Abstract
Blood circulation is the result of the beating of the heart, which provides the mechanical force to pump oxygenated blood to, and deoxygenated blood away from, the peripheral tissues. This depends critically on the preceding electrical activation. Disruptions in the orderly pattern of this propagating cardiac excitation wave can lead to arrhythmias. Understanding of the mechanisms underlying their generation and maintenance requires knowledge of the ionic contributions to the cardiac action potential, which is discussed in the first part of this review. A brief outline of the different classification systems for arrhythmogenesis is then provided, followed by a detailed discussion for each mechanism in turn, highlighting recent advances in this area.
Keywords: Arrhythmogenic mechanisms, Focal activity, Reentry, Ion channelopathy, Sudden cardiac death
1. Introduction
The heart beat provides the mechanical force for the pumping of oxygenated blood to, and deoxygenated blood away from, the peripheral tissues [1]. This depends critically on the orderly activation and recovery of electrical excitation through the myocardium. Disruptions of this can lead to arrhythmias. Understanding of the mechanisms underlying their generation and maintenance requires knowledge of the ionic contributions to the cellular action potential, which is discussed in the first part of this review. A brief outline of the different classification systems of arrhythmogenesis is then provided, followed by a discussion of each mechanism in turn, highlighting recent advances in this area.
2. Cardiac action potential and its ionic contributions
The cardiac action potential results from the sequential opening and closing of ion channel proteins that span the plasma membrane of individual myocytes. Its conduction through the heart depends on electrical coupling between these cells, which is mediated by gap junctions [2]. Differences in the expression and properties of ion channels result in heterogeneities in action potential waveforms in different cardiac regions and cell types, and in the normal unidirectional spread of the action potentials through the heart [3]. The cardiac action potential in humans has five different phases (from 0 to 4). Depolarization from the SA node brings the membrane potential to the threshold, opening the voltage-activated sodium channels [4]. This allows the sodium ions to diffuse down their electrochemical gradient from the extracellular space, across the membrane and into the cell. The resulting sodium current, INa, produces a positive feedback loop that causes further sodium channels to open, and depolarization of the membrane proceeds until the sodium Nernst potential is reached or when the channels are inactivated. This is responsible for the rapid upstroke, termed phase 0, of the action potential.
Early rapid repolarization then results from the activation of the fast and slow transient outward potassium currents, Ito,f and Ito,s, and is responsible for phase 1 of the action potential. This is followed by a prolonged plateau resulting from a balance between the inward currents mediated by the voltage-gated L-type calcium channel (ICa,L) and sodium–calcium exchanger (INCX), and the outward currents mediated by the voltage-gated delayed rectifier potassium channels (IK) [5]. The rapid and slow currents (IKr and IKs, respectively) make up IK. There is also contribution from the inward rectifying current (IK1). This plateau is responsible for phase 2 of the action potential. The driving force for potassium efflux remains high during the plateau phase due to a large difference between the membrane potential and the potassium Nernst potential. As the calcium channels become inactivated, the outward potassium currents predominate, causing further repolarization and bringing the membrane potential towards the potassium equilibrium potential. This is responsible for phase 3 of the action potential.
The membrane potential returns to its resting value after full repolarization, which corresponds to phase 4 of the action potential, and is normally polarized at values between −80 and −64 mV relative to the extracellular space [6]. This resting state is maintained mainly by the inward rectifier current, IK1. The weak inward rectifying ATP-dependent potassium channels (IK,ATP), activated by nucleotide diphosphates and inhibited by adenosine triphosphate, are also active during this phase. They are thought to provide a link between cellular metabolism and the membrane potential.
Pacemaker cells are distinct from other cell types in showing automaticity, a property resulting from both voltage- and calcium-dependent mechanisms [7]. The former involves the funny current (If) carried by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [8], which have several unusual characteristics, such as activation on hyperpolarization, permeability to both sodium and potassium ions, modulation by intracellular cyclic AMP, and a small single channel conductance. The latter involves spontaneous calcium release from the sarcoplasmic reticulum [9], which activates INCX. Its crucial role was demonstrated in mice with complete atrial-specific knockout of NCX, which showed no pacemaker activity [10]. Both mechanisms result in spontaneous depolarization that is responsible for the rising slope of the membrane potential.
3. Mechanisms of arrhythmias
Several schemes have been used to classify the mechanisms of cardiac arrhythmias. Traditionally, these have been divided into nonreentrant and reentrant activity [11]. An alternative scheme divided them into those occurring at the cellular and tissue levels [12]. A dynamics-based classification, focusing on the trigger-tissue substrate interactions, divided arrhythmogenic mechanisms into unstable calcium cycling, reduced repolarization reserve, and excess repolarization reserve [13].
4. Focal activity
Focal activity can arise from enhanced automaticity or triggered activity (Fig. 1).
Fig. 1.
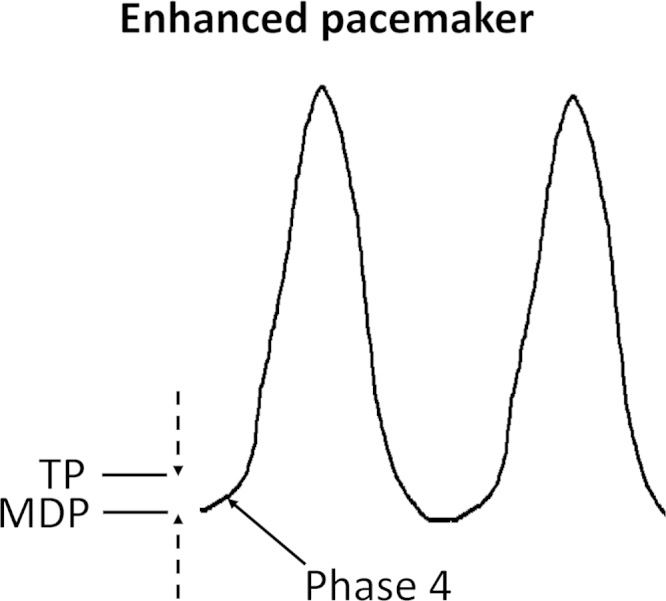
Enhanced pacemaker can occur via three mechanisms: a negative shift in the threshold potential (TP), a positive shift in the maximum diastolic potential (MDP), and an increased rate of phase 4 depolarization.
