Abstract
Background/aim
A post-marketing surveillance (PMS) study is being conducted to investigate the safety and effectiveness of the long-term use of dabigatran etexilate (dabigatran) in Japanese patients with nonvalvular atrial fibrillation (NVAF). Results of an interim analysis of this prospective cohort study including patient characteristics and adverse drug reactions (ADRs) collected up to September 17, 2014 are reported here.
Methods
Patients with NVAF who began to receive dabigatran for the first time from December 2011 to November 2013 were enrolled at 1042 study sites in Japan. Clinical parameters included patient characteristics, dabigatran dose strength, concomitant medications and outcome events. All outcome events were collected as serious and non-serious adverse events (AEs). ADRs were evaluated in this report. Pre-defined safety events of special interest for intensive survey were serious and non-serious outcome events such as myocardial infarction, as well as the total number of hemorrhage and gastrointestinal disorders.
Results
A total of 6772 patients were registered. The safety analysis set included 6148 patients; mean age was 70.8±9.9 (SD) years: 2323 patients (37.8%) were aged 75 years or older. Males accounted for 66.8% of the patients. Mean CHADS2 score was 1.8±1.3; the CHADS2 score was 0 in 13.6%, 1 in 31.3%, 2 in 25.9%, 3 in 14.9%, and 4 to 6 in 11.1% of the patients. Of the 6148 patients, 1701 patients (27.7%) were switchers from warfarin and 4407 patients (71.7%) were non-switchers (OAC naïve patients). Treatment adherence was assessed for the first 3 months from the start of treatment for this analysis. Total 5656 patients (92.0%) reported taking dabigatran twice daily (bid) every day according to the label recommendation. During the follow up period [mean duration of follow up: 498±259 days (corresponding to 8386 patient-years)], pre-defined safety events of special interest for intensive survey (reported as serious ADRs) were: myocardial infarction, reported in 5 patients (0.06 per 100 patient-years); serious hemorrhage, reported in 46 patients (0.55 per 100 patient-years); and gastrointestinal disorders (non-hemorrhagic), reported in 11 patients (0.13 per 100 patient-years). Fifteen patients had ADRs with fatal outcome.
Conclusions
The interim findings from this 6148 patient PMS study further corroborate the favorable safety profile of dabigatran as demonstrated previously in controlled clinical trials.
(ClinicalTrials.gov number, NCT01491178.)
Keywords: Atrial fibrillation, Anticoagulants, Post-marketing surveillance, Dabigatran, Japan
1. Introduction
Atrial fibrillation (AF) is an arrhythmia that is commonly seen and occurs more often in the elderly. In Japan, the overall prevalence of all types of AF based on the data from periodic health examinations was estimated to be 0.56% [1]. Considering the aging population in Japan, it is estimated that more than 1 million individuals would be suffering from the disease in the year 2030. The number of AF patients would increase when patients with paroxysmal AF are included. Nonvalvular atrial fibrillation (NVAF) associated with lifestyle-related diseases, such as hypertension and diabetes mellitus, is predominant among the patient population [1].
For the prevention of stroke in NVAF patients, appropriate antithrombotic therapy should be selected after a risk assessment of cerebral infarction [2]. In the past, warfarin was the only anticoagulant drug indicated for NVAF in Japan but less than 60% of high-risk NVAF patients are treated with the drug in real-world practice [3]. The major reason for under-use of warfarin is the difficulty in controlling anticoagulation intensity: it requires dose adjustments by routine monitoring of prothrombin time-international normalized ratio (PT-INR) because of its narrow therapeutic range, and it is subject to pharmacodynamic and pharmacokinetic interactions with concomitant drugs and diet.
Dabigatran etexilate (dabigatran) has been developed as a new anticoagulant (non-vitamin K antagonist oral anticoagulant; NOAC) to solve the above issues. Dabigatran is a direct competitive inhibitor of thrombin, which is the critical factor in the blood-clotting cascade, thereby suppressing the conversion of fibrinogen to fibrin, and leading to the prevention of thrombosis [4].
