Abstract
We report a case of acute odontogenic sepsis in a 59-year-old man, presenting with diffuse, descending necrotising mediastinitis complicated by pleural empyema. Despite surviving the odds, his recovery was complicated by severe dysphagia, resulting in gastrostomy feeding for 6 months. Until now, severe dysphagia following descending necrotising mediastinitis has been unreported.
Background
Descending necrotising mediastinitis (DNM) was first described by Pearse in 1938, who at the time reported a 49% mortality rate.1 Acute mediastinitis is a severe, life-threatening infection of the mediastinal connective tissues, interpleural spaces and surrounding thoracic organs. In most of the cases mediastinitis develop following sternotomies for cardiac surgery (incidence 1–2.65%),2 however, DNM is regarded as a rare complication of odontogenic infection.3
DNM typically follows a cervical necrotising fasciitis, with the most common origins of infection including odontogenic or peritonsillar abscesses. Typically, a polymicrobial infection is seen with mixed aerobic and anaerobic organisms reflecting the oropharyngeal commensal bacteria.4 Risk factors for DNM include diabetes mellitus, alcoholism, poor dental status, poor renal or hepatic function, immunosuppression, low socioeconomic status, chronic tonsillitis and severe oral candidiasis.5
Endo et al6 classified DNM according to the anatomical site of infection, which helps offer guidelines to the surgical management: type I, infection above the carina (localised form); and type II, infection below the carina (diffuse form), with a poorer outcome associated with type II.
Although DNM has been previously reported, we report a case of a man who presented with DNM complicated by severe dysphagia in the postoperative period. To the best of our knowledge, no such complications of DNM have been previously described. This article aims to raise awareness of DNM, and looks to revise the mechanism of swallowing and dysphagia.
Case presentation
A 59-year-old Caucasian man presented to the emergency department following a collapse. He gave a prior history of toothache for 1 week, for which he had sought treatment and was prescribed a short course of oral amoxicillin by his general dental practitioner. He collapsed 3 days later, reportingof general malaise, facial swelling, trismus and dysphagia.
His medical history was unremarkable, however, he lived alone, consumed over 10 units of alcohol per day, and had a 35 pack-year of smoking history.
On arrival to the emergency department, the patient was in acute septic shock (pulse 172, blood pressure 68/58, oxygen saturations 93% on 15 L/min, respiratory rate 40, temperature 38.7°C). Complete trismus coupled with large, tense, erythematous, bilateral submandibular and submental swellings, was seen. Tenderness to palpation extended past the clavicles. Gross dental caries and florid oropharyngeal candidiasis were also noted. The patient's speech was dysarthric secondary to the severe swelling, with drooling of saliva seen. Neurologically, his Glasgow Coma Scale was calculated at 13/15 (E3, V4, M6).
Investigations
The patient's respiratory efforts were increased and reduced chest expansion was noted on the left side. Chest X-ray confirmed a pleural effusion (figure 1), which, when tapped, drained 400 mLs of purulence. Haematological investigations revealed leucocytosis, acute kidney injury and a mixed metabolic and respiratory acidosis (white cell count 30.6×109/L, creatinine 225 μmol/L (baseline creatinine 88 μmol/L), urea 44.0 mmol/L, C reactive protein 694 mg/L, H+ 58.8 nmol/L, partial pressure of carbon dioxide 10.4 KPa, pO2 21.7 KPa (15 L/min O2), HCO3− 18.7 mmol/L, base excess −10.4 mmol/L, lactate 5.0 mmol/L).
Figure 1.
