Abstract
Objective
To determine the most effective treatment strategy among anticitrullinated protein antibodies (ACPA)-negative patients with early rheumatoid arthritis.
Methods
In the BeSt study, 184 ACPA-negative patients were randomised to: (1) sequential monotherapy, (2) step-up therapy, (3) initial combination including prednisone, (4) initial combination including infliximab. Treatment was targeted at the disease activity score (DAS) ≤2.4. Early response and 10-year outcomes were compared between the four strategy-arms in ACPA-negative patients.
Results
ACPA-negative patients achieved more short-term functional improvement from initial combination therapy than when on monotherapy (at month 3, mean Health Assessment Questionnaire (HAQ) 0.71 vs 0.98, p=0.006; at month 6, 0.59 vs 0.87, p=0.004). Functional ability over time was comparable between the strategy-arms (p=0.551) with a mean HAQ of 0.6 at year 10 (p=0.580 for comparison across the strategy-arms). 10-year radiographic progression was negligible (median 0.5) and comparable between the 4 strategy-arms (p=0.082). At year 10, remission was achieved by 11/40 (28%), 9/45 (20%), 17/56 (30%) and 17/43 patients (40%) in strategy-arms 1–4, respectively (p=0.434). Over time, similar remission percentages were achieved in all strategy-arms (p=0.815). 18%, 16%, 20% and 21% in strategy-arms 1 to 4 (p=0.742) were in drug-free remission at year 10, with a median duration of 60 months across the arms.
Conclusions
Initial combination therapy with methotrexate, sulfasalazine and prednisone, or methotrexate and infliximab, is the most effective treatment strategy for ACPA-negative patients, resulting in earlier functional improvement than when on initial methotrexate monotherapy. After 10 years of targeted treatment, in all strategy-arms favourable clinical outcomes were achieved and radiographic progression was limited.
Trial registration number
NTR262, NTR265.
Keywords: Rheumatoid Arthritis, Ant-CCP, Treatment
Key messages.
What is already known about this subject?
It has been suggested that anticitrullinated protein antibodies (ACPA)-negative patients with rheumatoid arthritis (RA) would not need intensive treatment because ACPA-negative RA are less likely to develop joint damage and more likely to achieve drug-free remission than the ACPA-positive patients with RA.
What does this study add?
In active ACPA-negative patients with RA we found that initial combination therapy is the most effective treatment strategy. It results in earlier functional improvement than that with initial monotherapy, without additional adverse events.
After 10 years of targeted treatment there were no differences between outcomes of the four different strategy arms and radiographic progression was low.
How might this impact on clinical practice?
Treatment of all patients with early and active RA should focus on rapid relief of symptoms, and there is no reason to weigh the initial treatment choice based on the presence of ACPA.
Introduction
In patients with rheumatoid arthritis (RA), presence of anti-citrullinated protein antibodies (ACPA) is associated with worse clinical and radiographic outcomes, compared to ACPA-negative RA.1–6 It has been proposed that ACPA-negative RA is another disease entity than ACPA-positive RA7–9 and therefore requires a different treatment approach.10 However, it is not clear which treatment strategy, in particular which initial treatment choice, is most effective in ACPA-negative patients with RA. ACPA-negative patients have been suggested to not require combination therapy and not benefit from corticosteroids,10 but respond better to antitumour necrosis factor α (anti-TNF-α) agents than ACPA-positive patients.11–13
In the BeSt study, recent-onset active patients with RA were included and treated without ACPA status being known. Patients were randomised to one of four dynamic treatment strategies, all aiming to achieve low disease activity (disease activity score: DAS≤2.4). In a previous analysis of the BeSt study, we found that there were no significant differences in clinical response between ACPA-negative and ACPA-positive patients.6 Here, we aim to determine in further detail what is the most effective treatment strategy for ACPA-negative patients. We investigated which treatment strategy resulted in the most rapid clinical response, and the most favourable long-term clinical and radiographic outcomes for ACPA-negative patients.
Patients and methods
Study design and patients
The BeSt study (Dutch acronym for treatment strategies), a multicentre randomised clinical trial, enrolled 508 patients to compare four dynamic treatment strategies in patients with active (at least 6 inflamed joints and either a high erythrocyte sedimentation rate (ESR) or a high patient visual analogue scale (VAS) for disease activity) recent-onset RA according to the 1987 revised American College of Rheumatology (ACR) criteria.14 More study details have been previously published.15 16 The medical ethics committees of all the participating centres approved the study protocol, and all patients gave written informed consent.