In the RE-LY study [5], a randomized global phase III study, which included Japanese patients, the favorable efficacy and safety of dabigatran compared to that of INR-adjusted warfarin was demonstrated. The Japanese Guidelines for Pharmacotherapy of AF (revised in 2013) [6] recommend dabigatran for NVAF patients whose CHADS2 scores are 1 or higher, taking into account the overall results of the RE-LY study and its sub-analysis, showing the efficacy and safety of dabigatran for patients with CHADS2 score of 1 or higher. In the United States [7] and Denmark [8], [9], large-scale investigations performed by the FDA and independent academic institutions, respectively, have meanwhile confirmed the effectiveness and safety of dabigatran in the real world.
In Japan, a post-marketing surveillance (PMS) study was started in December 2011 to investigate the safety and effectiveness of the long-term use of dabigatran in real-world practice; more than 6700 patients were enrolled. The aim of this ongoing prospective PMS study is to survey the safety and effectiveness of the long-term use of dabigatran in NVAF patients who receive dabigatran for the first time. It is conducted in compliance with “The Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices” (Law Number: Act No. 145 of 1960) and “Good post-marketing surveillance practice (GPSP)” (Japanese Ministry of Health and Welfare Ordinance No. 171: December 20, 2004). The interim analysis results of the PMS study collected up to September 17, 2014 are reported herein.
2. Material and methods
2.1. Subjects
Patients with NVAF who started dabigatran treatment from December 12, 2011 to November 30, 2013 were registered at 1042 study sites in Japan. This report is based on the data from the case report forms (CRFs) collected up to September 17, 2014.
2.2. Study methods
Electronic data capture system was used for the collection of data on demographics, clinical characteristics, dabigatran treatment status, concomitant medications, adverse events (AE), and adverse drug reactions (ADR; which are events with at least reasonably possible causal relationship with dabigatran) including outcome events of specific interest. In this report, ADRs out of the AEs reported are summarized. CHADS2 score [2] was calculated based on the patient conditions: congestive heart failure/left ventricular dysfunction, hypertension, age (≥75 years), diabetes mellitus, and previous stroke/transient ischemic attack. Myocardial infarction, hemorrhage, and gastrointestinal disorders were selected as the non-serious or serious safety events of special interest for intensive survey. Hemorrhage included all the serious and non-serious hemorrhagic events reported in the CRF. The following criteria were applied for major hemorrhage: symptomatic hemorrhage in a critical area or organ, such as intracranial, intramedullary, intraocular, retroperitoneal, intra-articular, and pericardial hemorrhage, and intramuscular hemorrhage with muscular compartment syndrome; hemorrhage with decrease in hemoglobin of 2 g/dL or more; and major hemorrhage requiring 5 units or more of blood transfusion (blood component transfusion of red blood cells or whole blood transfusion). Among any untoward medical events, the following AEs and ADRs were defined as “serious”: those that resulted in death, life-threatening events, those requiring hospitalization or prolongation of hospital stay, those that resulted in persistent or significant disability/incapacity, or congenital anomalies/birth defects. The exposure of each patient was the reported treatment duration if documented in the CRF or calculated from the first dabigatran treatment prescribed until September 17, 2014.
Patients who used warfarin within 7 days before the start of dabigatran treatment were defined as “Switchers (warfarin-experienced)”, and those who did not use warfarin within that period as “non-Switchers (warfarin-naïve)”.
Patients were followed for a maximum of 24 months from the index date (start of treatment). The CRFs were completed at the following time points: at baseline, before the start of dabigatran administration, and at 3 and 24 months after the start of treatment, at the time of serious ADR occurrence or at the time of discontinuation/dropout. The survey will continue until March 2016.
AEs reported in the study were handled as solicited reports. At the time of the interim analysis of this study, the drug exposure has been estimated based on all the data available at the cutoff date of the analysis. Final analysis at the end of the study will report the actual exposure based on all the data available.
2.3. Statistical analysis
Descriptive statistical methods were used for analyses. Data are expressed as mean±standard deviation (SD) or percentage.