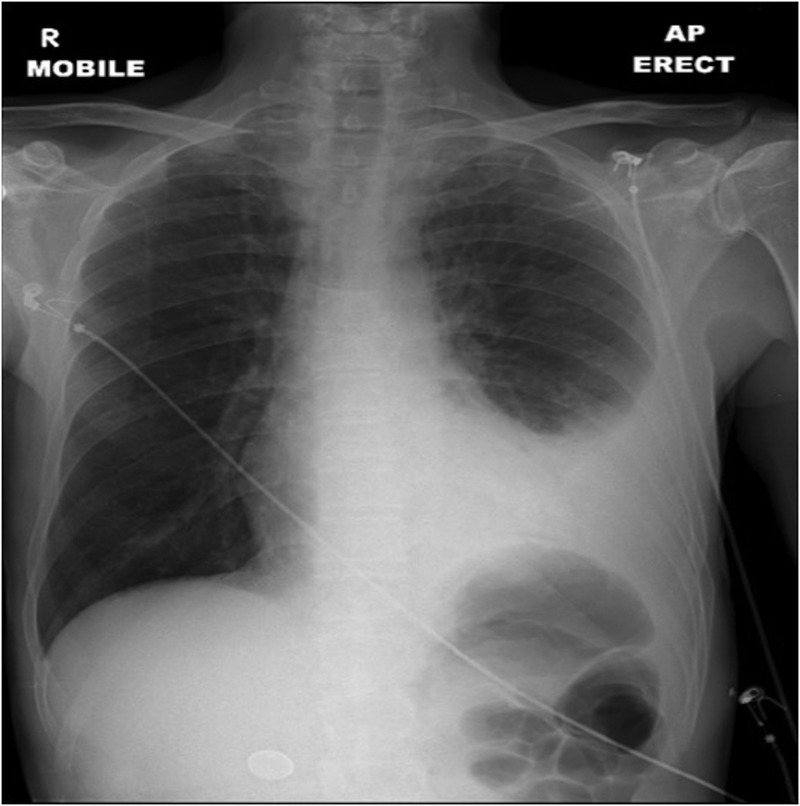
Erect chest X-ray demonstrating a large left-sided pleural effusion.
Differential diagnosis
The history and examination suggested that the pleural empyema was secondary to acute odontogenic infection. An urgent CT of the head, neck and thorax revealed a convoluted, contiguous bilateral fluid collection involving the submandibular, submental, parapharyngeal and paratracheal spaces, originating from the tooth apices, tracking into the middle and anterior mediastinum (figures 2 and 3). Infection extended below the carina perforating the left lower lung lobe, giving the diagnosis of type II DNM secondary to odontogenic infection.
Figure 2.

Contrast-enhanced axial CT demonstrating large fascial space collection at the level of hyoid (arrow).
Figure 3.
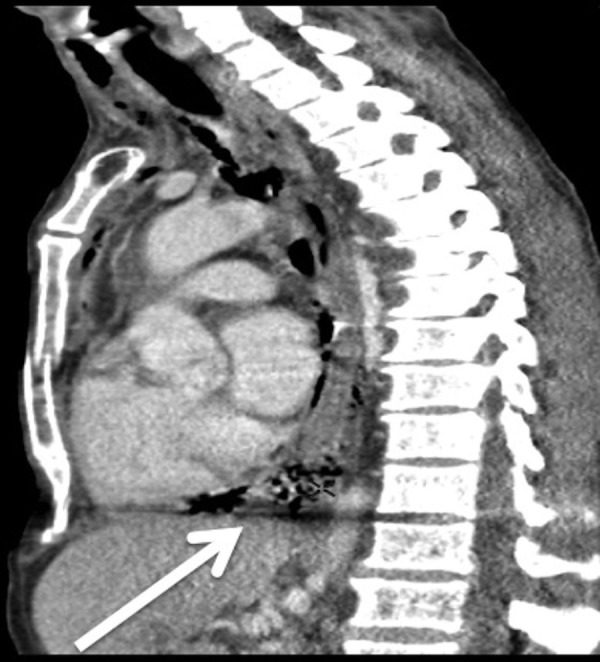
Contrast-enhanced sagittal CT demonstrating tracking infection and gas into the middle and anterior mediastinum (arrow).
