than 300 hospitals in the United States.
Participants Adults in hospital who experienced cardiac arrest with an initial shockable rhythm, including patients who had a first defibrillation within two minutes of the cardiac arrest and who remained in a shockable rhythm after defibrillation.
Intervention Epinephrine given within two minutes after the first defibrillation.
Main outcome measures Survival to hospital discharge. Secondary outcomes included return of spontaneous circulation and survival to hospital discharge with a good functional outcome. A propensity score was calculated for the receipt of epinephrine within two minutes after the first defibrillation, based on multiple characteristics of patients, events, and hospitals. Patients who received epinephrine at either zero, one, or two minutes after the first defibrillation were then matched on the propensity score with patients who were “at risk” of receiving epinephrine within the same minute but who did not receive it.
Results 2978patients were matched on the propensity score, and the groups were well balanced. 1510 (51%) patients received epinephrine within two minutes after the first defibrillation, which is contrary to current American Heart Association guidelines. Epinephrine given within the first two minutes after the first defibrillation was associated with decreased odds of survival in the propensity score matched analysis (odds ratio 0.70, 95% confidence interval 0.59 to 0.82; P<0.001). Early epinephrine administration was also associated with a decreased odds of return of spontaneous circulation (0.71, 0.60 to 0.83; P<0.001) and good functional outcome (0.69, 0.58 to 0.83; P<0.001).
Conclusion Half of patients with a persistent shockable rhythm received epinephrine within two minutes after the first defibrillation, contrary to current American Heart Association guidelines. The receipt of epinephrine within two minutes after the first defibrillation was associated with decreased odds of survival to hospital discharge as well as decreased odds of return of spontaneous circulation and survival to hospital discharge with a good functional outcome.
Introduction
Epinephrine (adrenaline) has been used in resuscitation after cardiac arrest for decades and the provision of epinephrine is currently suggested by both the American Heart Association (AHA) and the European Resuscitation Council (ERC) in both shockable and non-shockable rhythms.1 2 Despite this, the utility of epinephrine administration in patients with cardiac arrest remains controversial.3 4 5 6 7 8 9
The effect of epinephrine is thought to be mediated primarily through α-adrenergic effects10 leading to improved coronary perfusion pressure,11 which is associated with increased probability of return of spontaneous circulation in animals12 and humans.13 The effect on cerebral perfusion, however, remains controversial.11 14 Human interventional and observational studies in patients who have cardiac arrest outside hospital have consistently found improved return of spontaneous circulation but have yielded inconsistent results with regard to long term outcomes.15 16 17 18 We have previously found that delay in the first administration of epinephrine is associated with a decreased chance of good outcomes in both adults19 and children20 in hospital who experience cardiac arrest with an initial non-shockable rhythm but there is a lack of published studies in such patients presenting with a shockable rhythm (that is, pulseless ventricular tachycardia or ventricular fibrillation). As additional highly effective interventions are recommended in these patients (primarily early defibrillation), clinical decision making becomes even more complex and both the efficacy and the optimal timing of administration of epinephrine remain unknown. Currently, there are discrepant recommendations for treatment from the AHA and the ERC, with the AHA recommending epinephrine after the second defibrillation and the ERC after the third defibrillation.1 2 In addition, clinical practice patterns could include the provision of epinephrine even earlier, such as after the first defibrillation, in patients with a persistently shockable rhythm.
We used a large multicenter registry of cardiac arrest in inpatients in the United States to describe the use of epinephrine during cardiac arrest with a shockable rhythm and to assess compliance with current AHA guidelines. As the recommendation is based essentially on expert opinion as opposed to strong science, we then determined whether early administration of epinephrine (after the first defibrillation) is associated with survival to hospital discharge.
Methods
Study design and data source
This was an analysis of prospectively collected data from the Get With The Guidelines-Resuscitation (GWTG-R) registry. The GWTG-R registry is a national prospective quality improvement registry of inpatients with cardiac arrest sponsored by the AHA. The design for data collection and reliability has been described previously in detail.21 Data are collected on all inpatients with cardiac arrest who do not have prior do not resuscitate orders or cardiopulmonary resuscitation events that began outside the hospital. Cardiac arrest is defined as pulselessness requiring chest compressions and/or defibrillation, with a hospital-wide or unit based emergency response by acute care personnel. The registry uses Utstein-style templates for cardiac arrest, which are a set of standardized reporting guidelines used to define patient variables and outcomes.22 23 Integrity of the data is improved through certification of data entry personnel and the use of standardized software.24 We included data from January 2006, when the AHA guidelines for shockable rhythms changed to their current form,25 to September 2012, after which time the required data fields were no longer part of the registry. Hospital data were obtained from the American Hospital Association’s annual survey from 2013.26
All participating hospitals are required to comply with local regulatory guidelines. Because data are used primarily at the local site for quality improvement, sites are granted a waiver of informed consent under the common rule.
Patient involvement
No patients were involved in determining the research objective or the outcome measures; nor were they involved in the design, conduct, or interpretation of the study. There are no plans to involve patients in the dissemination of results.
Study population
We included adult patients with an index cardiac arrest and a documented initial shockable rhythm (that is, pulseless ventricular tachycardia or ventricular fibrillation). We included only patients who underwent documented first defibrillation within two minutes. This was done because current guidelines recommend defibrillation as the first line treatment (along with chest compressions) for such patients27 and because we considered rapid defibrillation as a surrogate marker for overall quality of resuscitation (currently used as a quality metric by the AHA). Furthermore, previous research has found an association between early defibrillation and improved outcome with comparable outcomes for a time to defibrillation of zero, one, and two minutes.28 We included only patients who had a documented shockable rhythm after the first defibrillation.
We excluded patients who received epinephrine before the first defibrillation, who had return of spontaneous circulation, or in whom resuscitation was terminated within the same minute as the first defibrillation, visitors/employees, patients with missing data on included covariates, those with missing time of epinephrine administration, and patients with missing data on survival (the primary outcome).
Epinephrine and outcomes
Epinephrine administration was defined as any bolus dose of epinephrine given during the cardiac arrest through an intravenous or intraosseous route. Endotracheal administration of epinephrine is not recorded in the registry. The time to epinephrine was defined as the time interval in minutes from the first defibrillation to the first bolus dose of epinephrine. The recording of the time of the first defibrillation and the first dose of epinephrine was done in whole minutes. As such, a time to epinephrine administration of zero minutes means that epinephrine was given within the same whole minute as when the patient was identified as pulseless; a time of one minute represents that epinephrine was given within the next whole minute, etc.
The primary outcome was survival to hospital discharge. Secondary outcomes were return of spontaneous circulation, defined as at least 20 minutes with a palpable pulse, and good functional outcome at the time of hospital discharge. Functional outcome was assessed with the use of the cerebral performance category score (score 1=mild or no neurological deficit, 2=moderate cerebral disability, 3=severe cerebral disability, 4=coma or vegetative state, and 5=brain death).29 A score of 1 or 2 was considered a good functional outcome and a score of 3-5 or death was considered a bad functional outcome, as commonly used in cardiac arrest research.30 31 32 The score was determined by abstractors reviewing the medical record.