4.1. Enhanced automaticity
Pacemaker cells are present in the SA node, atria, AV node, and the His-Purkinje system. In the human heart, the normal rate of discharge of the SA node is between 60 and 100 beats per min (bpm). Subsidiary pacemakers discharge at slower rates. They are usually latent and reset by the dominant pacemaker with the highest intrinsic rate of discharge (i.e., the SA node). For example, the AV node discharges at 40–60 bpm and the Purkinje system discharges at 20–40 bpm. Enhanced automaticity of pacemaker cells can increase the rate of action potential discharge (Fig. 1). This can result from three main mechanisms: a negative shift in the threshold potential (TP, top broken arrow), a positive shift in the maximum diastolic potential (MDP, bottom broken arrow), and an increased rate of phase 4 depolarization [14]. When this occurs in the SA node, it can lead to an increase in heart rate, termed sinus tachycardia. This can be physiological, due to increased sympathetic tone during exercise, or pathophysiological, due to hypovolemia, ischemia, or electrolyte disturbances. Moreover, tachycardia–bradycardia syndrome is alternating bradycardia and tachycardia, seen in patients with atrial fibrillation and sick sinus node syndrome [15]. Its underlying molecular mechanisms have not been fully elucidated. Recent evidence suggests a possible role of HCN channel downregulation in the SA node with a consequent decrease in If [16]. Furthermore, experiments in NCX knockout mice have demonstrated burst pacemaker activity, suggesting a possible contributory role of NCX in tachycardia–bradycardia syndrome [17]. Both HCN and NCX are responsible for the “voltage clock” of pacemaker activity. It is possible that dysfunctions of the proteins involved in the “calcium clock” mechanism, for example, ryanodine receptor and sarcoplasmic reticulum Ca2+-ATPase, may also contribute [18].
Enhanced automaticity can also occur in the AV node, under conditions of acute myocardial infarction, digitalis toxicity, isoprenaline administration, and recent cardiac surgery. When the discharge rate of the AV node is higher than the sinus rate, it can lead to abnormal rhythms called accelerated junctional rhythms [19]. These rhythms can occur at sites close to the atria, such as the pulmonary veins, superior vena cava, crista terminalis, coronary sinus, atrial septum, and the para-Hisian region that includes the tricuspid and mitral cannulae, leading to focal atrial tachycardia.
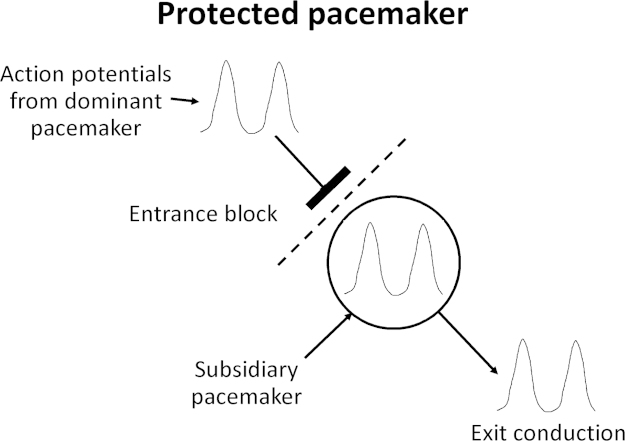
Alternatively, parasystole occurs when a latent pacemaker is protected from the dominant pacemaker by entrance block and becomes ectopic, discharging action potentials independently (Fig. 2). A region of entrance block can occur when the dominant pacemaker is surrounded by ischemic, infarcted, or otherwise compromised tissues that prevent conduction of action potentials to the latent pacemaker. When this happens, action potentials generated by the latent pacemaker can exit and activate the rest of the myocardium. Thus, a parasystolic focus is formed when there are both compromised conduction into the ectopic pacemaker and exit conduction [20].
Fig. 2.
Protected pacemaker. Entrance block of the dominant pacemaker allows exit conduction of the subsidiary pacemaker, which can generate action potentials that excite the rest of the myocardium.
Modulated parasystole is a variant of the above [21]. It results from incomplete entrance block of the ectopic pacemaker. In this situation, the dominant pacemaker or other cardiac tissues can exert electrotonic influences on the parasystolic focus. Electrotonic influences arriving early in the pacemaker cycle delay the firing of the parasystolic focus, whereas those arriving late in the cycle accelerate its firing. A special case of modulated parasystole occurs when the action potentials of the parasystolic focus exert electrotonic influence on the focus itself. This is termed automodulation [22]. As has been shown in the atria, a parasystolic impulse exerts electrotonic influences on the parasystolic focus itself during the supernormal phase, where the focus accelerates rather than delaying its discharge. Repetition of this mechanism would then result in a tachycardia [23].
4.2. Triggered activity
Triggered activity results from the premature activation of cardiac tissues by afterdepolarizations, which are depolarizations triggered by one or more preceding action potentials [24], [25].
