Abstract
A 40-year-old man with a history of orbital myositis (OM) presented to the emergency department with ventricular tachycardia requiring electrical cardioversion. Postcardioversion ECG showed right bundle branch block, while an echocardiogram revealed an ejection fraction of 20% and a dilated right ventricle. Cardiac MRI produced suboptimal images because the patient was having frequent arrhythmias. The rest of the work up, including coronary angiography, was unremarkable. Given the dilated right ventricle, we suspected arrhythmogenic right ventricular cardiomyopathy and discharged the patient with an implantable cardioverter-defibrillator. 1 week later, he was readmitted with cardiogenic shock; endomyocardial biopsy revealed giant cell myocarditis (GCM). To the best of our knowledge, this is the seventh case report of GCM described in a patient with OM. We recommend that clinicians maintain a high degree of suspicion for GCM in patients with OM presenting with cardiac problems.
Background
Giant cell myocarditis (GCM) is a rare life-threatening condition, with fewer than one hundred cases described in the English language literature. Without treatment, 1-year mortality approaches 70%.1 A limited number of case reports have described the association between GCM and orbital myositis (OM). We report this case to further elaborate on this association, with the hope of helping physicians recognise GCM early in this setting.
Case presentation
A 40-year-old Caucasian man presented to the emergency department with palpitations, dizziness and dyspnoea for 1 day. Three years prior, he had been evaluated for bilateral eye pain, redness and reduced eye movements. At that time, based on the MRI and lateral rectus muscle biopsy findings, he was diagnosed with OM. Since then he had intermittently been treated with systemic steroids and topical non-steroidal anti-inflammatory drugs, and his disease had been under control.
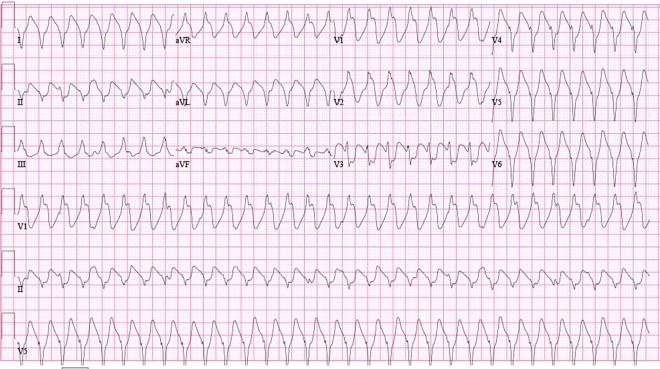
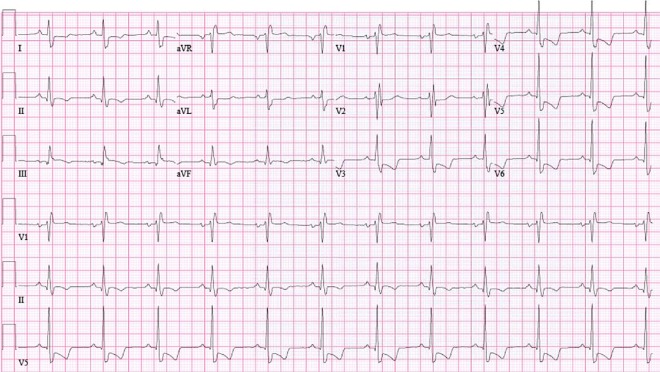
The ECG on presentation showed ventricular tachycardia (VT) with right bundle branch block (RBBB) morphology (figure 1). The patient was managed as a case of haemodynamically stable VT. After repeated boluses of amiodarone failed, he was electrically cardioverted. Postcardioversion ECG revealed normal sinus rhythm with RBBB and T wave inversions in lateral precordial leads (figure 2). Troponin-I level was 3.4 ng/mL. Echocardiogram performed soon after cardioversion showed an ejection fraction of 20% and a dilated right ventricle.
Figure 1.
ECG showing ventricular tachycardia with right bundle branch block morphology.
Figure 2.
ECG showing normal sinus rhythm with right bundle branch block and T wave inversions in lateral precordial leads.
The patient was admitted to the coronary care unit for further management. He underwent left heart catheterisation, which revealed no evidence of coronary artery disease. Cardiac MRI produced suboptimal images because of frequent arrhythmias. Endocrinology, autoimmune and infectious work up was unremarkable.
At this point, given the evidence of right ventricular dilation on echocardiogram, the main diagnostic consideration was arrhythmogenic right ventricular cardiomyopathy (ARVC). A plan was made to repeat the cardiac MRI.