3. Results
3.1. Clinical background
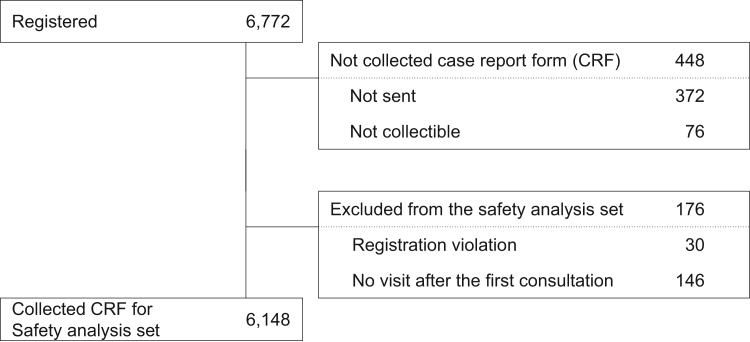
A total of 6772 patients were registered in the PMS study. The final sample size for this analysis included a total of 6148 patients, excluding those who were ineligible for registration or who did not visit the site after the first visit (Fig. 1). Three-month follow-up was completed in 6324 patients, and 24-month follow-up in 1213 patients.
Fig. 1.
Patient disposition.
Baseline demographics and clinical characteristics are shown in Table 1 (more detailed backgrounds are provided in the Supplementary table). The mean age was 70.8 years: 2323 patients (37.8%) were aged 75 years or older. Of those, 4104 patients (66.8%) were male. CHADS2 score of 1 was the most common among the patients (Supplementary Fig. 1). Hypertension was the most common comorbidity followed by renal impairment. A creatinine clearance (ClCr) of “50< to <80 mL/min” was seen most frequently (50.7%) in patients receiving 220 mg/day; whereas “≥80 mL/min” was seen most frequently (58.0%) in patients receiving 300 mg/day. ClCr of “30–50 mL/min” was seen in 1006 patients, which is the dose chosen (220 mg/day or 300 mg/day) criteria of dabigatran. Indeed, 925 (91.9%) of 1006 patients were administered 220 mg/day of the drug. There were 38 patients whose ClCr was “<30 mL/min”, a contraindication level for dabigatran use; nevertheless, most of them (n=28, 73.7%) were administered 220 mg/day of dabigatran (Supplementary Fig. 2).
Table 1.
Patient characteristics (safety analysis set).
| Variable | Initial daily dose of dabigatran etexilate |
Totala (n=6148) | |
|---|---|---|---|
| 220 mg (n=4560) | 300 mg (n=1473) | ||
| Age, years | 73.2±8.7 | 63.0±9.0 | 70.8±9.9 |
| <65 | 666 (14.6) | 791 (53.7) | 1467 (23.9) |
| 65–74 | 1747 (38.3) | 577 (39.2) | 2358 (38.4) |
| ≥75 | 2147 (47.1) | 105 (7.1) | 2323 (37.8) |
| ≥70 | 3295 (72.3) | 295 (20.0) | 3680 (59.9) |
| Women | 1697 (37.2) | 293 (19.9) | 2044 (33.2) |
| Creatinine clearance, mL/min | |||
| <30 | 28 (0.6) | 6 (0.4) | 38 (0.6) |
| 30–50 | 925 (20.3) | 37 (2.5) | 1006 (16.4) |
| 50< to <80 | 2314 (50.7) | 505 (34.3) | 2859 (46.5) |
| ≥80 | 1082 (23.7) | 854 (58.0) | 1953 (31.8) |
| Unknown | 211 (4.6) | 71 (4.8) | 292 (4.7) |
| mean±SD | 67.6±23.0 | 89.8±27.6 | 72.8±26.1 |
| CHADS2 score | |||
| 0 | 517 (11.3) | 309 (21.0) | 839 (13.6) |
| 1 | 1333 (29.2) | 561 (38.1) | 1923 (31.3) |
| 2 | 1237 (27.1) | 319 (21.7) | 1594 (25.9) |
| 3 | 731 (16.0) | 171 (11.6) | 919 (14.9) |
| 4 | 422 (9.3) | 63 (4.3) | 497 (8.1) |