Patients were randomised to: (1) sequential monotherapy, (2) step-up combination therapy, (3) initial combination including prednisone, (4) initial combination including infliximab. Strategy arm 1 and 2 both started with methotrexate (MTX) monotherapy. In strategy arm 3, patients started with MTX, sulfasalazine (SSA) and prednisone and in strategy arm 4, patients received MTX and infliximab. DAS were measured every 3 months. Treatment was targeted at low disease activity (DAS≤2.4). If low disease activity was not achieved, the next treatment step was taken. In case the DAS was ≤2.4 for ≥6 months, medication was tapered to a maintenance dose. If the DAS was then <1.6 for ≥6 months, medication was discontinued. As soon as DAS was ≥1.6, medication was restarted and further treatment steps were taken if DAS was >2.4 at a later visit.
ACPA were determined in a research setting using the anticyclic citrullinated peptide (anti-CCP2) test for 484 available serum samples that were collected at baseline and stored; for the remaining 24 patients, no serum sample was available. ACPA status did not influence treatment instructions according to the study protocol. For the current post hoc analysis, results of the four treatment strategies were compared within ACPA-negative patients.
Study end points
Primary outcomes were functional ability and radiographic joint damage progression. Functional ability was measured 3-monthly with the health assessment questionnaire (HAQ, range 0–3).17 Radiographic joint damage was assessed on radiographs of hands and feet, using the Sharp van der Heijde score (SHS, range 0–448).18 Radiographs were obtained yearly and were assessed in one session by two trained readers, blinded for patient identity, strategy arm and time order. Progression as a continuous measure was defined as an increase in SHS between two subsequent time points. Absence of progression was defined as <0.5 units increase in SHS and presence as ≥0.5 units increase in SHS.
DAS-remission percentages (defined as DAS<1.619), drug-free remission (DFR) percentages, toxicity and treatment response were secondary outcomes in this study. Toxicity included all reported (serious) adverse events ((S)AE). Treatment response to initial monotherapy and initial combination therapy were described for year 1 and 2 of follow-up. Treatment response was defined as success or failure from a specific treatment step. Success was defined as achieving and maintaining a DAS≤2.4 and failure was defined as a persistent DAS>2.4 or discontinuation of medication due to toxicity.
Early response was defined based on improvement in functional ability and the percentage of DAS-remission from 3 months after treatment start up to year 1. Radiographic progression during the first year was compared among the strategy arms. Long-term effect of the strategy arms was assessed based on the primary and secondary outcomes measured every 3 months or (for radiographic progression) yearly up to year 10.
Statistical analyses
Baseline characteristics and outcomes after 10 years were compared between the different treatment arms by the χ2 test, independent t test and analysis of variance, as appropriate. For the non-Gaussian distributed outcomes, the Kruskal-Wallis test or Mann-Whitney U test were used.
HAQ was compared at 3, 6, 9 and 12 months between the initial monotherapy arms (arm 1 and 2 combined) and the initial combination therapy arms (arm 3 and 4 combined) with an independent t test. Previous publications showed that arm 1 and 2 (monotherapy arms) had a similar response, and also responses in arm 3 and 4 (combination therapy arms) were comparable.15 20 Furthermore, HAQ was longitudinally analysed with linear mixed models (LMM). Determinants used for all longitudinal analysis were treatment group, time and its interaction term. This analysis was performed twice: for 1 year follow-up (0–1 year) to determine early response, and for the 10-year follow-up (0–10 year) to determine long-term outcomes. Generalised linear mixed models (GLMM) were used to analyse differences in DAS-remission percentages. Treatment group, time and its interaction term were entered as determinants. This analysis was also performed twice: for 0–1 year and for 0–10 year follow-up. The dropout rates were compared between the different treatment groups using Kaplan-Meier curves. Responses to the first, second and third treatment step in strategy arms 1 and 2, expressed as drug survival, are shown in the Kaplan-Meier curves.
SHS progression during the first year was compared with a Kruskal-Wallis test. SHS progression over 10 years was depicted in a cumulative probability plot, stratified for treatment strategy. SHS progression over time was analysed using a GLMM with SHS progression as binary outcome (defined as delta ≥0.5 units per year, yes/no). Treatment strategy, time and its interaction were entered as determinants.
On the one hand, the power calculation of the BeSt study was based on the total study population; we, however, only include a subpopulation (184 of 508). On the other hand, we performed multiple comparisons. These effects indicate that the p values should be interpreted in opposite directions. Therefore, we decided to adjust for neither of the effects.
Results
Baseline characteristics for 184 ACPA-negative patients (of 508 patients included in the BeSt study) were similar in the strategy arms. In agreement to the inclusion criteria, disease activity was high (mean±SD DAS 4.6±0.9) and functional ability considerably impaired (mean±SD HAQ 1.5±0.7; table 1). During the 10 year follow-up, 71/184 patients (39%) dropped out of the study, equally distributed among the strategy arms (p=0.738). 125/184 patients were both ACPA and rheumatoid factor (RF)-negative. There were also no significant differences in baseline characteristics between the treatment arms (see online supplementary table S1), nor in the comparison of ACPA-negative with RF-positive patients (data not shown).