Statistical analysis
Descriptive statistics were used to characterize the study population. Continuous variables are reported as medians with interquartile ranges and categorical variables are reported as counts with relative frequencies. Categorical data were compared with χ2 test and continuous data with the Wilcoxon rank sum test. The Cochran-Armitage test was used to assess for trends over time.
The goal of the main analysis was to determine survival to hospital discharge in those who received epinephrine within the two minutes after the first defibrillation (that is, before the recommended second defibrillation) compared with those who did not receive epinephrine or received epinephrine more than two minutes after the first defibrillation. To assess the adjusted relation we performed propensity score matching. The propensity score was calculated with multivariable logistic regression with generalized estimating equations with an exchangeable variance-covariance structure to account for clustering within hospitals. For the calculation of the propensity score, the dependent variable was administration of epinephrine within two minutes after the first defibrillation. We included all variables presented in tables 1, 2, and 3 in the propensity score model. These variables have been defined elsewhere.33 We included quadratic and cubic terms of age, and year of the cardiac arrest was treated as a categorical variable. We also included an interaction term between time to defibrillation and intubation within the first minute as these factors could theoretically affect (timing of) epinephrine.We chose all variables a priori based on prior work and/or clinical reasoning.33 34 35 36 37 38 39
Table 1.
Characteristics of patients in full cohort of patients with cardiac arrest in hospital according to timing of administration of epinephrine. Figures are numbers (percentage) of patients unless specified otherwise
| All patients (n=2974) | Epinephrine ≤2 min (n=1510) | No epinephrine ≤ 2 min (n=1464) | P value | |
|---|---|---|---|---|
| Median (IQR) age (years) | 65 (54-75) | 65 (54-76) | 64 (53-75) | 0.1 |
| Women | 1041 (35) | 532 (35) | 509 (35) | 0.8 |
| Race: | ||||
| White | 2285 (77) | 1133 (75) | 1152 (79) | 0.08 |
| Black | 397 (13) | 221 (15) | 176 (12) | |
| Other | 104 (4) | 59 (4) | 45 (3) | |
| Unknown | 188 (6) | 97 (6) | 91 (6) | |
| Type of admission: | ||||
| Medical-non-cardiac | 686 (23) | 390 (26) | 296 (20) | <0.001 |
| Medical-cardiac | 1584 (53) | 736 (49) | 848 (58) | |
| Surgical-non-cardiac* | 295 (10) | 184 (12) | 111 (8) | |
| Surgical-cardiac | 409 (14) | 200 (13) | 209 (14) | |
| Pre-existing conditions: | ||||
| Cardiac: | ||||
| History of myocardial infarction | 670 (23) | 321 (21) | 349 (24) | 0.09 |
| Myocardial infarction this admission | 979 (33) | 431 (29) | 548 (37) | <0.001 |
| History of heart failure | 614 (21) | 338 (22) | 276 (19) | 0.02 |
| Heart failure this admission | 505 (17) | 276 (18) | 229 (16) | 0.06 |
| Non-cardiac: | ||||
| Respiratory insufficiency | 1013 (34) | 554 (37) | 459 (31) | 0.002 |
| Diabetes mellitus | 848 (29) | 441 (29) | 407 (28) | 0.4 |
| Renal insufficiency | 742 (25) | 431 (29) | 311 (21) | <0.001 |
| Metastatic/hematologic malignancy | 242 (8) | 129 (9) | 113 (8) | 0.4 |
| Hypotension/hypoperfusion | 708 (24) | 397 (26) | 311 (21) | 0.001 |
| Pneumonia | 218 (7) | 121 (8) | 97 (7) | 0.2 |
| Baseline depression in CNS function | 242 (8) | 133 (9) | 109 (7) | 0.2 |
| Metabolic/electrolyte abnormality | 360 (12) | 205 (14) | 155 (11) | 0.01 |
| Septicemia | 311 (10) | 187 (12) | 124 (8) | <0.001 |
| Acute CNS non-stroke event | 148 (5) | 81 (5) | 67 (5) | 0.3 |
| Hepatic insufficiency | 172 (6) | 97 (6) | 75 (5) | 0.1 |
| Acute stroke | 96 (3) | 61 (4) | 35 (2) | 0.01 |
| Major trauma | 72 (2) | 54 (4) | 18 (1) | <0.001 |
IQR=interquartile range, CNS=central nervous system.
*Includes patients with admission type of “obstetric” (n=2) or “trauma” (n=60)
Table 2.
Hospital characteristics in full cohort of patients with cardiac arrest in hospital according to timing of administration of epinephrine. Figures are numbers (percentage) of patients
| All patients (n=2974) | Epinephrine ≤2 min (n=1510) | No epinephrine ≤2 min (n=1464) | P value | |
|---|---|---|---|---|
| Bed size: | ||||
| 1-249 | 450 (15) | 202 (13) | 248 (17) | 0.02 |
| 250-499 | 1029 (35) | 544 (36) | 485 (33) | |
| ≥500 | 1495 (50) | 764 (51) | 731 (50) | |
| Teaching status: | ||||
| Major | 1300 (44) | 687 (46) | 613 (42) | 0.04 |
| Minor | 787 (27) | 371 (25) | 416 (28) | |
| Non-teaching | 887 (30) | 452 (30) | 435 (30) | |
| Ownership: | ||||
| Private | 493 (17) | 241 (16) | 252 (17) | 0.8 |
| Government | 253 (9) | 130 (9) | 123 (8) | |
| Non-profit | 2200 (74) | 1125 (75) | 1075 (73) | |
| Military | 28 (1) | 14 (1) | 14 (1) | |
| Location: | ||||
| Rural | 116 (4) | 59 (4) | 57 (4) | 0.9 |
| Urban | 2858 (96) | 1451 (96) | 1407 (96) | |
| Geographical location: | ||||
| North east | 457 (15) | 206 (14) | 251 (17) | 0.07 |
| South east | 731 (25) | 354 (24) | 377 (25) | |
| Midwest | 701 (24) | 359 (25) | 342 (23) | |
| South central | 621 (21) | 296 (20) | 325 (22) | |
| West | 464 (16) | 249 (17) | 215 (14) | |
Table 3.