| 5 | 139 (3.0) | 14 (1.0) | 156 (2.5) |
| 6 | 30 (0.7) | 0 (0.0) | 30 (0.5) |
| Unknown | 151 (3.3) | 36 (2.4) | 190 (3.1) |
| mean±SD | 1.9±1.3 | 1.4±1.1 | 1.8±1.3 |
| Previous history | |||
| Stroke/TIA | 924 (20.3) | 273 (18.5) | 1218 (19.8) |
| Myocardial infarction | 233 (5.1) | 46 (3.1) | 283 (4.6) |
| Hemorrhagic events | 243 (5.3) | 35 (2.4) | 289 (4.7) |
| Gastrointestinal hemorrhage | 66 (1.4) | 9 (0.6) | 77 (1.3) |
| Gastrointestinal disorder | 101 (2.2) | 37 (2.5) | 142 (2.3) |
| Comorbidity | |||
| Heart failure | 863 (18.9) | 215 (14.6) | 1105 (18.0) |
| Hypertension | 3070 (67.3) | 908 (61.6) | 4054 (65.9) |
| Diabetes mellitus | 925 (20.3) | 297 (20.2) | 1236 (20.1) |
| Liver function disorder | 445 (9.8) | 160 (10.9) | 612 (10.0) |
| Renal impairment | 1934 (42.4) | 279 (18.9) | 2279 (37.1) |
| Gastrointestinal disorder | 720 (15.8) | 154 (10.5) | 887 (14.4) |
| Concomitant use of antiplatelets | 508 (11.1) | 108 ( 7.3) | 635 (10.3) |
TIA: transient ischemic attack.
Values are number (%) or mean±standard deviation.
Including 115 patients who received other dose levels.
The initial daily dose of dabigatran was 220 mg/day (110 mg twice daily) in approximately three-fourths of the patients, less than 220 mg/day in 109 patients (1.8%) and more than 300 mg/day (150 mg twice daily) in 6 patients (0.1%). Seventy years or older is the age cut-off for a dose reduction to 220 mg/day in the Japanese label. Indeed, 3295 (89.5%) of 3680 patients were administered 220 mg/day of dabigatran. Among the 2468 patients aged less than 70 years, only 47.7% were administered 300 mg/day of dabigatran. Antiplatelet agents were used concomitantly in 10.3% of the patients.
Treatment adherence was assessed for the first 3 months from the start of treatment for this analysis. 5656 Patients (92.0%) reported taking dabigatran every day for 3 months from the start of administration. The dosage of dabigatran did not affect the medication adherence (Table 2).
Table 2.
Medication adherence (3-month follow-up).
| Initial daily dose of dabigatran etexilate |
Total (n=6148) | |||
|---|---|---|---|---|
| 220 mg (n=4560) | 300 mg (n=1473) | Others (n=115) | ||
| Completely taken | 4218 (92.5) | 1332 (90.4) | 106 (92.2) | 5656 (92.0) |
| Often taken (≥2/3) | 165 (3.6) | 81 (5.5) | 3 (2.6) | 249 (4.1) |
| Sometimes taken (≥1/3 to <2/3) | 24 (0.5) | 13 (0.9) | 1 (0.9) | 38 (0.6) |
| Rarely taken (<1/3) | 26 (0.6) | 5 (0.3) | 1 (0.9) | 32 (0.5) |
| Unknown | 127 (2.8) | 42 (2.9) | 4 (3.5) | 173 (2.8) |
Values are number (%).