Table 1.
Baseline characteristics
| Sequential monotherapy | Step-up therapy | Initial combination with prednisone | Initial combination with infliximab | |
|---|---|---|---|---|
| N=40 | N=45 | N=56 | N=43 | |
| Age (years), mean±SD | 56±15 | 53±15 | 57±13 | 53±16 |
| Female, n (%) | 30 (75) | 36 (80) | 38 (68) | 32 (74) |
| Symptom duration (weeks), median (IQR) | 19 (12–41) | 30 (16–52) | 22 (11–41) | 19 (13–31) |
| DAS, mean±SD | 4.6±0.9 | 4.7±0.8 | 4.5±0.8 | 4.6±1.0 |
| HAQ, mean±SD | 1.5±0.7 | 1.4±0.5 | 1.5±0.6 | 1.5±0.8 |
| RF positive, n (%) | 12 (30) | 12 (27) | 22 (39) | 13 (30) |
| Erosive disease, n (%) | 27 (68) | 28 (62) | 36 (64) | 28 (65) |
| Smoker, n (%) | 14 (35) | 11 (24) | 16 (29) | 10 (23) |
Erosive disease: >0.5 erosion score on radiographs of hands and feet based on the Sharp van der Heijde score. Radiographs were assessed by two independent readers, and the mean score of both readers was used.
DAS, disease activity score; HAQ, health assessment questionnaire (scale 0–3); RF, IgM rheumatoid factor.
rmdopen-2015-000143supp.pdf (110.5KB, pdf)
Early response
During the first year, functional ability improved earlier in patients treated with initial combination therapy (arms 3 and 4) than in patients treated with initial monotherapy (arms 1 and 2; figure 1A). After 3 months, mean (SD) HAQ was 0.98 (0.63) in the monotherapy arms vs 0.71 (0.64) (p=0.006) in the combination therapy arms, and after 6 months, these were 0.87 (0.68) versus 0.59 (0.57) (p=0.004). In the monotherapy arms, 64% of patients had a HAQ improvement >0.22 points (minimal important difference 21) after 3 months and 68% after 6 months, compared to 81% of patients and 82%, respectively, in the combination therapy arms (p=0.012 at 3 months and p=0.026 at 6 months). Probably as a result of continued DAS≤2.4 targeted treatment adjustments, from 9 months of follow-up onwards, no differences in functional ability were found between the strategy arms. At 9 months, mean (SD) HAQ was 0.81 (0.71) in the monotherapy arms and 0.63 (0.57) in the combination therapy arms (p=0.067), and at year 1, these numbers were 0.69 (0.69) and 0.57 (0.54) (p=0.195), respectively. In ‘double negative’ (ACPA-negative and RF-negative) patients early decrease in HAQ was seen in all the strategy arms and was significantly different between monotherapy arms and combination therapy arms at 3 months (p=0.024; see online supplementary table S2). When the monotherapy arms and combination therapy arms were combined, HAQ improved earlier in patients treated with combination therapy at 3 and 6 months (p=0.003 and p=0.010, respectively (see online supplementary table S3).
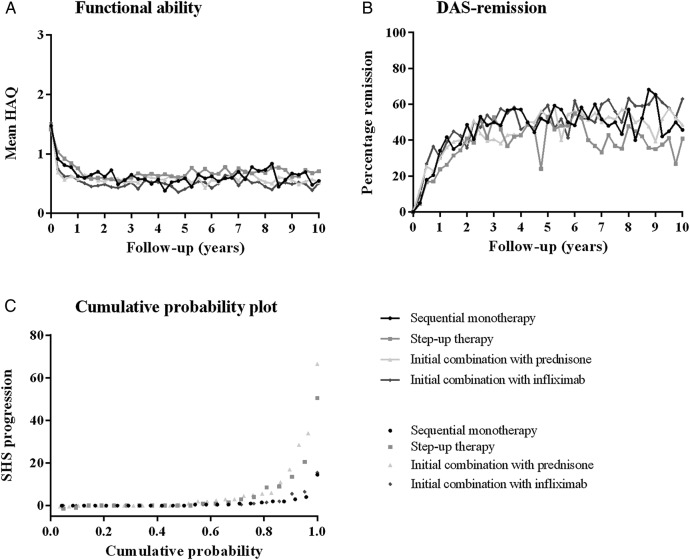
Figure 1.