Characteristic of cardiac arrest in full cohort of patients with cardiac arrest in hospital according to timing of administration of epinephrine. Figures are numbers (percentage) of patients
| All patients (n=2974) | Epinephrine ≤2 min (n=1510) | No epinephrine ≤2 min (n=1464) | P value | |
|---|---|---|---|---|
| Year of cardiac arrest: | ||||
| 2006 | 595 (20) | 275 (18) | 320 (22) | 0.02 |
| 2007 | 574 (19) | 283 (19) | 291 (20) | |
| 2008 | 496 (17) | 253 (17) | 243 (17) | |
| 2009 | 459 (15) | 246 (16) | 213 (15) | |
| 2010 | 320 (11) | 157 (10) | 163 (11) | |
| 2011 | 333 (11) | 178 (12) | 155 (11) | |
| 2012 | 197 (7) | 118 (8) | 79 (5) | |
| Interventions in place at time of arrest: | ||||
| Mechanical ventilation | 1065 (36) | 584 (39) | 481 (33) | <0.001 |
| ECG monitor | 2737 (92) | 1388 (92) | 1349 (92) | 0.8 |
| Pulse oximeter | 2322 (78) | 1195 (79) | 1127 (77) | 0.2 |
| Vasoactive agents* | 962 (32) | 503 (33) | 459 (31) | 0.3 |
| Antiarrhythmic drugs | 421 (14) | 190 (13) | 222 (15) | 0.04 |
| Arrest characteristics: | ||||
| Location: | ||||
| Emergency department | 430 (14) | 156 (10) | 274 (18) | <0.001 |
| Floor with telemetry | 376 (13) | 210 (14) | 166 (11) | |
| Floor without telemetry | 213 (7) | 118 (8) | 95 (6) | |
| Intensive care unit | 1529 (51) | 830 (55) | 699 (48) | |
| OR, PACU, or interventional area | 376 (13) | 171 (11) | 205 (14) | |
| Other | 50 (2) | 25 (2) | 25 (2) | |
| Time of day (night 11 pm to 6 59 am) | 820 (28) | 426 (28) | 394 (27) | 0.4 |
| Weekend (Friday 11 pm to Monday 7 am) | 899 (30) | 462 (31) | 437 (30) | 0.7 |
| Hospital-wide cardiac arrest response activated | 1943 (65) | 1035 (68) | 908 (62) | <0.001 |
| Witnessed | 2663 (90) | 1352 (90) | 1311 (90) | 0.9 |
| Initial rhythm: | ||||
| Pulseless VT | 1045 (35) | 513 (34) | 532 (36) | 0.12 |
| VF | 1929 (65) | 997 (66) | 932 (64) | |
| Time to defibrillation from loss of pulse (minutes): | ||||
| 0-1 | 2051 (69) | 984 (65) | 1067 (73) | <0.001 |
| 1-2 | 526 (18) | 297 (20) | 229 (16) | |
| 2-3 | 397 (13) | 229 (15) | 168 (11) | |
| Post-defibrillation rhythm: | ||||
| Pulseless VT | 940 (32) | 450 (30) | 490 (33) | 0.03 |
| VF | 2034 (68) | 1060 (70) | 974 (67) | |
| Intubation 0-1 min after loss of pulse | 141 (5) | 92 (6) | 49 (3) | <0.001 |
ECG=electrocardiogram, OR=operating room, PACU=post-anesthesia care unit, VT=ventricular tachycardia, VF=ventricular fibrillation.
*Includes dobutamine, dopamine (>3 μg/kg/min), epinephrine, nitroglycerin, norepinephrine, phenylephrine, vasopressin, and/or “other vasoactive agent(s).”
We next performed 1:1 matching on the propensity score using nearest neighbor matching with a maximum caliber of 0.01 of the propensity score. Patients who received epinephrine at either zero, one, or two minutes after the first defibrillation were separately matched on the propensity score with a patient who was “at risk” of receiving epinephrine within the same time frame. “At risk” patients included those still undergoing resuscitation (that is, patients who did not have return of spontaneous circulation or in whom resuscitation was terminated) and who did not receive epinephrine before or within the same minute, including patients who received epinephrine at a later time point (“as yet untreated” patients).40 The matching was performed separately for minutes zero, one, and two after the first defibrillation with replacement of controls to optimize the sample size. If we included a patient with return of spontaneous circulation within or before this time period, and who was therefore never “at risk” for receiving epinephrine, in the analysis this could bias the results towards a harmful effect of epinephrine because early return of spontaneous circulation (that is, short duration of arrest) is associated with improved survival.39 41 To assess the performance of the matching, we compared baseline categorical variables between the matched groups using the Cochran-Mantel-Haenszel test and calculated standardized differences.
Using the matched cohort, we next performed conditional logistic regression to assess the association between epinephrine administration and survival to hospital discharge. Given the potential importance of time to defibrillation, we included this variable in the regression model as a categorical variable. We report the results from the regression model as odds ratios with 95% confidence intervals. We performed similar conditional logistic regression analyses for the secondary outcomes of return of spontaneous circulation and good functional outcome. To compare the number of total defibrillations, the time to the second defibrillation, total dose of epinephrine, and the time to the end of resuscitation in the two groups we used Poisson regression with robust variance estimates while accounting for the correlation between matched participants. The results of these analyses are presented as relative increases with 95% confidence intervals.
Sensitivity analyses
We performed two predefined sensitivity analyses. First, we performed the propensity score matching without replacement of the control patients. Second, we accounted for missing data for 347 patients (10%) on covariates time of epinephrine administration or survival (fig 1) and for 140 patients (5%) on functional outcome. We imputed missing values for covariates and outcomes by using the fully conditional specification (FCS) method42 and created a total of 10 datasets. As time to epinephrine follows an approximate zero inflated Poisson distribution, we performed imputations for those receiving epinephrine using this distribution for all 10 datasets.20 43 We then performed the propensity score matching and conditional logistic regression on each of these 10 datasets and combined the results using SAS, version 9.4, (SAS Institute, Cary, NC) “proc mianalyze.” We also performed two post hoc sensitivity analyses. As guideline changes can take a substantial amount of time to be implemented,44 and because important changes were made in the 2005 guidelines, we conducted the analysis after excluding events in 2006 and 2007. In our second post hoc sensitivity analysis, we matched only patients who received epinephrine at zero or one minute after the first defibrillation as some patients could have received their second defibrillation during the two minute period.
Fig 1 Inclusion and exclusion of patients in study of timing of administration of epinephrine for inpatients with cardiac arrest. Out of 3777 patients who met all inclusion criteria, 2974 were included in main cohort
Post hoc analyses
We performed two additional post hoc analyses. In the first, we included the same patients as above but also included those who received epinephrine before the first defibrillation. We then restricted the population to those who received a second defibrillation and who received the second defibrillation between one and three minutes after the first defibrillation. In this cohort, we assessed whether receiving epinephrine at any time before the second defibrillation, compared with not receiving epinephrine before the second defibrillation, was associated with the various outcomes. In this analysis, we also utilized propensity score matching as above. In the second analysis, we broadened the inclusion criteria to include patients who had a documented first defibrillation within five minutes with no changes to the remaining inclusion/exclusion criteria. We then performed the main analysis in this patient cohort.
All hypothesis tests were two sided, with a significance level of P<0.05. All secondary analyses should be considered exploratory as we did not adjust for multiple comparisons. We conducted all statistical analyses using SAS software.