3.2. Clinical background of Switchers and non-Switchers
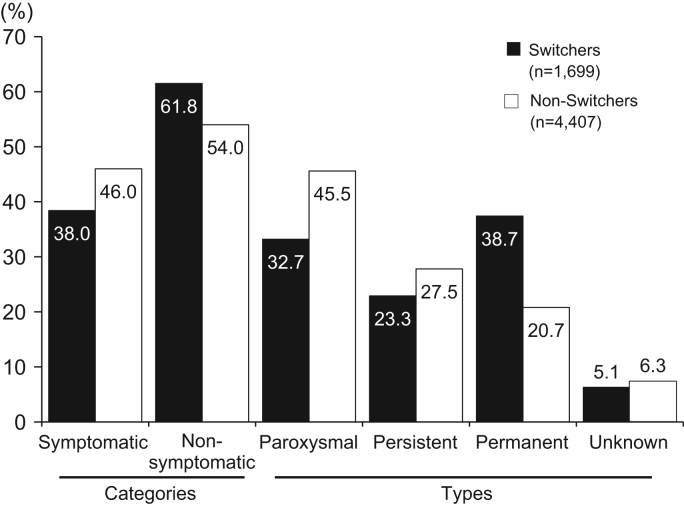
Switchers from warfarin were 1701 patients (27.7%), non-Switchers were 4407 patients (71.7%) and for 40 patients (0.7%) this is unknown. A CHADS2 score of 2 or more and permanent AF were more common in Switchers than in non-Switchers (Fig. 2, Fig. 3).
Fig. 2.
Distribution of CHADS2 score by use status of warfarin experience prior to dabigatran treatment (black=Switchers, white=non-Switchers).
Fig. 3.
Type of atrial fibrillation by use status of warfarin experience prior to dabigatran treatment (black=Switchers, white=non-Switchers).
3.3. Safety
During the mean follow up period of 498±259 days (8386 patient-years), the serious and non-serious ADRs of special interest for intensive survey were myocardial infarction in 5 patients (0.06 per 100 patient-years), serious hemorrhages in 46 patients (0.55 per 100 patient-years), and gastrointestinal disorders (non-hemorrhagic) in 11 patients (0.13 per 100 patient-years). Serious hemorrhagic events were gastrointestinal hemorrhages in 26 patients (0.31 per 100 patient-years), intracranial hemorrhages in 10 patients (0.12 per 100 patient-years), and other hemorrhages in 10 patients (0.12 per 100 patient-years) (Table 3). In spite of contraindication (ClCr<30 mL/min), one gastrointestinal hemorrhage was observed in a patient who received 220 mg/day. Fifteen patients (0.18 per 100 patient-years) had ADRs with fatal outcome. The causes of fatal outcome (in 15 patients) were as follows: hemorrhages in 4 patients (aortic aneurysm rupture, intracerebral hemorrhage, subdural hematoma, and gastrointestinal hemorrhage in 1 patient for each), cerebral infarction in 2 patients, pneumonia in 1 patient, anoxic encephalopathy in 1 patient, and cause of death unknown in 7 patients.
Table 3.
Serious adverse drug reactions (ADRs).
| Safety analysis set | n=6148 | |
|---|---|---|
| Follow-up period, mean±SD, days | 498±259 | |
| Cumulative exposure, patient-years | 8386 | |
| Safety events of special interest for intensive survey | No. of patients | Per 100 patient-years |
| Myocardial infarction | 5 | 0.06 |
| Serious hemorrhage | 46 | 0.55 |
| Gastrointestinal disordera (non-hemorrhagic) | 11 | 0.13 |
| Details of serious hemorrhage |
No. of patients |
Per 100 patient-years |
| Total serious hemorrhagic events | 46 | 0.55 |
| Gastrointestinal hemorrhagesb | 26 | 0.31 |
| Upper gastrointestinal hemorrhage | 15 | 0.18 |
| Lower gastrointestinal hemorrhage | 12 | 0.14 |
| Melena | 1 | 0.01 |
| Intracranial hemorrhages | 10 | 0.12 |
| Subdural hematoma | 5 | 0.06 |
| Intracerebral hemorrhage | 4 | 0.05 |
| Subarachnoid hemorrhage | 1 | 0.01 |
| Others | 10 | 0.12 |
Classified by MedDRA SOC cords.
Some events are counted in more than one category.
4. Discussion
This PMS study is a long-term, large-scale, prospective, non-interventional study in NVAF patients who received dabigatran for the first time in Japan, which is being conducted as a special drug use survey based on the law of Japan and the Ordinance of the Ministry of Health, Labor and Welfare. It is so far the largest of its kind for an NOAC registered in Japan.