(A) Functional ability, (B) DAS-remission percentages and (C) probability plot of radiographic joint damage progression from baseline to year 10 (completer analysis). (B) DAS-remission was defined as disease activity score (DAS) <1.6.19 Percentages reflect the number of patients in DAS-remission as part of the completers. More patients missed the visits before the yearly visits at year 5 and 10, because they were running behind on their schedule. Low attendance makes the DAS-remission percentages at these visits difficult to interpret. Mean disease activity did not show this decrease (data not shown). (C) Patients in strategy arms 1 and 4 had numerically less progression compared to strategy arms 2 and 3, statistically not significant (p=0.639). In strategy arms 1 and 4, patients with progression (defined as ≥0.5 SHS) had moderate disease activity during early visits (mean DAS±SD 2.99±1.14 at 3 months and 2.45±1.13 at 6 months) and 46% were rheumatoid factor (RF) positive. In strategy arms 2 and 3, patients with progression (defined as ≥0.5 SHS) had also moderate disease activity at early visits (mean DAS±SD 2.99±1.16 at 3 months and 2.46±1.14 at 6 months) and 42% were RF-positive. HAQ, health assessment questionnaire (range 0–3); SHS, Sharp van der Heijde score.
In the longitudinal analysis, over the first year of follow-up, level of functioning was similar between the four strategy arms (p=0.236). For ‘double negative’ patients, similar results were obtained (data not shown).
During the first year, higher percentages of DAS-remission (DAS<1.6) were found in strategy arms 3 and 4 than in strategy arms 1 and 2, but these were not significantly different (figure 1B): after 3 months, 5% in the monotherapy arms compared to 11% in the combination therapy arms achieved DAS-remission (p=0.119); after 6 months, 17% versus 25% (p=0.161); after 9 months, 18% versus 27% (p=0.116); and after 1 year, 27% versus 29% (p=0.833). Over the first year, no differences were found between the four strategy arms (p=0.472). There was no difference in CDAI remission (≤2.8) between the strategy arms during the first year (data not shown).
Radiographic progression during year 1 was low as expected, with median (IQR) progression scores of 0 (0–0), 0 (0–1), 0 (0–1) and 0 (0–0.5) in strategy arms 1–4, respectively (p=0.259).
Long-term outcomes
At year 10, mean (SD) DAS has decreased from 4.6 (0.9) at baseline to 1.6 (0.8), and HAQ from 1.5 (0.7) to 0.6 (0.6; more details in table 2). Over the 10-year follow-up, no differences in clinical outcomes were found. Functional ability was similar among the four strategy arms (p=0.551; figure 1A). The same was true for DAS-remission percentages (p=0.851; figure 1B). Similar results were obtained for double negative patients (data not shown). There was no difference in CDAI, DAS and HAQ during 10 years follow-up for patients who were treated with steroids from the beginning (arm 3) versus patients who were not treated with steroids from the beginning (arm1, 2 and 4) (data not shown).
Table 2.
Clinical and radiographic outcomes in the different strategy arms at year 10
| Sequential monotherapy | Step-up therapy | Initial combination with prednisone | Initial combination with infliximab | p Value | |
|---|---|---|---|---|---|
| N=40 | N=45 | N=56 | N=43 | ||
| Drop-out, n (%) | 14 (35) | 20 (44) | 21 (38) | 16 (37) | 0.738 |
| DAS, mean±SD | 1.7±0.9 | 1.8±0.8 | 1.6±0.8 | 1.4±0.8 | 0.431 |
| HAQ, mean±SD | 0.5±0.5 | 0.7±0.7 | 0.5±0.5 | 0.5±0.5 | 0.580 |
| DAS-remission, n (%) | 11 (28) | 9 (20) | 17 (30) | 17 (40) | 0.434 |
| Drug-free remission, n (%) | 7 (18) | 7 (16) | 11 (20) | 9 (21) | 0.742 |
| On initial treatment step, n (%) | 10 (25) | 7 (16) | 18 (32) | 15 (35) | 0.161 |
| Use of infliximab, n (%) | 3 (8) | 3 (7) | 4 (7) | 4 (9) | 0.978 |
| Use of prednisone, n (%) | 0 (0) | 0 (0) | 3 (5) | 2 (5) | 0.226 |
| SHS progression, year 0–10 Median (IQR) |
0.3 (0–1.4) | 0 (0–6.3) | 1.0 (0–5.3) | 0 (0–1.3) | 0.639 |
| SHS progression ≥5 units, n (%) | 1 (3) | 5 (11) | 8 (14) | 3 (7) | 0.132 |
| SHS progression ≥10 units, n (%) | 1 (3) | 3 (7) | 5 (9) | 1 (2) | 0.324 |
DAS, disease activity score; HAQ, health assessment questionnaire (scale 0–3); SHS, Sharp van der Heijde score.
During 10 years, DFR was achieved by 16/40 (40%), 15/45 (33%), 20/56 (36%) and 21/43 patients (49%) in strategy arms 1 to 4, respectively (p=0.453). In 5/16, 4/15, 6/20 and 7/21 patients in strategy arms 1 to 4, respectively (p=0.993), DFR was lost during follow-up. Of these patients 4/5, 3/4, 2/6 and 3/7 patients in strategy arms 1 to 4, respectively (p=0.704), achieved clinical DAS-remission again, with a median (IQR) of 1.0 (0.3–3.5) since loss of DFR. Only 1 patient in strategy arm 3 and 2 patients in strategy arm 4 achieved DFR after restart of medication. Table 2 shows DFR percentages at year 10.