Results
Characteristics of study population
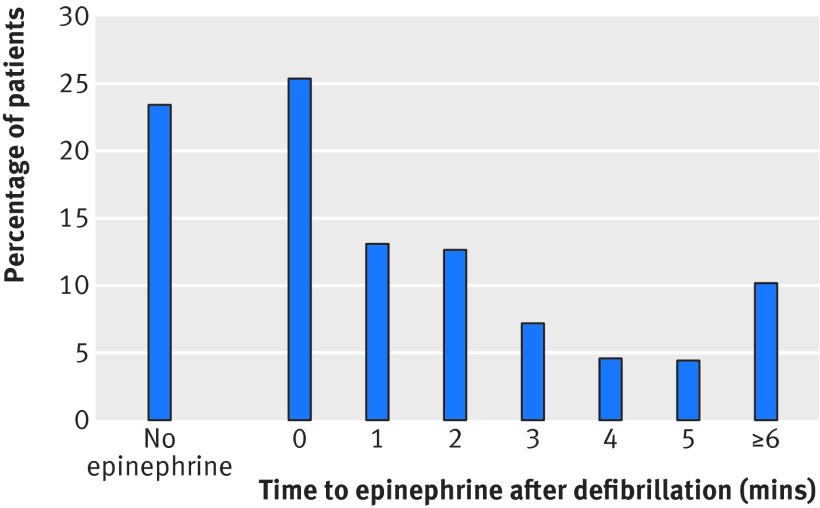
We included 2974 patients from 310 hospitals in the main cohort (fig 1). The median age was 65 (interquartile range 54-75), 1041 (35%) were women, and 1167 (39%)survived to hospital discharge. Tables 1, 2, and 3 provide additional characteristics for patients, hospitals, and events for the overall group. Overall, 692 (23%) patients did not receive epinephrine at any time during the resuscitation. Figure 2 shows the timing of epinephrine administration in relation to the first defibrillation.
Fig 2 Distribution of timing of epinephrine in relation to first defibrillation. 692 (23%) patients did not receive epinephrine at any time during resuscitation and 1510 (51%) received epinephrine within two minutes after first defibrillation
Epinephrine within first two minutes after first defibrillation
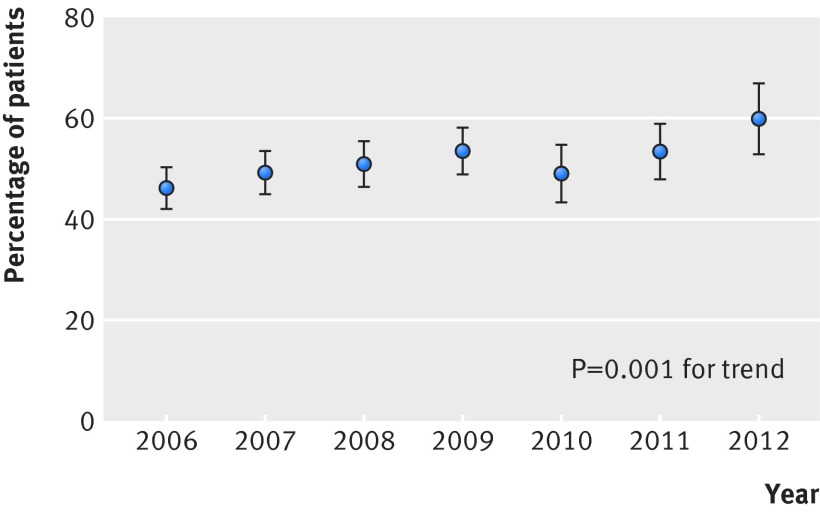
In total 1510 (51%) patients received epinephrine within two minutes after the first defibrillation. Tables 1, 2 and 3 show the characteristics of these patients and those not receiving epinephrine within this timeframe. Over time, there was an increase in the incidence of epinephrine given within two minutes after the first defibrillation (275/595 (46%) in 2006 to 118/197 (60%) in 2012, P=0.001 for a linear trend, fig 3, table 3).This increase over time remained in multivariable analysis when we adjusted for variables included in the tables (odds ratio 1.07 per year, 95% confidence interval 1.03 to 1.12; P=0.001).
Fig 3 Proportion of patients with early administration (within two minutes after defibrillation) of epinephrine per year, showing significant increase over time in both bivariable and multivariable analysis (both P=0.001 for linear trends)
Early epinephrine administration within two minutes after the first defibrillation was associated with a decreased likelihood of survival in unadjusted analysis (464/1510 (31%) v 703/1464 (48%), odds ratio 0.48, 95% confidence interval 0.41 to 0.56; P<0.001). Early epinephrine administration was also associated with decreased likelihood of return of spontaneous circulation (1018/1510 (67%) v 1158/1464 (79%), 0.55, 0.46 to 0.65; P<0.001) and good functional outcome (357/1445 (25%) v 567/1389 (41%), 0.48, 0.41 to 0.56; P<0.001).
Propensity score matched cohort
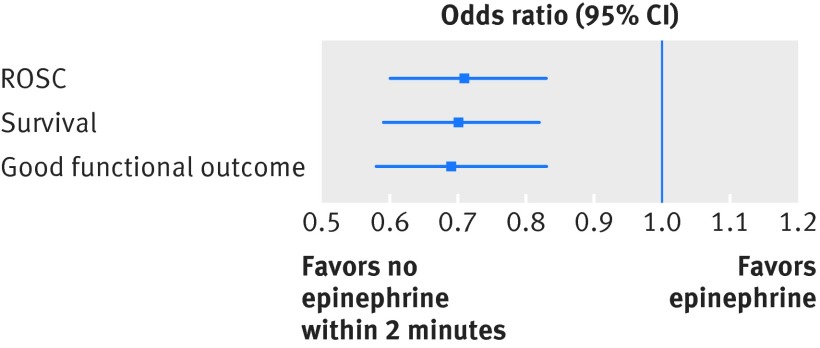
We matched 2978 patients on the propensity score for receipt of epinephrine within the first two minutes after defibrillation. Characteristics of the two groups in the matched cohort are displayed in appendix 1, and the distributions of propensity scores before and after matching are displayed in appendix 2. The two groups were well balanced on all included variables (all P>0.05 with standardized differences between −0.1 and 0.1). In the matched cohort, epinephrine administration within two minutes after the first defibrillation was still associated with decreased odds of survival (odds ratio 0.70, 95% confidence interval 0.59 to 0.82; P<0.001). Epinephrine administration also remained associated with a decreased odds of return of spontaneous circulation (0.71, 0.60 to 0.83; P<0.001) and good functional outcome (0.69, 0.58 to 0.83; P<0.001), see figure 4. These associations remained significant when we analyzed patients with return of spontaneous circulation (n=1974) for survival (0.79, 0.64 to 0.98; P=0.03) and good functional outcome (0.77, 0.61 to 0.96; P=0.02).