On the third day of admission, the patient again developed VT and had to be electrically cardioverted. Given this second episode of VT, an implantable cardioverter-defibrillator (ICD) was placed. Repeat echocardiogram revealed an ejection fraction of 35–40%, with hypokinetic basal inferior and inferolateral segments. The patient was started on metoprolol, enalapril and amiodarone. He remained stable and was discharged home with a diagnosis of recurrent VT secondary to possible ARVC. Unfortunately, cardiac MRI could not be repeated because of the ICD placement.
One week later, the patient was readmitted with 1 day of nausea, vomiting and dyspnoea. His blood pressure (BP) was 98/69 mm Hg and pulse 96/min, and he was saturating at 96% on room air. His examination was remarkable for an elevated jugular venous pressure and cool extremities. His troponin-I level was 12.10 ng/mL (up from 4.1 ng/mL during the previous admission), serum creatinine 1.7 mg/dL (up from a baseline of 1.2 mg/dL) and total bilirubin was 1.3 mg/dL (up from a baseline of 0.7 mg/dL). ECG was unchanged from the previous admission, with normal sinus rhythm, RBBB and T wave inversions in lateral precordial leads. Repeat echocardiogram showed an ejection fraction of 15%. The patient was admitted to the intensive care unit, and management was initiated on lines of cardiogenic shock with diuretics and inotropic support.
While in the intensive care unit, the patient continued to worsen. By the third day of admission, his serum creatinine had gone up to 3.4 mg/dL and total bilirubin had risen to 2.8 mg/dL. Despite inotropic support, his BP was plummeting (systolic BP down to early 80s). An intra-aortic balloon pump was placed. The patient was shifted to another centre for heart transplantation evaluation and bridging with extracorporeal membrane oxygenation (ECMO). He underwent endomyocardial biopsy and ECMO support was initiated. The biopsy revealed myocyte necrosis, severe inflammation and multinucleated giant cells (figure 3). These findings were consistent with previously published histological features of GCM.2 3 Before further treatment decisions could be taken, the patient started to bleed from the ECMO cannulation site. Attempts at suturing the bleeding site failed and, before a vascular closure device could be engaged, the patient developed cardiopulmonary arrest. Attempts to resuscitate him failed and he was pronounced dead, 7 days after being shifted to the outside facility.
Figure 3.
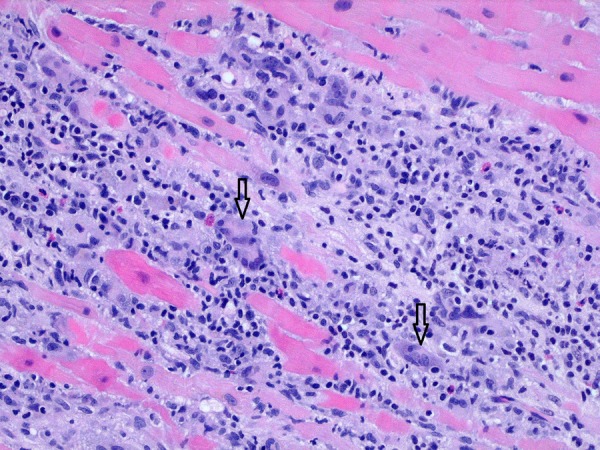
Endomyocardial biopsy showing myocyte necrosis, severe inflammation and multinucleated giant cells (black arrows).
Discussion
GCM as a distinct disorder was first described by Saltykow, in 1905.4 The presenting features include heart failure (75%), ventricular arrhythmias (9%) and heart block (3%); of note, sustained VT develops in half the patients at some point during the disease course.5 Sustained monomorphic VT at a rate of 100–200/min is the most common arrhythmia, and it may not lead to immediate haemodynamic instability.6 Nineteen per cent of GCM cases are associated with other autoimmune disorders.5 Endomyocardial biopsy is an effective means for diagnosing GCM, with a reported yield of 92% after two biopsies.7 Without treatment, mortality is high.1 Currently, the mainstay of treatment includes steroids, calcineurin inhibitors, antimetabolites and cytolytic therapies.8 Even though some patients will require a heart transplant, with contemporary combination immunosuppressant regimens, the 5-year transplant-free survival rate has been reported at 72%.9 ECMO and ventricular assist devices are frequently employed as bridging therapies for patients awaiting transplant.