4.2.1. Early afterdepolarizations
Early afterdepolarizations (EADs) can develop before full repolarization, corresponding to phase 2 or phase 3 of the cardiac action potential in humans (Fig. 3). They are usually but not exclusively associated with prolonged action potential durations (APDs), which occur when the inward current is greater in amplitude than the outward current. Several factors can tip the balance towards the inward direction. These include increases in the late sodium current (INa), the calcium current (ICa), or INCX, or decreases in the repolarizing potassium currents (IKr, IKs, IK1). Two mechanisms have been proposed for the EADs that are associated with prolongations in APDs and occur during phase 2 of the action potential. Firstly, depolarizing shifts in the membrane potential can reactivate the L-type calcium channels [26], resulting in increased ICa,L that further depolarizes the membrane. This sets up a positive feedback loop, triggering an action potential. Secondly, at membrane potentials negative to the threshold of ICa,L activation (but before full repolarization), spontaneous calcium release from the sarcoplasmic reticulum can activate INCX [27], resulting in membrane depolarization. The intermittent nature of EADs has recently been examined, demonstrating that it is due to slow changes in [Na+]i and potentially explaining why arrhythmias do not occur all the time [28].
Fig. 3.
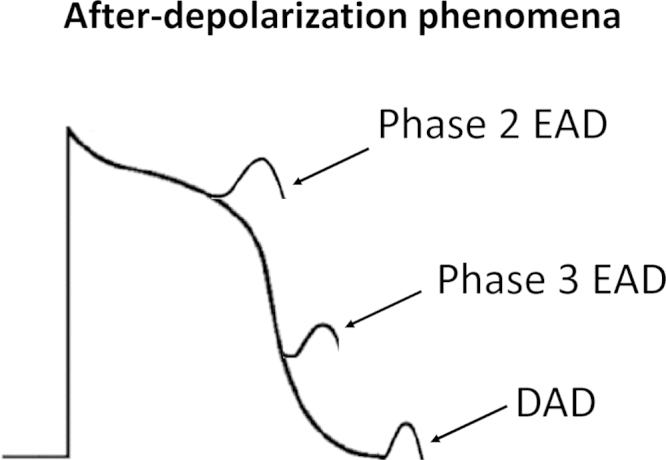
Afterdepolarization phenomena: early afterdepolarization (EAD) occurs early (phase 2) or late (phase 3), and delayed afterdepolarization (DAD) occurs during phase 4 of the action potential.
EADs have also been associated with shortening in APDs, occurring late in phase 3 of the action potential [29]. Here, an abbreviated APD permits normal calcium release from the sarcoplasmic reticulum. If the intracellular calcium concentration ([Ca2+]i) remains elevated when the membrane potential is negative to the equilibrium potential for NCX, INCX can be activated, causing membrane depolarization. These late EADs are clinically relevant, as they can occur immediately after termination of other types of tachycardia, such as atrial flutter, AT, VT, and VF [30]. In such instances, repolarization time is shortened and a transient increase in sarcoplasmic calcium release can be induced when reverting to sinus rhythm.
Whatever be the underlying mechanism, if the change in membrane potential brought about by the EAD is sufficiently large, it will activate INa, resulting in triggered activity. EADs and their resulting triggered activity are thought to underlie the arrhythmogenesis observed in heart failure and long QT syndromes [31].
4.2.2. Delayed afterdepolarizations
Delayed afterdepolarizations (DADs) were first described as oscillatory afterpotentials [32]. They can develop after full repolarization, corresponding to phase 4 of the cardiac action potential in humans (Fig. 3). DADs are observed under conditions of intracellular calcium overload, which can result from exposure to digitalis, catecholamines, hypokalemia, and hypercalcemia, and in hypertrophy and heart failure. The proposed mechanism for the genesis of DADs is as follows: high levels of intracellular calcium induce spontaneous calcium release from the sarcoplasmic reticulum, activating three calcium-sensitive currents—the nonselective cationic current, INS, the sodium–calcium exchange current, INCX, and the calcium-activated chloride current, ICl,Ca. Together, these constitute the transient inward current (ITI) that is responsible for membrane depolarization [33]. If the depolarization produced by the DAD is sufficiently large, INa is activated, leading to triggered activity. DAD-induced triggered activity is thought to underlie the arrhythmogenesis observed in catecholaminergic polymorphic ventricular tachycardia (CPVT) [34].
It is worth noting that DADs and late EADs are somewhat similar [30]. Both occur under conditions of intracellular calcium overload and involve spontaneous release of calcium from the sarcoplasmic reticulum. The difference appears to be the timing of this release [35], which occurs during the repolarizing phase of the action potential in the case of late EADs, and at the resting membrane potential for DADs. Indeed, for atrial fibrillation, both EADs and DADs have been implicated as the mechanisms of arrhythmogenesis [36].
5. Reentry
Re-entry occurs when an action potential fails to extinguish itself and reactivates a region that has recovered from refractoriness [37]. It can be divided into two types: (i) reentry that occurs in the presence of an obstacle, around which an action potential can travel (circus-type); and (ii) reentry that occurs without an obstacle (reflection or phase 2).
5.1. Reentry involving an obstacle (circus-type)
This occurs when an action potential travels around an anatomical or functional obstacle and reexcites its site of origin (Fig. 4).
Fig. 4.
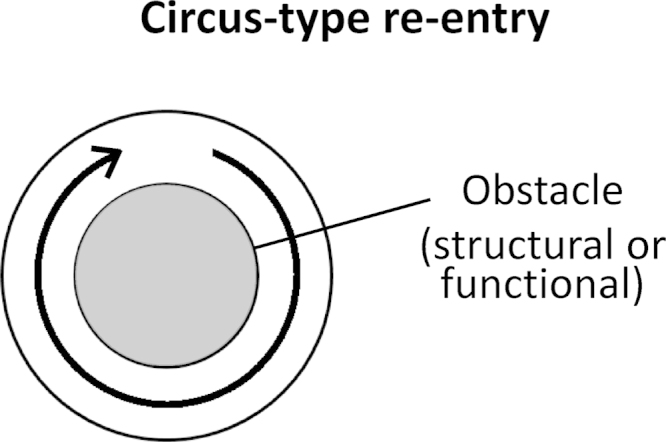
Circus-type reentry requires a structural or functional obstacle (gray center) around which an action potential can circulate.