Fig 4 Graphical representation of odds ratios in propensity matched cohort for outcomes survival, return of spontaneous circulation (ROSC), and good functional outcome epinephrine administration compared with no epinephrine administration ≤2 min after first defibrillation. Error bars represent 95% confidence intervals
Patients who received early epinephrine had a similar number of total defibrillations as those not receiving early epinephrine (median 3 (interquartile range 2-5) in both groups; relative increase 1.03, 95% confidence interval 0.98 to 1.09; P=0.25). In each group 87% of patients had a second defibrillation (P=0.91). There was no difference in the time from the first defibrillation to the second defibrillation (median 2 minutes (1-3) in both groups; relative increase: 0.77, 0.58 to 1.03; P=0.08). Patients who received early epinephrine had a higher total dose of epinephrine (3 mg (1-4) v 1 mg (0-3); relative increase: 1.57, 1.47 to 1.68; P<0.001). Time to return of spontaneous circulation was similar in those who did and did not receive early epinephrine (13 minutes (7-27) v 13 minutes (6-26); relative increase 1.02, 0.93 to 1.13; P=0.63). The time to termination of resuscitation efforts in patients who did not achieve return of spontaneous circulation was also similar (22 minutes (14-32) v 21 minutes (14-29); relative increase 1.05, 0.95 to 1.17; P=0.32).
Sensitivity analyses
We included 2310 patients in the matched cohort without replacement of controls. The association between early epinephrine administration and survival remained in this cohort (odds ratio 0.73, 95% confidence interval 0.62 to 0.87; P<0.001). The association between early epinephrine and return of spontaneous circulation (0.72, 0.60 to 0.87; P<0.001) and functional outcome (0.68, 0.56 to 0.83; P<0.001) also remained. The full cohort used for multiple imputation to account for missing data included 3321 patients. Between 3330 and 3519 patients were matched in the 10 created datasets. The combined estimate for the matched cohorts was similar to that reported in the main analysis for survival (0.72, 0.61 to 0.85; P<0.001), return of spontaneous circulation (0.76, 0.62 to 0.94; P=0.01), and good functional outcome (0.75, 0.63 to 0.99; P=0.001).
In our first post hoc sensitivity analysis, which excluded events in 2006 and 2007, we matched 1878 patients. The association between early epinephrine administration and survival (odds ratio 0.70, 0.57 to 0.85; P<0.001), return of spontaneous circulation (0.76, 0.62 to 0.84; P=0.01), and functional outcome (0.71, 0.57 to 0.89; P=0.003) remained. In our second post hoc sensitivity analysis, we matched only the 1418 patients in the zero and one minute timeframes (that is, excluding minute two). The association between early epinephrine administration and survival (0.72, 0.57 to 0.90; P=0.004) and functional outcome (0.70, 0.54 to 0.90; P=0.007) remained. The association between early epinephrine administration and return of spontaneous circulation was not significant in this sensitivity analysis (0.82, 0.65 to 1.03; P=0.09).
Additional analyses
There were 1758 patients in the cohort of patients with epinephrine administration any time before the second defibrillation, of whom 1252 were propensity score matched. The groups were well matched. In this analysis, we looked at epinephrine administration at any time before the second defibrillation compared with no epinephrine administration before the second defibrillation. We found that early epinephrine administration was also associated with decreased odds of survival (odds ratio 0.54, 95% confidence interval 0.42 to 0.69; P<0.001), return of spontaneous circulation (0.55, 0.42 to 0.72; P<0.001), and good functional outcome (0.55, 0.42 to 0.72; P<0.001) in this analysis.
In the cohort of patients with the first defibrillation within five minutes, we matched 3520 patients for propensity score. In this cohort, the results remained similar. Early epinephrine administration was associated with decreased odds of survival (odds ratio 0.77, 95% confidence interval 0.66 to 0.89; P<0.001), return of spontaneous circulation (0.73, 0.63 to 0.85; P<0.001), and good functional outcome (0.77, 0.66 to 0.91; P=0.002).
Discussion
For in hospital patients with cardiac arrest and a shockable rhythm, administration of epinephrine within the first two minutes after the initial defibrillation was common and was associated with decreased chance of return of spontaneous circulation, survival, and survival with a good functional outcome compared with those who were not given epinephrine within this period. This association remained when we used time dependent propensity score matching and in sensitivity analyses.
Epinephrine background and guideline recommendation
Epinephrine, a potent α- and β-adrenergic agonist with inotropic, chronotropic, and vasoconstrictive effects,45 has been used during cardiac arrest for decades. This practice is partly based on findings from animal studies from the 1960s,6 46 47 and epinephrine has been recommend since 1974 when the American Heart Association (AHA) published its first recommendations on cardiac arrest.48 The most recent AHA guidelines state that it is “reasonable to consider” epinephrine every three to five minutes for patients with a non-shockable rhythm and every three to five minutes after the second defibrillation in patients with a shockable rhythm.1 In the current study, we found that over half of our included patients received epinephrine within two minutes after the first defibrillation (contrary to current guidelines). The true proportion of patients treated contrary to guidelines is even larger as we excluded 439 who received epinephrine before the initial defibrillation. Furthermore, we found that the proportion of patients who received early epinephrine has been increasing over the years (from 46% in 2006 to 60% in 2012), a finding that remains largely unexplained.
Comparison with previous studies
The published evidence in humans regarding the use of epinephrine in cardiac arrest with shockable rhythms is scant, especially in those who experience cardiac arrest as inpatients and in the early stages of arrest.4 To our knowledge, one randomized controlled trial has compared intravenous drug administration during cardiac arrest outside hospital with no intravenous drug administration,49 and only one randomized controlled trial has been published directly comparing epinephrine with placebo.15 In this trial, Jacobs and colleagues analyzed 534 patients with cardiac arrest outside hospital and found that those who were randomized to epinephrine had markedly increased rate of return of spontaneous circulation and admission to hospital, but they also found no significant difference in survival or survival with a good functional outcome, although the study was underpowered to detect such differences.15 In the group of patients with an initial shockable rhythm, which was the focus of our investigation, the findings were similar to those of the non-shockable group, although the magnitude of the benefit regarding return of spontaneous circulation was somewhat less compared with in the non-shockable group.15 In Jacobs and colleagues’ study, epinephrine was administered after the third unsuccessful defibrillation, consistent with European and Australian guidelines.2 15 Furthermore, all patients had cardiac arrests outside hospital, and the mean response time of emergency providers was about 10 minutes, making any comparison with the current study difficult or essentially impossible.
Recently, several large observational studies regarding the use of epinephrine in cardiac arrest outside hospital have been published.16 17 50 In a large Japanese study utilizing a similar statistical approach as here, Nakahara and colleagues found that administration of epinephrine in the prehospital setting was associated with increased overall survival but found no difference in good functional recovery among patients with a shockable rhythm.17 While these findings are different from the current report, Nakahara and colleagues also examined a different population of patients (cardiac arrest outside hospital), a different algorithm, and a different time of epinephrine administration. As noted by other investigators,51 there are profound differences between patients with cardiac arrest in and outside hospital, both in term of patients’ characteristics, underlying etiology, treatment and timing of treatment, and outcomes. As such, the efficacy of interventions such as epinephrine might vary between these populations.
Potential mechanisms
There are multiple potential reasons for why epinephrine could be detrimental in early cardiac arrest with a shockable rhythm. Patients with a shockable rhythm more often have a cardiac cause of their arrest.52 Epinephrine’s β-adrenergic effects could increase myocardial oxygen demand, which, despite increased coronary perfusion pressure, can lead to increased myocardial damage and increased myocardial dysfunction after cardiac arrest.53 54 Epinephrine can also decrease blood flow to other organs including the kidneys,55 lungs,56 and brain14 as well as reduce microcirculatory blood flow.57 All these findings, however, are from controlled animal studies and are not consistent between studies.11 As such, their translation to complex heterogeneous patients with various underlying etiologies and interventions is questionable.