Switchers had a higher risk of stroke with a CHADS2 score of at least 2, whereas non-switchers mostly had a CHADS2 score of 0 or 1. Permanent AF was most common in Switchers, whereas paroxysmal AF was most common in non-Switchers. Medication adherence was good with >90% measured for the first 3 months of treatment period.
In the RE-LY study [5], patients with a CHADS2 score of 2 accounted for the highest proportion of about 35%; in the Asian subgroup [10] in particular, patients with CHADS2 scores ≥3 accounted for 36.8%. In the present PMS study, however, patients with CHADS2 scores of 0 or 1 accounted for 44.9%, indicating that more NVAF patients at a lower risk for stroke were observed in real clinical practice than in the RE-LY study.
The J-RHYTHM Registry [11], which is a Japanese non-interventional study, showed quite comparable risk scores at baseline for stroke [mean CHADS2 score: 1.8±1.3 (this study) vs. 1.7±1.2; proportion of patients with CHADS2 scores of 0 or 1: 44.9% and 49.6%, respectively]. The Fushimi AF Registry [12] is a non-interventional study to enroll AF patients in Fushimi-ku, Kyoto that is assumed to represent a typical urban community in Japan. The mean CHADS2 score was higher at 2.09±1.35 and the proportion of patients with CHADS2 scores of 0 or 1 was lower at 36.9% in the Fushimi AF Registry than in the present study.
According to the labeling of dabigatran in Japan, patients to be considered for the prescription of the lower dose of 220 mg/day are as follows: (1) aged ≥70 years, (2) with moderate renal impairment (ClCr 30–50 mL/min), (3) concomitant use of a p-glycoprotein inhibitor, and (4) history of hemorrhage or hemorrhagic tendency. In the present study, 89.5% of the patients aged ≥70 years used the low dose (220 mg/day), whereas only 53.9% of the patients aged <65 years and 47.7% of the patients aged <70 used the higher dose (300 mg/day) of dabigatran. This suggests that low-dose administration is preferably selected regardless of the dosage chosen criteria for age in the label (Table 1).
Low dose of dabigatran was given to 91.9% of the patients with ClCr levels of 30–50 mL/min, and also used in 84.9% of the patients diagnosed with renal impairment as comorbidity (Table 1). Of note, 38 patients with ClCr levels of <30 mL/min were administered dabigatran despite contraindication.
Among the patients with a history of hemorrhage, 84% were administered the low-dose, whereas 12% were administered the high-dose of dabigatran (Table 1). As described above, low-dose administration was selected for more patients (85–90% or more) than recommended in the labeling of dabigatran.
There are several limitations to the present interim analysis. All the results are based on an interim analysis performed on the data obtained from 6148 patients with a mean follow-up period of 498 days. Safety events will be reported in the final analysis of the study. Medication adherence was assessed by the individual treating doctors, and was not assessed quantitatively.
The period of this interim analysis study is longer than the published periods for other NOACs, also reflecting the fact that dabigatran was the first launched NOAC in Japan. The PMS study will be continued until March 2016 and the final analyses on the safety and effectiveness are due to be reported.
5. Conclusions
The selected dosage of dabigatran is chosen predominantly in accordance with the label recommendation. The main prescribed dosage strength is 220 mg/day of dabigatran. The assessment of predefined serious safety outcomes for intensive survey revealed an incidence of myocardial infarction of 0.06 per 100 patient-years and 0.55 per 100 patient-years for serious hemorrhage, and 0.13 per 100 patient-years for gastrointestinal disorders. Among NVAF patients newly treated with dabigatran or those switching from warfarin to dabigatran, the favorable safety profile of dabigatran, as demonstrated previously in randomized clinical trials, is corroborated in the real world setting in Japan.
Source of funding
The PMS study was conducted under the sponsorship of Nippon Boehringer Ingelheim.
Conflicts of interest
Dr. H. Inoue has received research funding from Boehringer Ingelheim and Daiichi-Sankyo, and remuneration from Daiichi-Sankyo, Bayer Healthcare, Bristol-Myers Squibb and Boehringer Ingelheim.