5.1.1. Anatomical obstacle
The ring model was the first example of circus-type reentry involving an anatomical obstacle. It emerged from experiments using disks made from sub-umbrella tissue of a jellyfish [38]. Mayer made the following observations. The disks were paralyzed when they were separated from their sense organs. They did not pulsate in seawater, but did so when ring-like cuts were made on the tissue. Upon mechanical stimulation, the disks then showed “rhythmical pulsations so regular and sustained as to recall the movement of clockwork” [38]. Later, Mines used a ring-like preparation of the tortoise heart, demonstrating that it was possible to initiate circus-type reentry by electrical stimulation [39]. If an excitation wave has a high propagation rate and a long duration, the whole circuit would be excited at the same time, causing the excitation to die out. In contrast, one with slower conduction and a shorter duration would permit the tissue ahead of the excitation wave to recover from refractoriness, which can therefore be reexcited, resulting in circus-type reentry. Mines predicted, “A circulating excitation of this type may be responsible for some cases of paroxysmal tachycardia as observed clinically.” He also proposed three criteria for this type of reentry: (a) an area of unidirectional block must exist; (b) the excitation wave propagates along a distinct pathway, returns to its point of origin, and starts again; and (c) interruption of the circuit at any point would terminate this circus movement.
It was recognized that conduction of the excitation must be sufficiently slow to allow the tissue ahead in the circuit to recover from refractoriness so that it can be reexcited. Thus, both the conduction velocity (CV) and the refractory period determine whether reentrant arrhythmogenesis occurs. It is useful to describe this excitation as a propagating wave, with a wave front that represents action potential depolarization, and a tail that represents repolarization [40]. The length of this excitation wave is given by the product of its CV and refractory period [41], and must be smaller than the length of the circuit in order for reentry to be successful. Often, it is assumed that the end of the refractory period coincides with the end of the APD.
5.1.2. Functional obstacle
The possibility of circus-type reentry occurring without an anatomical obstacle was later suggested [42]. Direct evidence for this came from experiments demonstrating that premature electrical stimuli can induce tachycardia in isolated rabbit atrial preparations [43]. Electrical stimulation was applied at the center of the atrial tissue. Electrical activation initiated by regular stimuli spread normally throughout the tissue. In contrast, an impulse initiated by premature stimuli only propagates in the direction of shortened refractory periods and does so at a reduced CV. The unidirectional block of the premature impulse is caused by spatial dispersion in the refractory periods [44]. Furthermore, it was proposed that the center of the tissue was held above threshold by the electrotonic influences of the depolarization wave front, which revolved around this area, rendering it inexcitable. The excitation wave would then continue to revolve around this functional obstacle. Subsequent experiments then obtained transmembrane potential recordings, which led to the leading circle model [45]. Here, the circuit is defined entirely by the electrophysiological properties of the tissue. The smallest circuit permitting successful reentry, which was called the leading circle, is one in which the circulating wave front can just reexcite the tissue ahead that is still in the relative refractory period.
The CV of the propagating action potential depends on the wave curvature. For a planar wave, each cell activates one cell downstream. For a wave front curving inwards (concave), each cell will be activating less than one cell downstream. This source–sink mismatch will increase the depolarizing current available for each downstream cell, resulting in a greater rate of voltage rise, and therefore, a higher CV compared to that of a planar wave. The opposite is true for a wave front curving outwards (convex), where each cell will be activating more than one cell downstream, and thus the CV will be smaller than that of a planar wave. If the curvature is sufficiently convex, conduction block can result [46]. The point where the activation and repolarization wave fronts meet is called the phase singularity [47]. This corresponds to a nonexcited point because all phases of the wave meet here.
A variation of functional reentry termed spiral wave reentry was later described. A spiral wave is a two-dimensional wave of excitation emitted by a self-organizing source of functional reentrant activity, termed a rotor. The three-dimensional equivalent of a spiral wave is a scroll wave. Spiral waves were described earlier in the Belousov–Zhabotinsky chemical reaction, in which cerium ions catalyze the oxidation of malonic acid by bromate. In this reaction, the ratio of cerium (IV) to cerium (III) undergoes repeated temporal oscillations, generating spiral waves with alternating colors. Spiral waves have been subsequently reproduced in models of cardiac tissue and demonstrated in thin slices of epicardial muscle using a potentiometric dye, which changes its spectral properties in response to voltage changes. Experiments have shown that the phase singularity is excitable but remains nonexcited, and therefore acts as a functional obstacle around which the spiral wave can circulate [48].
Spiral waves are not fixed in space but can drift through the tissue [49]. This drift phenomenon is accompanied by a Doppler effect, in which the frequency of excitation at a given measurement site depends on the location of this site relative to the drifting spiral wave [50]. Thus, the sites in front of the wave are excited faster than those behind the wave. This may be the underlying mechanism of torsade de pointes [51], in which the periodic torsion of the QRS axis has been attributed to two widely separated foci discharging at different frequencies. Two counter-rotating spiral waves separated by a small distance can produce reentry in a figure-of-eight configuration, which was first demonstrated in the canine heart using a healed myocardial infarction model [52].
5.2. Reentry not involving an obstacle
Reentry can also occur without circus movement. This can be divided into reflection and phase 2 reentry.
5.2.1. Reflection
The possibility of reflection was first suggested by a report that investigated the role of slowed action potential conduction in reentrant excitation using excised canine Purkinje fibers [53]. Depressed excitability in discrete segments of the fibers was produced by increasing the extracellular potassium concentrations. The authors made the following observations. An action potential traveling in the forward direction was sometimes followed by a return extrasystole that traveled in the backward direction through the original route. This only occurred when the initiating impulse reached an area of depressed excitability. It was noted that the return extrasystole could arise from circus movement within the depressed segment, a mechanism proposed earlier [54].