Although it was not the primary focus of our study, we found that those receiving early epinephrine received a higher cumulative dose during their cardiac arrest (3 mg (interquartile range 1-4) v 1 mg (0-3); P<0.001). Previous observational studies have found that a high cumulative epinephrine dose is associated with worse outcomes,58 59 and it is possible that the cumulative dose in the current study could be a driver of outcomes. It also remains possible that the early administration of epinephrine could have interfered with the administration or quality of other interventions, such as subsequent defibrillations, chest compressions, or airway management. In contrast to epinephrine, defibrillation has minimal, if any, side effects. Therefore, one could postulate that an effective treatment with limited side effects might be superior to another with important side effects, particularly early in the arrest period (that is, what has been termed, the “electrical phase” of cardiac arrest).60 In other words, during the electrical phase of cardiac arrest many patients with shockable rhythms could have return of spontaneous circulation with defibrillation, without the need for an intervention such as epinephrine with potential side effects. This contrasts with non-shockable rhythms, where treatments are limited to cardiopulmonary resuscitation and attempts to deal with the underlying etiology of arrest. Of note, in one animal study epinephrine had no effect on subsequent success of defibrillation, although animals treated with epinephrine had increased return of spontaneous circulation.61 An ongoing large randomized controlled trial comparing epinephrine with placebo in cardiac arrest outside hospital (“Paramedic2”),62 currently enrolling in the United Kingdom, will help answer many lingering questions, but the translatability to cardiac arrest in hospital, where interventions are usually administered much earlier, is unknown.
Limitations of study
The design and limitations of the current study should be taken into account in the interpretation of the findings. Despite adjustment for multiple patient, event, and hospital factors, and with the timing of epinephrine taken into account, this is an observational study and no strong conclusions can be made regarding the causal effect of early epinephrine administration. Unknown and/or unmeasured confounders, such as the quality of cardiopulmonary resuscitation, could have changed our results if they had been included in the analysis. Despite inclusion of data from more than 300 hospitals, the sample size was inadequate for making inference about epinephrine administration at later time points (such as after the second and third defibrillation). As such, these findings should not be extrapolated to later time points, nor to settings outside hospital. Furthermore, most of our included patients were monitored at the time of the arrest. This might not be comparable with other settings and countries,63 which could limit the generalizability of our findings.
The GWTG-R registry does not collect information on endotracheal administration of epinephrine. In the unlikely event of this occurring in the “no early epinephrine group,” however, we believe this would bias our results towards to null. Misclassification of variables could have influenced our results, given the nature of this data registry. Misclassification related to timing of interventions would be especially concerning,64 65 although we suspect that errors would more likely occur for absolute times rather than relative times (that is, differences between two time points), which we used for this study. Furthermore, we believe that any misclassification, including those related to timing, would be unrelated to outcomes and would therefore most likely bias the results towards the null (that is, this potential bias is unlikely to explain our current finding).
Conclusion
In conclusion, we found that early administration of epinephrine after the first defibrillation (that is, contrary to guidelines) was common (>50%) in patients who experience cardiac arrest in hospital with a shockable rhythm. Moreover, the provision of epinephrine at this time point was associated with a decreased chance of good outcome, including decreased in hospital survival. These findings might be relevant to guideline developers, educators, and clinicians involved with the care of such patients.
What is already known on this topic
There is conflicting evidence regarding the effectiveness of epinephrine in patients with cardiac arrest outside hospital
Little is known about the effectiveness of epinephrine in patients in hospital who experience cardiac arrest with a shockable rhythm, particularly early during the arrest
What this study adds
Early administration of epinephrine after the first defibrillation (that is, contrary to guidelines) was common in inpatients with cardiac arrest and a shockable rhythm (> 50%)
Provision of epinephrine at this time point was associated with a decreased chance of good outcome including decreased survival in hospital
Web Extra.
Web extra material supplied by author
Appendix 1: Characteristics of study population in main propensity score matched cohorts
Appendix 2: Distribution of propensity scores before and after matching
Get with the Guidelines-Resuscitation Investigators
MWD, Paul S Chan (Saint Luke’s Mid America Heart Institute), Steven M Bradley (VA Eastern Colorado Healthcare System), Girotra Saket (University of Iowa Carver College of Medicine), Monique L Anderson (Duke Clinical Research Institute), Matthew M Churpek (University of Chicago), Ahamed H Idris (University of Texas Southwestern Medical Center), Dana P Edelson (University of Chicago), Robert T Faillace (Geisinger Healthcare System), Romergryko Geocadin (Johns Hopkins University School of Medicine), Raina Merchant (University of Pennsylvania School of Medicine), Vincent N Mosesso Jr (University of Pittsburgh School of Medicine), Joseph P Ornato and Mary Ann Peberdy (Virginia Commonwealth University), Sarah M Perman (University of Colorado, School of Medicine), Mindy Smyth (retired).
Contributors: LWA and MWD were responsible for study concept and design, acquisition of data, and drafting of the manuscript. LWA and TK performed the statistical analysis. All authors interpreted the data, critically revised the manuscript for important intellectual content and approved the final version for submission. All authors agree to be accountable for all aspects of the work. LWA and MWD are guarantors.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships except as noted below with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. MWD is a paid consultant for the American Heart Association.
Ethical approval: All participating hospitals in the Get With The Guidelines-Resuscitation registry are required to comply with local regulatory guidelines. Because data are used primarily at the local site for quality improvement, sites are granted a waiver of informed consent under the common rule.
Transparency: The lead author affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant) have been explained.
Data sharing: No additional data available.