Median (IQR) total SHS progression after 10 years of targeted treatment was low and similar between the four treatment groups in the study completers (p=0.639; table 2). Figure 1C shows the cumulative probability of SHS progression per strategy arm in ACPA-negative patients who completed follow-up. Over time, based on a generalised linear mixed model that takes into account all included patients, no difference in SHS progression (defined as delta ≥0.5 units per year) was found between the randomisation strategy arms: with strategy arm 1 as reference, ORs (95% CI) were 1.98 (0.60 to 6.47) for arm 2, 2.89 (0.96 to 8.72) for arm 3 and 1.66 (0.50 to 5.47) for arm 4 (p=0.082).
Response to initial monotherapy
Response to initial monotherapy in strategy arms 1 and 2 was explored during year 1 and 2. Eighteen out of 84 patients (21%) achieved the treatment target of low disease activity after 3 months, but 64/84 patients (76%) failed to respond to initial MTX monotherapy (and had to increase MTX dose according to the study protocol). Two patients stopped MTX because of an AE (nausea and headache; figure 2A). At 6 months, 39/84 patients (46%) achieved a DAS≤2.4 on MTX monotherapy. Thirty-six patients failed due to a DAS>2.4 (despite MTX dose increase at 3 months) and two patients failed due to an AE (not specified).
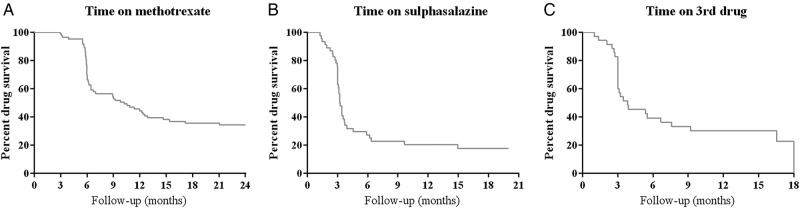
Figure 2.
Kaplan-Meier Curves showing drug survival in strategy arms 1 and 2. (A) Initial methotrexate monotherapy, n=84; (B) Switching to sulfasalazine monotherapy in strategy arm 1, adding sulfasalazine to methotrexate in strategy arm 2, n=46; (C) Switching to leflunomide monotherapy in strategy arm 1, adding hydroxychloroquine to methotrexate and sulpsalazine in strategy arm 2, n=35. Discontinuation of drugs is due to insufficient response, toxicity or other reasons. The lines indicate the percentage of patients in strategy arms 1 and 2 who are treated according to the concerned treatment step.
The second treatment step was taken in 46/84 patients: switching to (in strategy arm 1) or adding (in strategy arm 2) SSA. In 9/46 patients (20%), a DAS≤2.4 was achieved by this step (figure 2B). Failure on SSA therapy occurred in 33/46 patients because of a DAS>2.4, and in 4/46 patients because of an AE (skin/mucous, infection, nausea and malaise).
In total, 35/84 patients continued to the third treatment step during 2 years of follow-up: switching to leflunomide monotherapy (in strategy arm 1) or adding hydroxychloroquine to MTX and SSA (in strategy arm 2). In 9/35 patients (26%), a DAS≤2.4 was achieved (figure 2C). During 2 years of follow-up, 21/35 patients (60%) continued to the next treatment step due to a DAS>2.4. Five patients failed due to an AE (3 times gastrointestinal, malaise and skin/mucous). DAS-components that contributed to failure due to DAS>2.4 per treatment step are shown in online supplementary table S4.
After 1 year, 7/40 patients (18%) in strategy arm 1 continued to combination therapy (MTX and infliximab). During year 2, two additional patients continued to combination therapy. In strategy arm 2, 24/45 patients (53%) used combination therapy (MTX and SSA, step 3 in the study protocol) at the end of year 1. During year 2, only one more patient failed on monotherapy and continued to combination therapy. The difference in percentages of patients in combination therapy between strategy arms 1 and 2 can be explained by the design of the protocol: in strategy arm 1, the first option to receive combination therapy was the 3rd step after initial MTX treatment, while it was already the 2nd step in strategy arm 2.
Response to initial combination therapy
By the end of year 1, in strategy arm 3 (MTX, SSA and prednisone) 18/56 patients (32%) had tapered combination therapy to monotherapy of which three restarted with MTX during the second year. In strategy arm 4, 17/43 patients (40%) had discontinued infliximab. One of them restarted infliximab during the second year. For more detailed treatment responses to initial combination therapy during 2-year follow-up (strategy arms 3 and 4) flow charts are shown in the online supplementary figures S1 and S2.