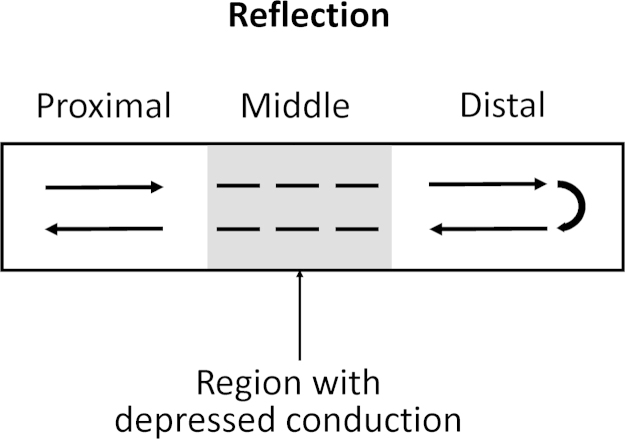
Later, reflection was demonstrated as a mechanism of reentrant arrhythmogenesis using the sucrose gap model [55]. Experiments used ion-free isotonic sucrose solution to create a central inexcitable gap in canine Purkinje fibers, thereby dividing them into three segments (Fig. 5). Electrical stimulation at the proximal segment elicits an action potential. This excitation is transmitted across the gap to the distal segment after a delay. However, this cannot be active in the form of action potentials because the extracellular space is ion-free, but instead involves passive spread of the local current (electrotonic current) across the low-resistance intracellular pathway. When depolarization reaches threshold, an action potential is initiated in the distal segment. This in turn generates electrotonic currents in the retrograde direction. With a further delay, the proximal region can be reexcited when it has recovered from refractoriness, resulting in a return extrasystole, completing reflection [55]. Successful segment-type reflection requires a balance between the conduction delay and the cellular membrane excitability [56]. A slightly different model of reflection involved immersion of the Purkinje fibers in a solution containing high concentrations of potassium and lactic acid to mimic the extracellular milieu that is present during ischemia [57]. This also renders the central segment inexcitable. Segment-type reflection has been demonstrated in isolated atrial [58] and ventricular [59] tissues.
Fig. 5.
Reflection. Stimulation of the proximal segment elicits an action potential. Its conduction across the middle segment cannot take place actively as the extracellular region is ion-free. Instead, it involves electrotonic current spread intracellularly. After a delay, when the membrane potential reaches threshold at the distal segment, another action potential is generated.
Recently, a different type of reflection, called the expansion-type, has been demonstrated [60]. Antegrade propagation of an impulse occurs from a narrow isthmus region to an expanded distal region. The activation wave front has an outward curvature (convex) and stimulates a higher number of cells in the expanded region, where the source–sink mismatch is greatest. This causes the direction of electrotonic currents to be reversed, in turn prolonging the action potential. This in turn initiates retrograde propagation along the same path.
5.2.2. Phase 2 reentry
Phase 2 reentry is another mechanism that does not depend on circus-type movement [61]. Its concept emerged from experiments that introduced pinacidil, an activator of the ATP-regulated potassium current, IK,ATP, to canine ventricular tissues. The canine ventricular action potentials have a “spike and dome” morphology. Pinacidil increases IK,ATP, resulting in the shortening of APDs and thus loss of the action potential dome. However, this effect is much more prominent in the epicardium than in the endocardium, possibly because of a smaller endocardial Ito, and any changes produced there by pinacidil would be less dramatic. Propagation of the action potential dome from sites where it is maintained to sites where it is abolished can then result in an extrasystole [62]. Electrotonic currents can flow from sites with longer APDs to sites with shorter APDs, and can cause reexcitation when the latter sites have recovered from refractoriness [63]. The arrhythmogenesis in Brugada syndrome is thought to involve phase 2 reentry, where the resulting premature beat initiates spontaneous polymorphic VT. Heterogeneity in action potential repolarization between cardiac regions leading to phase 2 reentry occurs following exposure to the sodium channel inhibitor flecainide and under conditions of raised [Ca2+]i and ischemia [64]. Phase 2 reentry, e.g., from ischemia, can also initiate circus-type reentry [65].
The concept of prolonged repolarization-dependent reexcitation (PRDR) proposed earlier [66] is also similar to that of phase 2 reentry. PRDR requires an area of myocardium with prolonged repolarization connected to another area with a normal repolarization time-course. For example, EADs can prolong repolarization and the resulting triggered activity in Purkinje fibers can conduct to the connecting ventricular muscle [67]. However, in PRDR, prolonged APDs per se do not cause reexcitation of the regions with shorter APDs, as they do in phase 2 reentry. Rather, secondary depolarizations such as EADs or their resulting triggered activity in the affected region provide an additional current source. Together, the local circuit currents generated by the APD difference and by the EAD provide the necessary depolarizing currents for an extrasystole in the affected region. Furthermore, in PRDR, the interaction is between sites with prolonged and normal APDs, whereas in phase 2 reentry, it is between sites with normal and shortened APDs [64]. Nevertheless, both mechanisms require an increased transmural heterogeneity in the time courses of repolarization.
6. Trigger versus substrate?
Traditional views have considered trigger and substrate as independent entities [68]. Examples of triggers are EADs and DADs, which can induce ectopic beats. Substrate refers to electrophysiological abnormalities predisposing to reentry, including increased dispersion of conduction or repolarization, and abnormal restitution [69]. However, evidence suggests that the trigger itself can produce the necessary substrate for reentry, e.g., DADs inducing unidirectional block [70]. Conversely, reentry may be the triggering event for an arrhythmia. Thus, phase 2 reentry can produce closely coupled ectopic beats capable of initiating circus-type reentry in Brugada syndrome [64].