References
- 1.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(Suppl 3):S729-67. 10.1161/CIRCULATIONAHA.110.970988 pmid:20956224. [DOI] [PubMed] [Google Scholar]
- 2.Deakin CD, Nolan JP, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation 2010;81:1305-52. 10.1016/j.resuscitation.2010.08.017 pmid:20956049. [DOI] [PubMed] [Google Scholar]
- 3.Perkins GD, Cottrell P, Gates S. Is adrenaline safe and effective as a treatment for out of hospital cardiac arrest?BMJ 2014;348:g2435 10.1136/bmj.g2435 pmid:24709574. [DOI] [PubMed] [Google Scholar]
- 4.Lin S, Callaway CW, Shah PS, et al. Adrenaline for out-of-hospital cardiac arrest resuscitation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation 2014;85:732-40. 10.1016/j.resuscitation.2014.03.008 pmid:24642404. [DOI] [PubMed] [Google Scholar]
- 5.Perkins GD, Nolan JP. Early adrenaline for cardiac arrest. BMJ 2014;348:g3245 10.1136/bmj.g3245 pmid:24874448. [DOI] [PubMed] [Google Scholar]
- 6.Callaway CW. Questioning the use of epinephrine to treat cardiac arrest. JAMA 2012;307:1198-200. 10.1001/jama.2012.313 pmid:22436961. [DOI] [PubMed] [Google Scholar]
- 7.McCartney M. Adrenaline in cardiac arrest: it’s unethical for patients not to know. BMJ 2014;349:g5258 10.1136/bmj.g5258 pmid:25148860. [DOI] [PubMed] [Google Scholar]
- 8.Atiksawedparit P, Rattanasiri S, McEvoy M, Graham CA, Sittichanbuncha Y, Thakkinstian A. Effects of prehospital adrenaline administration on out-of-hospital cardiac arrest outcomes: a systematic review and meta-analysis. Crit Care 2014;18:463 10.1186/s13054-014-0463-7 pmid:25079607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamoorthy V, Vavilala MS, Fettiplace MR, Weinberg G. Epinephrine for cardiac arrest are we ng more harm than good?Anesthesiology 2014;120:792-4. 10.1097/ALN.0000000000000032 pmid:24135580. [DOI] [PubMed] [Google Scholar]
- 10.Otto CW, Yakaitis RW, Blitt CD. Mechanism of action of epinephrine in resuscitation from asphyxial arrest. Crit Care Med 1981;9:321-4. 10.1097/00003246-198104000-00008 pmid:7214942. [DOI] [PubMed] [Google Scholar]
- 11.Michael JR, Guerci AD, Koehler RC, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984;69:822-35. 10.1161/01.CIR.69.4.822 pmid:6697465. [DOI] [PubMed] [Google Scholar]
- 12.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med 1985;14:521-8. 10.1016/S0196-0644(85)80774-5 pmid:3994075. [DOI] [PubMed] [Google Scholar]
- 13.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990;263:1106-13. 10.1001/jama.1990.03440080084029 pmid:2386557. [DOI] [PubMed] [Google Scholar]
- 14.Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med 2009;37:1408-15. 10.1097/CCM.0b013e31819cedc9 pmid:19242339. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation 2011;82:1138-43. 10.1016/j.resuscitation.2011.06.029 pmid:21745533. [DOI] [PubMed] [Google Scholar]
- 16.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 2012;307:1161-8. 10.1001/jama.2012.294 pmid:22436956. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara S, Tomio J, Takahashi H, et al. Evaluation of pre-hospital administration of adrenaline (epinephrine) by emergency medical services for patients with out of hospital cardiac arrest in Japan: controlled propensity matched retrospective cohort study. BMJ 2013;347:f6829 10.1136/bmj.f6829 pmid:24326886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto Y, Maeda T, Goto Y. Effects of prehospital epinephrine during out-of-hospital cardiac arrest with initial non-shockable rhythm: an observational cohort study. Crit Care 2013;17:R188 10.1186/cc12872 pmid:24004456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WY, Kwak MK, Ko BS, et al. Factors associated with the occurrence of cardiac arrest after emergency tracheal intubation in the emergency department. PLoS One 2014;9:e112779 10.1371/journal.pone.0112779 pmid:25402500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen LW, Berg KM, Saindon BZ, et al. American Heart Association Get With the Guidelines–Resuscitation Investigators. Time to Epinephrine and Survival After Pediatric In-Hospital Cardiac Arrest. JAMA 2015;314:802-10. 10.1001/jama.2015.9678 pmid:26305650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 2003;58:297-308. 10.1016/S0300-9572(03)00215-6 pmid:12969608. [DOI] [PubMed] [Google Scholar]
- 22.Cummins RO, Chamberlain D, Hazinski MF, et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital ‘Utstein style’. A statement for healthcare professionals from the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Australian Resuscitation Council, and the Resuscitation Councils of Southern Africa. Resuscitation 1997;34:151-83. 10.1016/S0300-9572(97)01112-X pmid:9141159. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs I, Nadkarni V, Bahr J, et al. International Liason Committee on Resusitation. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004;63:233-49. 10.1016/j.resuscitation.2004.09.008 pmid:15582757. [DOI] [PubMed] [Google Scholar]
- 24.Peberdy MA, Ornato JP, Larkin GL, et al. National Registry of Cardiopulmonary Resuscitation Investigators. Survival from in-hospital cardiac arrest during nights and weekends. JAMA 2008;299:785-92. 10.1001/jama.299.7.785 pmid:18285590. [DOI] [PubMed] [Google Scholar]
- 25. ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005;112(Suppl):IV1-203.pmid:16314375. [DOI] [PubMed] [Google Scholar]
- 26.American Hospital Association. AHA Annual Survey Database™ Fiscal Year 2013 web page. Secondary AHA Annual Survey Database™ Fiscal Year 2013 web page 2014. http://www.ahadataviewer.com/book-cd-products/aha-survey/.
- 27.Deakin CD, Morrison LJ, Morley PT, et al. Advanced Life Support Chapter Collaborators. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2010;81(Suppl 1):e93-174. 10.1016/j.resuscitation.2010.08.027 pmid:20956032. [DOI] [PubMed] [Google Scholar]
- 28.Chan PS, Krumholz HM, Nichol G, Nallamothu BK. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med 2008;358:9-17. 10.1056/NEJMoa0706467 pmid:18172170. [DOI] [PubMed] [Google Scholar]
- 29.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;i:480-4. 10.1016/S0140-6736(75)92830-5 pmid:46957. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen N, Wetterslev J, Friberg H. TTM Trial Steering Group. Targeted temperature management after cardiac arrest. N Engl J Med 2014;370:1360.pmid:24693900. [DOI] [PubMed] [Google Scholar]
- 31.Donnino MW, Miller JC, Bivens M, et al. A pilot study examining the severity and outcome of the post-cardiac arrest syndrome: a comparative analysis of two geographically distinct hospitals. Circulation 2012;126:1478-83. 10.1161/CIRCULATIONAHA.111.067256 pmid:22879369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker LB, Aufderheide TP, Geocadin RG, et al. American Heart Association Emergency Cardiovascular Care Committee Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation 2011;124:2158-77. 10.1161/CIR.0b013e3182340239 pmid:21969010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng TJ, Andersen LW, Saindon BZ, et al. American Heart Association’s Get With The Guidelines®-Resuscitation Investigators. The administration of dextrose during in-hospital cardiac arrest is associated with increased mortality and neurologic morbidity. Crit Care 2015;19:160 10.1186/s13054-015-0867-z pmid:25887120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen LW, Bivens MJ, Giberson T, et al. The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation 2015;94:49-54. 10.1016/j.resuscitation.2015.05.015 pmid:26044753. [DOI] [PubMed] [Google Scholar]