Dr. S. Uchiyama has received consultancy fees from Bayer and Boehringer Ingelheim and research grants from Bayer, Boehringer Ingelheim, and Daiichi-Sankyo.
Dr. H. Atarashi has received consultancy fees from Eisai and Otsuka Pharmaceuticals, research funding from Daiichi-Sankyo and Boehringer Ingelheim, and lecture fees from Bayer Healthcare, Bristol-Myers Squibb and Boehringer Ingelheim.
Dr. K. Okumura received research funding from Boehringer Ingelheim and Daiichi-Sankyo, and remuneration from Boehringer Ingelheim, Bayer Healthcare, Daiichi-Sankyo and Pfizer.
Dr. Y. Koretsune has received research funding from Boehringer Ingelheim and Daiichi-Sankyo, and remuneration from Nippon Boehringer Ingelheim, Bayer Yakuhin, Bristol-Myers Squibb and Daiichi-Sankyo.
Dr. M. Yasaka has received remuneration from Nippon Boehringer Ingelheim, Bayer Yakuhin, Bristol-Myers Squibb, Otsuka Pharmaceuticals and Daiichi Sankyo.
Dr. Yamashita received research funding from Boehringer Ingelheim and Daiichi-Sankyo, and remuneration from Boehringer Ingelheim, Daiichi-Sankyo, Bayer Healthcare, Pfizer, Bristol-Myers Squibb, Eisai and Ono Pharmaceuticals.
Ms. M. Ohnishi, Mr. N. Yagi, and Mr. T. Fukaya are employees of Nippon Boehringer Ingelheim.
Acknowledgment
We sincerely thank the patients and doctors who provided valuable data through their participation in the special drug use survey on dabigatran etexilate.
The authors meet the authorship criteria recommended by the International Committee of Medical Journal Editors (ICMJE). The authors are responsible for the content and editorial decisions of the paper and were involved in all stages of the manuscript preparation.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2015.11.008.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Inoue H., Fujiki A., Origasa H. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–107. doi: 10.1016/j.ijcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. J Am Med Assoc. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvie I.M., Newton N., Welner S.A. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Umer Usman M.H., Raza S., Raza S. Advancement in antithrombotics for stroke prevention in atrial fibrillation. J Interv Card Electrophysiol. 2008;22:129–137. doi: 10.1007/s10840-008-9210-9. [DOI] [PubMed] [Google Scholar]
- 5.Connolly S.J., Ezekowitz M.D., Yusuf S., the RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.JCS Joint Working Group Guideline for Pharmacotherapy of Atrial Fibrillation (JCS 2013) Circ J. 2014;78:1997–2021. doi: 10.1253/circj.cj-66-0092. [DOI] [PubMed] [Google Scholar]
- 7.Graham D.J., Reichman M.E., Wernecke M. Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 8.Larsen T.B., Rasmussen L.H., Gorst-Rasmussen A. Myocardial ischemic events in׳real world׳ patients with atrial fibrillation treated with dabigatran or warfarin. Am J Med. 2014;127:329–336. doi: 10.1016/j.amjmed.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Larsen T.B., Gorst-Rasmussen A., Rasmussen L.H. Bleeding events among new starters and switchers to dabigatran compared with warfarin in atrial fibrillation. Am J Med. 2014;127:650–656. doi: 10.1016/j.amjmed.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Hori M., Connolly S.J., Zhu J., the RE-LY Investigators Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 11.Atarashi H., Inoue H., Okumura K., the J-RHYTHM Registry Investigators Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J-RHYTHM Registry. Circ J. 2011;75:1328–1333. doi: 10.1253/circj.cj-10-1119. [DOI] [PubMed] [Google Scholar]
- 12.Akao M., Chun Y.H., Wada H., the Fushimi AF Registry investigators Current status of clinical background of patients with atrial fibrillation in a community-based survey: the Fushimi AF Registry. J Cardiol. 2013;61:260–266. doi: 10.1016/j.jjcc.2012.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material