Toxicity
During 10 years of follow-up, in total 1265 AEs were reported in 36/40, 39/45, 55/56 and 41/43 patients in strategy arms 1–4, respectively (p=0.113). The most common AE in all groups were upper airway infections, elevated liver enzymes, nausea and other gastrointestinal symptoms. SAE were reported in 25/40, 29/45, 27/56 and 22/43 patients in strategy arms 1 to 4, respectively (p=0.300; table 3). Ten patients died during the study; one in strategy arm 1, four in strategy arm 2, one in strategy arm 3 and four in strategy arm 4 (p=0.220) (details in table 3). (S)AE during year 1, when most patients in strategy arms 3 and 4 were still on combination therapy, are reported in table 3.
Table 3.
Number of reported adverse events and serious adverse events
| Sequential monotherapy | Step-up combination therapy | Initial combination with prednisone | Initial combination with infliximab | p Value | |
|---|---|---|---|---|---|
| N=40 | N=45 | N=56 | N=43 | ||
| 0–1 year follow-up | |||||
| AE, n* | 31 | 51 | 41 | 34 | 0.414 |
| SAE, n* | 3 | 3 | 6 | 1 | 0.400 |
| 0–10 year follow-up | |||||
| Total AE, n* | 293 | 292 | 368 | 312 | 0.872 |
| Patients with AE, n (%) | 36 | 39 | 55 | 41 | 0.113 |
| Total SAE, n* | 50 | 33 | 60 | 43 | 0.183 |
| Patients with SAE, n (%) | 25 (63) | 19 (42) | 27 (48) | 22 (51) | 0.300 |
| Patients with serious infection, n (%) | 9 (23) | 5 (11) | 5 (9) | 3 (7) | 0.124 |
| Patients with malignancy, n (%) | 3 (8) | 2 (4) | 8 (14) | 6 (14) | 0.310 |
| Deceased, n† | 1 | 4 | 1 | 4 | 0.220 |
*More events per patient possible.
†Causes of death, group 1: 1 ischaemic colon after complicated diverticulitis surgery; group 2: 1 lung carcinoma, 1 stomach cancer, 2 unknown; group 3: 1 lung carcinoma; group 4: 1 oesophagus carcinoma, 1 cardiac arrest, 1 lung carcinoma, 1 unknown.
AE, adverse event; SAE, serious adverse event.
Discussion
Previous literature suggests that ACPA-negative and ACPA-positive patients with RA may represent two different disease entities, which may require different treatment strategies.7–10 On one hand, as ACPA-negative patients are less likely to develop joint damage and more likely to achieve DFR,2 5 6 22 they may not need intensive treatment. On the other hand, with similar disease activity, functional disability is not related to ACPA status6 23 and to alleviate symptoms rapidly, the initial treatment choice is important. Roughly 50% of active patients with RA fail to achieve low disease activity within 6 months on methotrexate monotherapy.24 In RA (2010 criteria) and patients with UA in the PROMPT study, we showed that methotrexate was as effective as placebo in ACPA-negative patients.25 To establish the best initial treatment strategy in ACPA-negative patients with RA, we performed the current analysis in the BeSt study. Based on our results, all four strategy arms starting with either monotherapy or combination therapy have a comparable long-term effectiveness, with the only difference that an earlier functional improvement was achieved following initial combination therapy with the option to taper to monotherapy. Radiographic progression was generally low as expected in ACPA-negative patients and after 10 years of targeted treatment, without difference between the strategy arms.
These results expand on our previous report that compared clinical response between ACPA-positive and ACPA-negative patients in the BeSt study.6 Initial combination therapy appears to result in earlier clinical response in both groups of patients, and during subsequent treatment adjustments targeted at low disease activity (DAS≤2.4), clinical outcomes are roughly similar from month 9 of follow-up onwards. In this analysis, we showed that also ACPA-negative patients benefit from initial combination therapy, with a better functional ability at 3 and 6 months follow-up compared to patients treated with initial methotrexate monotherapy. This was also seen in seronegative (ACPA-negative and RF-negative) patients. This indicates that RF does not seem to predict treatment response. In ACPA-positive and ACPA-negative patients, treatment choices depend on positive effects that one aims to achieve, in relation to the possible negative effects. If treatment aims mainly at preventing long-term debilitating joint damage, one may argue that ACPA-negative patients require less intensive treatment and maybe a less stringent treatment target than the ACPA-positive patients. Likewise, ACPA-positive patients may require more intensive treatment and possibly a more stringent treatment target. If rapid relief of symptoms is the aim of initiating treatment, then initial combination therapy has the highest success rate. In the BeSt study, all patients were selected on having active RA, with ≥6/66 swollen and ≥6/68 painful joints and either an ESR>28 mm/h or a high VAS (≥20 mm) of global health. At baseline, ACPA-negative patients had an even slightly higher DAS and more severe functional disability than ACPA-positive patients.6 Compared to the 1987 criteria used in the BeSt study, the 2010 criteria instigate that primarily ACPA-negative patients with high tender and swollen joint counts will be classified as having RA.