- 35.Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK. American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med 2009;169:1265-73. 10.1001/archinternmed.2009.196 pmid:19636027. [DOI] [PubMed] [Google Scholar]
- 36.Chan PS, Nallamothu BK, Krumholz HM, et al. American Heart Association Get with the Guidelines–Resuscitation Investigators. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med 2013;368:1019-26. 10.1056/NEJMoa1200657 pmid:23484828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012;367:1912-20. 10.1056/NEJMoa1109148 pmid:23150959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med 2010;38:101-8. 10.1097/CCM.0b013e3181b43282 pmid:19770741. [DOI] [PubMed] [Google Scholar]
- 39.Kim WY, Giberson TA, Uber A, Berg K, Cocchi MN, Donnino MW. Neurologic outcome in comatose patients resuscitated from out-of-hospital cardiac arrest with prolonged downtime and treated with therapeutic hypothermia. Resuscitation 2014;85:1042-6. 10.1016/j.resuscitation.2014.04.005 pmid:24746783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Propert K, Rosenbaum P. Balanced Risk Set Matching. J Am Stat Assoc 2001;96:870-882. 10.1198/016214501753208573. [DOI] [Google Scholar]
- 41.Chan PS, Spertus JA, Krumholz HM, et al. Get With the Guidelines-Resuscitation Registry Investigators. A validated prediction tool for initial survivors of in-hospital cardiac arrest. Arch Intern Med 2012;172:947-53. 10.1001/archinternmed.2012.2050 pmid:22641228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219-42. 10.1177/0962280206074463 pmid:17621469. [DOI] [PubMed] [Google Scholar]
- 43.Pahel BT, Preisser JS, Stearns SC, Rozier RG. Multiple imputation of dental caries data using a zero-inflated Poisson regression model. J Public Health Dent 2011;71:71-8. 10.1111/j.1752-7325.2010.00197.x pmid:20880027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berdowski J, Schmohl A, Tijssen JG, Koster RW. Time needed for a regional emergency medical system to implement resuscitation Guidelines 2005--The Netherlands experience. Resuscitation 2009;80:1336-41. 10.1016/j.resuscitation.2009.08.011 pmid:19766376. [DOI] [PubMed] [Google Scholar]
- 45.Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation 2008;118:1047-56. 10.1161/CIRCULATIONAHA.107.728840 pmid:18765387. [DOI] [PubMed] [Google Scholar]
- 46.Redding JS, Pearson JW. Resuscitation from asphyxia. JAMA 1962;182:283-6.pmid:13973494. [PubMed] [Google Scholar]
- 47.Redding JS, Pearson JW. Resuscitation from ventricular fibrillation. Drug therapy. JAMA 1968;203:255-60. 10.1001/jama.1968.03140040007002 pmid:5694097. [DOI] [PubMed] [Google Scholar]
- 48.Standards for cardiopulmonary resuscitation (CPR) and emergency cardiac care (ECC). 3. Advanced life support. JAMA1974;227(Suppl):852-60. [PubMed] [Google Scholar]
- 49.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA 2009;302:2222-9. 10.1001/jama.2009.1729 pmid:19934423. [DOI] [PubMed] [Google Scholar]
- 50.Dumas F, Bougouin W, Geri G, et al. Is epinephrine during cardiac arrest associated with worse outcomes in resuscitated patients?J Am Coll Cardiol 2014;64:2360-7. 10.1016/j.jacc.2014.09.036 pmid:25465423. [DOI] [PubMed] [Google Scholar]
- 51.Fredriksson M, Aune S, Bång A, et al. Cardiac arrest outside and inside hospital in a community: mechanisms behind the differences in outcome and outcome in relation to time of arrest. Am Heart J 2010;159:749-56. 10.1016/j.ahj.2010.01.015 pmid:20435182. [DOI] [PubMed] [Google Scholar]
- 52.Terman SW, Hume B, Meurer WJ, Silbergleit R. Impact of presenting rhythm on short- and long-term neurologic outcome in comatose survivors of cardiac arrest treated with therapeutic hypothermia. Crit Care Med 2014;42:2225-34. 10.1097/CCM.0000000000000506 pmid:25014063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Callaway CW. Epinephrine for cardiac arrest. Curr Opin Cardiol 2013;28:36-42. 10.1097/HCO.0b013e32835b0979 pmid:23196774. [DOI] [PubMed] [Google Scholar]
- 54.Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation 1995;92:3089-93. 10.1161/01.CIR.92.10.3089 pmid:7586280. [DOI] [PubMed] [Google Scholar]
- 55.Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation 1988;78:382-9. 10.1161/01.CIR.78.2.382 pmid:3396175. [DOI] [PubMed] [Google Scholar]
- 56.Lindberg L, Liao Q, Steen S. The effects of epinephrine/norepinephrine on end-tidal carbon dioxide concentration, coronary perfusion pressure and pulmonary arterial blood flow during cardiopulmonary resuscitation. Resuscitation 2000;43:129-40. 10.1016/S0300-9572(99)00129-X pmid:10694173. [DOI] [PubMed] [Google Scholar]
- 57.Fries M, Weil MH, Chang YT, Castillo C, Tang W. Microcirculation during cardiac arrest and resuscitation. Crit Care Med 2006;34(Suppl):S454-7. 10.1097/01.CCM.0000247717.81480.B2 pmid:17114977. [DOI] [PubMed] [Google Scholar]
- 58.Behringer W, Kittler H, Sterz F, et al. Cumulative epinephrine dose during cardiopulmonary resuscitation and neurologic outcome. Ann Intern Med 1998;129:450-6. 10.7326/0003-4819-129-6-199809150-00004 pmid:9735082. [DOI] [PubMed] [Google Scholar]
- 59.Arrich J, Sterz F, Herkner H, Testori C, Behringer W. Total epinephrine dose during asystole and pulseless electrical activity cardiac arrests is associated with unfavourable functional outcome and increased in-hospital mortality. Resuscitation 2012;83:333-7. 10.1016/j.resuscitation.2011.10.027 pmid:22079948. [DOI] [PubMed] [Google Scholar]
- 60.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA 2002;288:3035-8. 10.1001/jama.288.23.3035 pmid:12479769. [DOI] [PubMed] [Google Scholar]
- 61.Otto CW, Yakaitis RW, Ewy GA. Effect of epinephrine on defibrillation in ischemic ventricular fibrillation. Am J Emerg Med 1985;3:285-91. 10.1016/0735-6757(85)90048-8 pmid:4004996. [DOI] [PubMed] [Google Scholar]
- 62.Warwick Medical School. PARAMEDIC2. Secondary PARAMEDIC2 2015. http://www2.warwick.ac.uk/fac/med/research/hscience/ctu/trials/critical/paramedic2/.
- 63.Nolan JP, Soar J, Smith GB, et al. National Cardiac Arrest Audit. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014;85:987-92. 10.1016/j.resuscitation.2014.04.002 pmid:24746785. [DOI] [PubMed] [Google Scholar]
- 64.Kaye W, Mancini ME, Truitt TL. When minutes count--the fallacy of accurate time documentation during in-hospital resuscitation. Resuscitation 2005;65:285-90. 10.1016/j.resuscitation.2004.12.020 pmid:15919564. [DOI] [PubMed] [Google Scholar]
- 65.Peace JM, Yuen TC, Borak MH, Edelson DP. Tablet-based cardiac arrest documentation: a pilot study. Resuscitation 2014;85:266-9. 10.1016/j.resuscitation.2013.10.013 pmid:24157630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Characteristics of study population in main propensity score matched cohorts
Appendix 2: Distribution of propensity scores before and after matching