Since treatment can significantly alter the GCM disease course, it is of paramount importance that the diagnosis is made promptly and treatment initiated in a timely fashion. Unfortunately, in our case, the right ventricular dilation on the echocardiogram performed immediately after cardioversion was a distraction that made us think more along the lines of ARVC. Cardiac MRI could have provided us more clues in favour of GCM; however, it produced suboptimal images due to frequent arrhythmias, and we were unable to repeat it later because of the ICD. Looking back at the case, we relied too heavily on the echocardiogram that was performed immediately after cardioversion. Transient myocardial stunning after resuscitation can lead to cardiac dysfunction, but recovery is expected within a matter of days.10 In our case, as well, the right ventricular dilation was likely secondary to myocardial stunning and it was not demonstrated on subsequent echocardiograms. Similarly, the presence of VT with RBBB morphology should have also brought into suspicion the presumed diagnosis of ARVC because VT in ARVC most commonly presents with LBBB morphology.11 12 We therefore recommend that physicians keep GCM as a differential in patients presenting with new onset heart failure and/or refractory ventricular arrhythmias. In such cases, endomyocardial biopsy should be considered for further evaluation.13
The other important teaching point in our case is the association of GCM and OM. There have been six previous case reports describing this association. Klein et al described a case of a 65-year-old woman who presented with eye symptoms 1 month before succumbing to a fatal arrhythmia;14 diagnoses of myositis and myocarditis were established based on pathology. Kattah et al described a case of a young woman who developed eye symptoms and 18 months later died of a fatal arrhythmia. In her case, diagnosis of myositis and GCM was established after autopsy.15 Leib et al described a case of a 22-year-old woman who was diagnosed with OM and 1 month later admitted with cardiogenic shock that was found to be secondary to GCM.16 She did well after a heart transplant. Interestingly, Stevens et al17 also described a very similar case of a young woman who was diagnosed with OM and 1 month later presented with cardiogenic shock due to GCM. She also required a heart transplant. Kollmeier et al described a case of a 32-year-old woman who presented with symptoms consistent with OM and 2 months later was admitted with cardiogenic shock;18 unfortunately, she died and the diagnosis of OM and GCM was established after autopsy. Lind-Ayres et al described a case of a 14-year-old girl who was diagnosed with OM and 2 months later presented with heart failure attributed to GCM. The patient did well on immunosuppressants and did not require a heart transplant.19
To the best of our knowledge, our case is the seventh report of GCM occurring in a patient with OM. However, two aspects make our case unique. Firstly, this is the only such case described in a male patient. Secondly, the 3-year interval between the onset of eye symptoms and cardiac symptoms in our case is the longest among all such cases. In most of the previous cases, cardiac symptoms developed within a few months of the eye symptoms.
Numerous investigators have described the role of T-cells in the development of GCM.1 20 21 Abnormal T-cell responses perhaps provide the pathophysiological link between GCM and OM, since T-cells have also, in part, been implicated in the development of the latter. However, more research is needed to better understand the mechanisms connecting these two disorders. A better insight into these mechanisms will also help us understand which patients suffering from OM are more likely to develop GCM, and who are therefore candidates for closer monitoring.
Learning points.
Giant cell myocarditis (GCM) is a rare but potentially fatal disorder that requires prompt diagnosis and treatment.
GCM should be considered in patients presenting with new onset heart failure and/or refractory ventricular arrhythmias.
If GCM is suspected, there should be a low threshold for performing endomyocardial biopsy since it is safe and sensitive for diagnosing GCM.
Physicians should be wary of relying heavily on echocardiograms performed soon after resuscitation. Such echocardiograms can show cardiac dysfunction due to transient myocardial stunning; therefore, any abnormal findings should be followed up with repeat echocardiograms to assess for resolution.
Orbital myositis (OM) has been reported as a harbinger of GCM, with a lag time ranging from a few days to a few years. All patients with OM should be promptly assessed for GCM if they develop cardiac problems.