Rapid symptom relief, associated with less work disability26 is an important treatment target. We have shown that only a minority of ACPA-negative patients respond to MTX monotherapy (despite a dose increase after 3 months), and that in case of failure, the response to SSA is even poorer. DAS components revealed a substantial inflammatory element in these failing patients. In contrast, a rapid decrease in disease activity is observed following initial combination therapy, with accompanied improvement in functional ability. These results point towards the favourable effects of initial combination therapy in patients with ACPA-negative RA. Registration of AEs and SAEs during the BeSt study did not show more toxicity in the initial combination strategy arms compared to the initial monotherapy arms.16 This may be related to the fact that after a rapid improvement, tapering and discontinuation was often possible. The earliest possibility to taper prednisone in strategy arm 3 (at week 28) was possible in 66% of patients and 32% subsequently tapered to SSA monotherapy. In strategy arm 4, discontinuation of infliximab to MTX monotherapy (by protocol possible first at month 9) occurred in 33% of patients and after 12 months, in 40%. To meet concerns on possible adverse effects of high-dose corticosteroids, although not objectified in this trial, more recent studies have shown that the initial dose of prednisone need not be as high as that used in the COBRA trial27 and subsequently in the BeSt study to achieve similar rapid suppression of disease activity.28–30
Given the fact that our data are derived from a subpopulation of the BeSt trial, there are several caveats. First, in the smaller ACPA-negative population, in relation to the power calculations carried out for the complete BeSt population, we may not have had sufficient power to detect differences between the treatment arms. To partly overcome this, we combined the results of arms 1 and 2, which used the same medication for the first 6 months of the trial, and results of arms 3 and 4. Although small numbers and lack of power may have resulted in underestimation of any differences between the treatment strategies, the significant differences between the 3 and 6 months efficacy of initial monotherapy and initial combination therapy remain. Second, the BeSt study only included patients with a high disease activity, including at least 6/66 swollen and 6/68 painful joints and either a high ESR or a high patient VAS for disease activity. Thus, it is unclear whether our conclusions would apply for ACPA-negative patients with less active disease. If symptoms are mild and functional impairment slight, patients may want to risk a delay in improvement to avoid combination therapy.
In conclusion, for ACPA-negative patients with RA, initial combination therapy with methotrexate and either sulfasalazine plus prednisone, or infliximab is the most effective treatment strategy. It results in early functional improvement, without additional AEs, compared to initial methotrexate monotherapy. We suggest that treatment of all patients with early and active RA should focus on rapid relief of symptoms, and that there is no reason to weigh the initial treatment choice based on the presence of ACPA.
Acknowledgments
The authors would like to thank all patients for their contribution as well as the following rheumatologists who participated in the BeSt study group: J van Aken (Spaarne Hospital, Hoofddorp); WM de Beus (Medical Center Haaglanden, Leidschendam); C Bijkerk (Reinier de Graaf Gasthuis, Delft); MHW de Bois (Medical Center Haaglanden, Leidschendam); H Boom (Spaarne Hospital, Hoofddorp); M de Buck (Medical Center Haaglanden, Leidschendam); G Collée (Medical Center Haaglanden, Leidschendam); BAC Dijkmans (retired); JAPM Ewals (retired); F Fodili (Fransiscus Hospital, Roosendaal); AH Gerards (Vlietland Hospital, Schiedam); RJ Goekoop (Haga Hospital, The Hague); YPM Goekoop-Ruiterman (Haga Hospital, The Hague); BAM Grillet (Zorgsaam, Terneuzen); JHLM van Groenendael (Franciscus Hospital, Roosendaal); KH Han (MCRZ Hospital, Rotterdam); JB Harbers (Fransiscus Hospital, Roosendaal); AL Huidekoper (Bronovo Hospital, The Hague); MV van Krugten (Admiraal de Ruyter Hospital, Vlissingen); L Lard (Medical Center Haaglanden, Leidschendam); H van der Leeden (retired); MF van Lieshout-Zuidema (Spaarne Hospital, Hoofddorp); A Linssen (retired); MC Lodder (Kennemer Gasthuis, Haarlem); PAHM van der Lubbe (Vlietland Hospital, Schiedam); C Mallée (Kennemer Gasthuis, Haarlem); ETH Molenaar (Groene Hart Hospital, Gouda); M van Oosterhout (Groene Hart Hospital, Gouda); AJ Peeters (Reinier de Graaf Gasthuis, Delft); HK Ronday (Haga Hospital, The Hague); D van Schaardenburg (VU Medical Center, Amsterdam); AA Schouffoer (Haga Hospital, The Hague); PEH Seys (retired); PBJ de Sonnaville (Admiraal de Ruyter Hospital, Goes); I Speyer (Bronovo Hospital, The Hague); KSS Steen (Kennemer Gasthuis, Haarlem); JPh Terwiel (retired); AE Voskuyl (VU Medical Center, Amsterdam); ML Westedt (Bronovo Hospital, The Hague); S ten Wolde (Kennemer Gasthuis, Haarlem); D van Zeben (Sint Franciscus Gasthuis, Rotterdam). The authors would also like to thank all other rheumatologists and trainee rheumatologists who enrolled patients in the BeSt study, and all research nurses for their contributions.