Treatment
Surgical management involved an awake fibre optic intubation, surgical tracheostomy (releasing a copious volume of foul smelling purulence from the pretracheal space), bilateral neck dissections via carotid endarterectomy incisions, blunt dissection to the level of the clavicles, debridement of necrotic tissue, extensive lavage with warmed normal saline (0.9% NaCl), dental clearance and chest drains on the advice of the cardiothoracic surgeons.
Empirical intravenous piperacillin with tazobactam, teicoplanin, metronidazole and clindamycin, was initially prescribed for aggressive broad spectrum antibiotic cover on the advice of the microbiologist. Urgent Gram staining identified large numbers of Gram-positive cocci, Gram-negative bacilli and yeasts. The main pathogens isolated from tissue and blood cultures were identified as Streptococcus intermedius and Candida albicans. Following sensitivities, the antibiotics were then adjusted to intravenous co-amoxiclav, metronidazole, clarithromycin and clindamycin, with the addition of intravenous fluconazole to cover the candidaemia. A nasogastric tube was placed for enteral feeding. The patient required vasoactive support until day 9 and was discharged from intensive care to the ward on day 15 having been decannulated on day 14. Antibiotics were stopped on day 17.
Outcome and follow-up
Recovery was slow and despite being neurologically intact he had lost his ability to swallow. His speech and cranial nerves were unaffected. Assessment by the speech and language therapy (S<) team determined a grade 7 (severe) dysphagia. Detailed radiographic imaging and videofluoroscopy demonstrated normal oral and oesophageal phases of deglutition, but impairment in the pharyngeal phase. After 2 weeks of physiotherapy, the dysphagia had not improved. On day 36, a percutaneous endoscopic gastrostomy (PEG) was placed and the patient was discharged home after PEG training, on day 41. Despite the detrimental and psychological effects of his dysphagia and PEG feeding, the patient engaged in a comprehensive and long-term physiotherapy programme with the S< team. At 6 months, his swallowing had improved and was deemed safe, and the gastrostomy was removed.
Discussion
There is emerging evidence that the incidence and severity of odontogenic infections may be rising in the UK7 and to the best of our knowledge, there have been no previous reports of severe long-term dysphagia associated with DNM and odontogenic sepsis.
Normal swallowing is a complex series of coordinated fine neuromuscular events, divided into oral, pharyngeal and oesophageal phases. The oral phase consists of a preparatory phase where a bolus is primed for swallowing and an oral phase proper, which initiates the swallowing mechanism. The oral phase of swallowing is largely voluntary, under the control of cranial nerves V (trigeminal), VII (facial) and XII (hypoglossal).
The pharyngeal phase begins the involuntary component of the swallowing mechanism. It starts immediately at the end of the oral phase and is initiated by the swallowing centre in the medulla via cranial nerves IX (glossopharyngeal) and X (vagus). Four key components are required and consist of (1) closure of the nasopharynx with the soft palate to prevent nasal reflux, (2) elevation and closure of the larynx to prevent aspiration, (3) contraction of the pharyngeal constrictors and (4) opening of the cricopharyngeus muscle.8 The final oesophageal phase of swallowing is characterised by a primary peristaltic wave travelling the length of the oesophagus.
Given the sophisticated nature of deglutition, which involves multiple anatomical structures, there is considerable opportunity for dysphagia to occur. A large number of conditions exist that can cause dysphagia. Examples limited to the oesophagus include achalasia, diffuse spasm, strictures, foreign bodies, tumours, oesophagitis and gastro-oesophageal reflux disease. Oropharyngeal dysphagia is associated with conditions that weaken the muscles involved in swallowing, inhibiting the passage of food from the oral cavity towards the oesophagus. Neurological conditions such as multiple sclerosis and Parkinson's disease can cause dysphagia, as well as oropharyangeal cancers and pharyngeal diverticula. In addition, dysphagia is commonly seen following chemoradiation in patients with postoperative head and neck cancer. This, in part, is due to a combination of hyposalivation, mucositis, distorted anatomy following ablative surgery and scarring secondary to the surgery and radiotherapy itself.