7. Future directions
Novel antiarrhythmic agents that have been developed are mainly for the management of atrial fibrillation, targeting atrial-specific ion channels [71]. An example is vernakalant, a multichannel blocker of the ultra-rapid (IKur) and acetylcholine-activated (IK, ACh) potassium currents. This has minimal effects on the ventricles [72], and is thus less likely to induce malignant ventricular arrhythmias. However, there has been no recently licensed drug for the treatment of ventricular arrhythmias, although ranolazine, a late INa inhibitor licensed for angina, has demonstrated efficacy in experimental studies [73]. There is a need to develop new drugs for several reasons. First, implantable cardioverter-defibrillators (ICDs) are not able to prevent the occurrence of ventricular arrhythmias, but can only terminate them, and are not without significant morbidity. Yet, they are the only form of treatment shown to increase life expectancy. Secondly, current pharmacological agents possess significant cardiac and extracardiac side effects, the most concerning of which are their proarrhythmic properties [74].
A potential solution is the rational design of agents that target the abnormal proteins concerned. Thus, EADs observed in long QT syndrome type 3 could be prevented by drugs inhibiting the late INa [75]. DADs underlying CPVT may be abolished by blocking the ryanodine receptors, which mediate calcium release from the sarcoplasmic reticulum [76], or the sodium–calcium exchanger [77]. Cardiac-specific KATP channels, which are activated by ATP depletion during ischemia, are currently under investigation [78]. Furthermore, understanding the physiology of arrhythmogenesis, for example, the substrates that sustain reentry, can guide drug development. Thus, heterogeneities in action potential conduction or repolarization, observed in a number of ion channelopathies, may be reduced by gap junction openers [79].
In summary, this article reviewed the physiological mechanisms of non-reentrant and reentrant arrhythmias. Their generation and maintenance are important in both congenital and acquired arrhythmic syndromes. In the future, antiarrhythmic drugs have the potential to match device-based therapy and catheter ablation in terms of efficacy. Research efforts on their development therefore warrant further exploration.
Conflict of interest
Disclosure of potential conflicts of interest: GT received funding from Xention Discovery for his research. This did not influence the content of this review article in any manner.
Acknowledgments
The author received a BBSRC Doctoral CASE Studentship at the Department of Biochemistry, University of Cambridge, in conjunction with Xention Discovery, for his PhD studies. This manuscript is based, in part, on his doctoral thesis. He thanks Dr. Antony Workman of University of Glasgow, and Prof. Sarah Lummis, of University of Cambridge, for their helpful comments on an earlier draft of his thesis.
References
- 1.Whitteridge G. E. & S.Livingstone; Edinburgh and London, UK: 1964. The anatomical lectures of William Harvey. [Google Scholar]
- 2.Kanno S., Saffitz J.E. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. doi: 10.1016/s1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 3.Nerbonne J.M., Guo W. Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias. J Cardiovasc Electrophysiol. 2002;13:406–409. doi: 10.1046/j.1540-8167.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Kunze D.L., Lacerda A.E., Wilson D.L. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985;86:691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 6.Amos G.J., Wettwer E., Metzger F. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491:31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakatta E.G., Vinogradova T., Lyashkov A. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart׳s pacemaker. Ann NY Acad Sci. 2006;1080:178–206. doi: 10.1196/annals.1380.016. [DOI] [PubMed] [Google Scholar]
- 8.Baruscotti M., Bucchi A., Difrancesco D. Physiology and pharmacology of the cardiac pacemaker ("funny") current. Pharmacol Ther. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Vinogradova T.M., Maltsev V.A., Bogdanov K.Y. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann NY Acad Sci. 2005;1047:138–156. doi: 10.1196/annals.1341.013. [DOI] [PubMed] [Google Scholar]
- 10.Groenke S., Larson E.D., Alber S. Complete atrial-specific knockout of sodium-calcium exchange eliminates sinoatrial node pacemaker activity. Plos One. 2013;8:e81633. doi: 10.1371/journal.pone.0081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antzelevitch C., Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin. 2011;3:23–45. doi: 10.1016/j.ccep.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anumonwo J.M., Pandit S.V. Ionic mechanisms of arrhythmogenesis. Trends Cardiovasc Med. 2015;25:487–496. doi: 10.1016/j.tcm.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss J.N., Garfinkel A., Karagueuzian H.S. Perspective: a dynamics-based classification of ventricular arrhythmias. J Mol Cell Cardiol. 2015;82:136–152. doi: 10.1016/j.yjmcc.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalife J., Delmar M., Anumonwo J. 2nd ed. Wiley-Blackwell; 2009. Basic mechanisms of cardiac arrhythmias. Basic cardiac electrophysiology for the clinician. [Google Scholar]
- 15.Adan V., Crown L.A. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003;67:1725–1732. [PubMed] [Google Scholar]
- 16.Yeh Y.H., Burstein B., Qi X.Y. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576–1585. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 17.Torrente A.G., Zhang R., Zaini A. Burst pacemaker activity of the sinoatrial node in sodium-calcium exchanger knockout mice. Proc Natl Acad Sci USA. 2015;112:9769–9774. doi: 10.1073/pnas.1505670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maltsev V.A., Lakatta E.G. Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart Lung Circ. 2007;16:335–348. doi: 10.1016/j.hlc.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Azevodo I.M., Watanabe Y., Dreifus L.S. Atrioventricular junctional rhythm: classification and clinical significance. Chest. 1973;64:732–740. doi: 10.1378/chest.64.6.732. [DOI] [PubMed] [Google Scholar]