Acknowledgments
The authors are grateful to all colleagues at the Department of Medicine, John H. Stroger, Jr. Hospital of Cook County.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cooper LT., Jr Giant cell myocarditis: diagnosis and treatment. Herz 2000;25:291–8. 10.1007/s000590050023 [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, Pomerance A, Teare RD. Idiopathic giant cell myocarditis—a distinctive clinico-pathological entity. Br Heart J 1975;37:192–5. 10.1136/hrt.37.2.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okura Y, Dec GW, Hare JM et al. . A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol 2003;41:322–9. 10.1016/S0735-1097(02)02715-8 [DOI] [PubMed] [Google Scholar]
- 4.Kean BH, Hoekenga MT. Giant cell myocarditis. Am J Pathol 1952;28:1095–105. [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper LT, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis—natural history and treatment. Multicenter giant cell myocarditis study group investigators. N Engl J Med 1997;336:1860–6. 10.1056/NEJM199706263362603 [DOI] [PubMed] [Google Scholar]
- 6.Granér M, Lommi J, Kupari M et al. . Multiple forms of sustained monomorphic ventricular tachycardia as common presentation in giant-cell myocarditis. Heart 2007;93:119–21. 10.1136/hrt.2005.079053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandolin R, Lehtonen J, Salmenkivi K et al. . Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 2013;6:15–22. 10.1161/CIRCHEARTFAILURE.112.969261 [DOI] [PubMed] [Google Scholar]
- 8.Shih JA, Shih JA. Small steps for idiopathic giant cell myocarditis. Curr Heart Fail Rep 2015;12:263–8. 10.1007/s11897-015-0260-x [DOI] [PubMed] [Google Scholar]
- 9.Maleszewski JJ, Orellana VM, Hodge DO et al. . Long-term risk of recurrence, morbidity and mortality in giant cell myocarditis. Am J Cardiol 2015;115:1733–8. 10.1016/j.amjcard.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 10.Kern KB, Hilwig RW, Rhee KH et al. . Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol 1996;28:232–40. 10.1016/0735-1097(96)00130-1 [DOI] [PubMed] [Google Scholar]
- 11.Miljoen H, State S, de Chillou C et al. . Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 2005;7:516–24. 10.1016/j.eupc.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Marcus FI, Abidov A. Arrhythmogenic right ventricular cardiomyopathy 2012: diagnostic challenges and treatment. J Cardiovasc Electrophysiol 2012;23:1149–53. 10.1111/j.1540-8167.2012.02412.x [DOI] [PubMed] [Google Scholar]
- 13.Cooper LT, Baughman KL, Feldman AM et al. . The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007;116:2216–33. 10.1161/CIRCULATIONAHA.107.186093 [DOI] [PubMed] [Google Scholar]
- 14.Klein BR, Hedges TR, Dayal Y et al. . Orbital myositis and giant cell myocarditis. Neurology 1989;39:988–90. 10.1212/WNL.39.7.988 [DOI] [PubMed] [Google Scholar]
- 15.Kattah JC, Zimmerman LE, Kolsky MP et al. . Bilateral orbital involvement in fatal giant cell polymyositis. Ophthalmology 1990;97:520–5. 10.1016/S0161-6420(90)32554-X [DOI] [PubMed] [Google Scholar]
- 16.Leib ML, Odel JG, Cooney MJ. Orbital polymyositis and giant cell myocarditis. Ophthalmology 1994;101:950–4. 10.1016/S0161-6420(94)31232-2 [DOI] [PubMed] [Google Scholar]
- 17.Stevens AW, Grossman ME, Barr ML. Orbital myositis, vitiligo, and giant cell myocarditis. J Am Acad Dermatol 1996;35:310–12. 10.1016/S0190-9622(96)90656-8 [DOI] [PubMed] [Google Scholar]
- 18.Kollmeier M, Brodhun M, Sliwka U et al. . [Diplopia and cardiogenic shock]. Nervenarzt 2006;77:187–8. 90–1 10.1007/s00115-005-1931-8 [DOI] [PubMed] [Google Scholar]
- 19.Lind-Ayres MR, Abramowsky C, Mahle WT. Pediatric giant cell myocarditis and orbital myositis. Pediatr Cardiol 2009;30:510–12. 10.1007/s00246-008-9338-5 [DOI] [PubMed] [Google Scholar]
- 20.Laufs H, Nigrovic PA, Schneider LC et al. . Giant cell myocarditis in a 12-year-old girl with common variable immunodeficiency. Mayo Clin Proc 2002;77:92–6. 10.4065/77.1.92 [DOI] [PubMed] [Google Scholar]
- 21.Pinderski LJ, Fonarow GC, Hamilton M et al. . Giant cell myocarditis in a young man responsive to T-lymphocyte cytolytic therapy. J Heart Lung Transplant 2002;21:818–21. 10.1016/S1053-2498(01)00396-5 [DOI] [PubMed] [Google Scholar]