In the case described above, iatrogenic causes cannot be excluded. Furthermore, dysphagia and aspiration following a period of prolonged endotracheal intubation is a well-documented sequelae of tracheostomy.8 However, the above patient had an extensive collection of purulence within the neck, involving multiple fascial planes. It would therefore stand to reason that, given the severe paratracheal space involvement, opportunity existed for local structures to become involved in the local inflammatory response. We postulate that the chronic inflammatory changes within the fascial spaces caused fibrosis and scarring of the hypopharyngeal muscles. This would account for the impairment of the pharyngeal phase of swallowing seen on videofluoroscopy of this patient. Furthermore, the gradual response seen with physiotherapy would help support the theory of a musculoskeletal impairment.
DNM remains a rare but potentially fatal complication of cervicofacial space infection, with a 64% mortality rate when combined with sepsis.1 Early recognition and investigation, combined with aggressive surgical and medical therapy in a multidisciplinary setting, offers the best chance of survival and overall prognosis.
Learning points.
Deglutination is a complex series of finely coordinated neuromuscular events.
Many pathophysiological causes of dysphagia exist that can affect the gastrointestinal tract anywhere from the stomach to the brain.
Dental sepsis complicated by fascial space involvement is considered a surgical emergency, owing to potential airway compromise.
Descending necrotising mediastinitis secondary to acute odontogenic sepsis, although rare, is a potentially fatal condition and carries a mortality rate of 64%.
Footnotes
Contributors: PG wrote and edited the case report. JM performed the surgery described and supervised the writing of the report.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sarna T, Sengupta T, Miloro M et al. Cervical necrotizing fasciitis with descending mediastinitis: literature review and case report. J Oral Maxillofac Surg 2012;70:1342–50. 10.1016/j.joms.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 2.Kocher GJ, Hoksch B, Caversaccio M et al. Diffuse descending necrotizing mediastinitis: surgical therapy and outcome in a single-centre series. Eur J Cardiothorac Surg 2012;42:e66–72. 10.1093/ejcts/ezs385 [DOI] [PubMed] [Google Scholar]
- 3.Ho MW, Dhariwal DK, Chandrasekhar J et al. Use of interventional radiology in the management of mediastinitis of odontogenic origin. Br J Oral Maxillofac Surg 2006;44:538–42. 10.1016/j.bjoms.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Karkas A, Chahine K, Schmerber S et al. Optimal treatment of cervical necrotizing fasciitis associated with descending necrotizing mediastinitis. Br J Surg 2010;97:609–15. 10.1002/bjs.6935 [DOI] [PubMed] [Google Scholar]
- 5.Byers J, Lowe T, Goodall CA. Acute cervico-facial infection in Scotland 2010: patterns of presentation, patient demographics and recording of systemic involvement. Br J Oral Maxillofac Surg 2012;50:626–30. 10.1016/j.bjoms.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Endo S, Murayama F, Hasegawa T et al. Guideline of surgical management based on diffusion of descending necrotizing mediastinitis. Jpn J Thorac Cardiovasc Surg 1999;47:14–9. 10.1007/BF03217934 [DOI] [PubMed] [Google Scholar]
- 7.Burnham R, Bhandari R, Bridle C. Changes in admission rates for spreading odontogenic infection resulting from changes in government policy about the dental schedule and remunerations. Br J Oral Maxillofac Surg 2011;49:26–8. 10.1016/j.bjoms.2009.10.033 [DOI] [PubMed] [Google Scholar]
- 8.Kronenberger MB, Meyers AD. Dysphagia following head and neck cancer surgery. Dysphagia 1994;9:236–44. 10.1007/BF00301917 [DOI] [PubMed] [Google Scholar]