- 20.Gussak I., Antzelevitch C., Hammill S.C. Humama Press; Totowa, New Jersey: 2003. Cardiac repolarization: bridging basic and clinical science (contemporary cardiology) [Google Scholar]
- 21.Jalife J., Antzelevitch C., Moe G.K. The case for modulated parasystole. Pacing Clin Electrophysiol. 1982;5:911–926. doi: 10.1111/j.1540-8159.1982.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 22.Satullo G., Donato A., Luzza F. Atrial parasystole and tachycardia. Modulation and automodulation of a parasystolic focus. Chest. 1992;102:622–625. doi: 10.1378/chest.102.2.622. [DOI] [PubMed] [Google Scholar]
- 23.Saoudi N., Castellanos A., Touboul P. Automodulation des para systolies ventriculaires: la courbe de phase reponse triphasique. Arch Mal Coeur. 1989;82:201–206. [PubMed] [Google Scholar]
- 24.January C.T., Chau, Makielski J.C. Triggered activity in the heart: cellular mechanisms of early after-depolarizations. EuruHeart J. 1991;12:4–9. doi: 10.1093/eurheartj/12.suppl_f.4. [DOI] [PubMed] [Google Scholar]
- 25.Cranefield P.F. Action potentials, afterpotentials, and arrhythmias. Circ Res. 1977;41:415–423. doi: 10.1161/01.res.41.4.415. [DOI] [PubMed] [Google Scholar]
- 26.January C.T., Riddle J.M. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. CirCRes. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 27.Szabo B., Sweidan R., Rajagopalan C.V. Role of Na+:Ca2+ exchange current in Cs(+)-induced early afterdepolarizations in Purkinje fibers. J Cardiovasc Electrophysiol. 1994;5:933–944. doi: 10.1111/j.1540-8167.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 28.Xie Y., Liao Z., Grandi E. Slow [Na]i changes and positive feedback between membrane potential and [Ca]i underlie intermittent early afterdepolarizations and arrhythmias. Circ Arrhythm Electrophysiol. 2015 doi: 10.1161/CIRCEP.115.003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson E., Lazzara R., Szabo B. Sodium–calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Burashnikov A., Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maruyama M., Lin S.F., Xie Y. Genesis of phase 3 early afterdepolarizations and triggered activity in acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 2011;4:103–111. doi: 10.1161/CIRCEP.110.959064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrier G.R., Saunders J.H., Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973;32:600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- 33.Guinamard R., Chatelier A., Demion M. Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004;558:75–83. doi: 10.1113/jphysiol.2004.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priori S.G., Napolitano C., Tiso N. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 35.Fink M., Noble D. Pharmacodynamic effects in the cardiovascular system: the modeller׳s view. Basic Clin Pharmacol Toxicol. 2010;106:243–249. doi: 10.1111/j.1742-7843.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 36.Workman A.J., Marshall G.E., Rankin A.C. Transient outward K+ current reduction prolongs action potentials and promotes afterdepolarisations: a dynamic-clamp study in human and rabbit cardiac atrial myocytes. J Physiol. 2012;590:4289–4305. doi: 10.1113/jphysiol.2012.235986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janse M.J., Wit A.L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 38.Mayer AG. Rhythmical pulsation in scyphomedusae. Washington, DC; 1906.
- 39.Mines G.R. On dynamic equilibrium in the heart. J Physiol. 1913;46:349–383. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss J.N., Chen P.S., Qu Z. Ventricular fibrillation: how do we stop the waves from breaking? Circ Res. 2000;87:1103–1107. doi: 10.1161/01.res.87.12.1103. [DOI] [PubMed] [Google Scholar]
- 41.Wiener N., Rosenblueth A. The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle. Arch Inst Cardiol Mex. 1946;16:205–265. [PubMed] [Google Scholar]
- 42.Garrey W.E. The nature of fibrillary contraction of the heart: Its relation to tissue mass and form. Am J Physiol. 1914;33:397–414. [Google Scholar]
- 43.Allessie M.A., Bonke F.I., Schopman F.J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Circ Res. 1973;33:54–62. [PubMed] [Google Scholar]
- 44.Allessie M.A., Bonke F.I., Schopman F.J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block, as studied with multiple microelectrodes. Circ Res. 1976;39:168–177. doi: 10.1161/01.res.39.2.168. [DOI] [PubMed] [Google Scholar]
- 45.Allessie M.A., Bonke F.I., Schopman F.J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 46.Cabo C., Pertsov A.M., Baxter W.T. Wave-front curvature as a cause of slow conduction and block in isolated cardiac muscle. Circ Res. 1994;75:1014–1028. doi: 10.1161/01.res.75.6.1014. [DOI] [PubMed] [Google Scholar]
- 47.Winfree A.T. Electrical instability in cardiac muscle: phase singularities and rotors. J Theor Biol. 1989;138:353–405. doi: 10.1016/s0022-5193(89)80200-0. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda T., Uchida T., Hough D. Mechanism of spontaneous termination of functional reentry in isolated canine right atrium. Evidence for the presence of an excitable but nonexcited core. Circulation. 1996;94:1962–1973. doi: 10.1161/01.cir.94.8.1962. [DOI] [PubMed] [Google Scholar]
- 49.Pertsov A.M., Davidenko J.M., Salomonsz R. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res. 1993;72:631–650. doi: 10.1161/01.res.72.3.631. [DOI] [PubMed] [Google Scholar]
- 50.Davidenko J.M., Pertsov A.V., Salomonsz R. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992;255:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- 51.Dessertenne F. La tachycardie ventriculaire a deux foyers opposes variable. Arch Mal Coeur. 1966;56:263–272. [PubMed] [Google Scholar]
- 52.El-Sherif N., Smith R.A., Evans K. Canine ventricular arrhythmias in the late myocardial infarction period. 8. Epicardial mapping of reentrant circuits. Circ Res. 1981;49:255–265. doi: 10.1161/01.res.49.1.255. [DOI] [PubMed] [Google Scholar]
- 53.Wit A.L., Hoffman B.F., Cranefield P.F. Slow conduction and reentry in the ventricular conducting system. I. Return extrasystole in canine Purkinje fibers. Circ Res. 1972;30:1–10. doi: 10.1161/01.res.30.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt F.O., Erlanger J. Directional differences in the conduction of the impulse through heart muscle and their possible relation to extrasystolic and fibrillary contractions. Am J Physiol. 1928;87:326–347. [Google Scholar]
- 55.Antzelevitch C., Jalife J., Moe G.K. Characteristics of reflection as a mechanism of reentrant arrhythmias and its relationship to parasystole. Circulation. 1980;61:182–191. doi: 10.1161/01.cir.61.1.182. [DOI] [PubMed] [Google Scholar]
- 56.Tung L. Expanding on forty years of reflection. J Physiol. 2011;589:2107–2108. doi: 10.1113/jphysiol.2011.209239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antzelevitch C., Moe G.K. Electrotonically mediated delayed conduction and reentry in relation to "slow responses" in mammalian ventricular conducting tissue. Circ Res. 1981;49:1129–1139. doi: 10.1161/01.res.49.5.1129. [DOI] [PubMed] [Google Scholar]
- 58.Lukas A., Antzelevitch C. Reflected reentry, delayed conduction, and electrotonic inhibition in segmentally depressed atrial tissues. Can J Physiol Pharmacol. 1989;67:757–764. doi: 10.1139/y89-121. [DOI] [PubMed] [Google Scholar]
- 59.Rozanski G.J., Jalifé J., Moe G.K. Reflected reentry in nonhomogeneous ventricular muscle as a mechanism of cardiac arrhythmias. Circulation. 1984;69:163–173. doi: 10.1161/01.cir.69.1.163. [DOI] [PubMed] [Google Scholar]
- 60.Auerbach D.S., Grzda K.R., Furspan P.B. Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers. J Physiol. 2011;589:2363–2381. doi: 10.1113/jphysiol.2010.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Diego J.M., Antzelevitch C. Pinacidil-induced electrical heterogeneity and extrasystolic activity in canine ventricular tissues. Does activation of ATP-regulated potassium current promote phase 2 reentry? Circulation. 1993;88:1177–1189. doi: 10.1161/01.cir.88.3.1177. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu W., Aiba T., Kamakura S. Mechanisms of disease: current understanding and future challenges in Brugada syndrome. Nat Clin Pr Cardiovasc Med. 2005;2:408–414. doi: 10.1038/ncpcardio0268. [DOI] [PubMed] [Google Scholar]
- 63.Moe G.K. Evidence for reentry as a mechanism of cardiac arrhythmias. Rev Physiol Biochem Pharmacol. 1975;72:55–81. doi: 10.1007/BFb0031546. [DOI] [PubMed] [Google Scholar]
- 64.Lukas A., Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc Res. 1996;32:593–603. [PubMed] [Google Scholar]
- 65.Kuo C.S., Munakata K., Reddy C.P. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- 66.Brugada P., Wellens H.J. Early afterdepolarizations: role in conduction block, "prolonged repolarization-dependent reexcitation," and tachyarrhythmias in the human heart. Pacing Clin Electrophysiol. 1985;8:889–896. doi: 10.1111/j.1540-8159.1985.tb05908.x. [DOI] [PubMed] [Google Scholar]
- 67.Li Z.Y., Maldonado C., Zee-Cheng C. Purkinje fibre-papillary muscle interaction in the genesis of triggered activity in a guinea pig model. Cardiovasc Res. 1992;26:543–548. doi: 10.1093/cvr/26.5.543. [DOI] [PubMed] [Google Scholar]
- 68.Coumel P. Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol. 1993;4:338–355. doi: 10.1111/j.1540-8167.1993.tb01235.x. [DOI] [PubMed] [Google Scholar]
- 69.Koller M.L., Riccio M.L., Gilmour R.F.J. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 70.Liu M.B., de Lange E., Garfinkel A. Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm. 2015;12:2115–2124. doi: 10.1016/j.hrthm.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mason P.K., DiMarco J.P. New pharmacological agents for arrhythmias. Circ Arrhythm Electrophysiol. 2009;2:588–597. doi: 10.1161/CIRCEP.109.884429. [DOI] [PubMed] [Google Scholar]
- 72.Dorian P., Pinter A., Mangat I. The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. J Cardiovasc Pharmacol. 2007;50:35–40. doi: 10.1097/FJC.0b013e3180547553. [DOI] [PubMed] [Google Scholar]
- 73.Antzelevitch C., Belardinelli L., Zygmunt A.C. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams E.S., Viswanathan M.N. Current and emerging antiarrhythmic drug therapy for ventricular tachycardia. Cardiol Ther. 2013;2:27–46. doi: 10.1007/s40119-013-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belardinelli L., Liu G., Smith-Maxwell C. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. doi: 10.1124/jpet.112.198887. [DOI] [PubMed] [Google Scholar]
- 76.Savio-Galimberti E., Knollmann B.C. Channel activity of cardiac ryanodine receptors (RyR2) determines potency and efficacy of flecainide and R-propafenone against arrhythmogenic calcium waves in ventricular cardiomyocytes. Plos One. 2015;10:e0131179. doi: 10.1371/journal.pone.0131179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sipido K.R., Varro A., Eisner D. Sodium calcium exchange as a target for antiarrhythmic therapy. Handb Exp Pharmacol. 2006:159–199. doi: 10.1007/3-540-29715-4_6. [DOI] [PubMed] [Google Scholar]
- 78.Englert H.C., Gerlach U., Goegelein H. Cardioselective K(ATP) channel blockers derived from a new series of m-anisamidoethylbenzenesulfonylthioureas. J Med Chem. 2001;44:1085–1098. doi: 10.1021/jm000985v. [DOI] [PubMed] [Google Scholar]
- 79.Kjolbye A.L., Dikshteyn M., Eloff B.C. Maintenance of intercellular coupling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol. 2008;294:H41–H49. doi: 10.1152/ajpheart.01089.2006. [DOI] [PubMed] [Google Scholar]