Footnotes
Contributors: GA and IM performed the statistical analysis, interpreted the data and drafted the manuscript. LD, NR and GSB contributed to the acquisition of the data and revised the manuscript. PK, WL and TH participated in the study design, contributed to the acquisition of the data and were involved in revising the manuscript. CA participated in the study design, contributed to the acquisition of the data, and was involved in the analysis and interpretation of the data, and helped to draft the manuscript. All authors read and approved the final version of the manuscript.
Funding: The study was designed by the investigators and supported by a government grant from the Dutch Insurance Companies, with additional funding from Schering-Plough B.V. and Janssen B.V. Data collection, trial management, data analysis and preparation of the manuscript were performed by the authors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The medical ethics committees of all participating centres approved the study protocol and all patients gave written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: GA and IM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. There is no additional unpublished data from this study.
References
- 1.Bas S, Genevay S, Meyer O et al. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology (Oxford) 2003;42:677–80. 10.1093/rheumatology/keg184 [DOI] [PubMed] [Google Scholar]
- 2.de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Verpoort KN et al. Progression of joint damage in early rheumatoid arthritis: association with HLA-DRB1, rheumatoid factor, and anti-citrullinated protein antibodies in relation to different treatment strategies. Arthritis Rheum 2008;58:1293–8. 10.1002/art.23439 [DOI] [PubMed] [Google Scholar]
- 3.Lindqvist E, Eberhardt K, Bendtzen K et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 2005;64:196–201. 10.1136/ard.2003.019992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer O, Labarre C, Dougados M et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 2003;62:120–6. 10.1136/ard.62.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronnelid J, Wick MC, Lampa J et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005;64:1744–9. 10.1136/ard.2004.033571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Broek M, Dirven L, Klarenbeek NB et al. The association of treatment response and joint damage with ACPA-status in recent-onset RA: a subanalysis of the 8-year follow-up of the BeSt study. Ann Rheum Dis 2012;71:245–8. 10.1136/annrheumdis-2011-200379 [DOI] [PubMed] [Google Scholar]
- 7.Daha NA, Toes RE. Rheumatoid arthritis: are ACPA-positive and ACPA-negative RA the same disease? Nat Rev Rheumatol 2011;7:202–3. 10.1038/nrrheum.2011.28 [DOI] [PubMed] [Google Scholar]
- 8.Klareskog L, Ronnelid J, Lundberg K et al. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol 2008;26:651–75. 10.1146/annurev.immunol.26.021607.090244 [DOI] [PubMed] [Google Scholar]
- 9.van der Helm-van Mil AH, Huizinga TW. Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res Ther 2008;10:205 10.1186/ar2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seegobin SD, Ma MH, Dahanayake C et al. ACPA-positive and ACPA-negative rheumatoid arthritis differ in their requirements for combination DMARDs and corticosteroids: secondary analysis of a randomized controlled trial. Arthritis Res Ther 2014;16:R13 10.1186/ar4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canhao H, Rodrigues AM, Mourao AF et al. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford) 2012;51:2020–6. 10.1093/rheumatology/kes184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher BA, Plant D, Lundberg K et al. Heterogeneity of anticitrullinated peptide antibodies and response to anti-tumor necrosis factor agents in rheumatoid arthritis. J Rheumatol 2012;39:929–32. 10.3899/jrheum.111315 [DOI] [PubMed] [Google Scholar]
- 13.Potter C, Hyrich KL, Tracey A et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis 2009;68:69–74. 10.1136/ard.2007.084715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 15.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2008;58(2 Suppl):S126–35. 10.1002/art.23364 [DOI] [PubMed] [Google Scholar]
- 16.Klarenbeek NB, Guler-Yuksel M, van der Kooij SM et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis 2011;70:1039–46. 10.1136/ard.2010.141234 [DOI] [PubMed] [Google Scholar]
- 17.Siegert CE, Vleming LJ, Vandenbroucke JP et al. Measurement of disability in Dutch rheumatoid arthritis patients. Clin Rheumatol 1984;3:305–9. 10.1007/BF02032335 [DOI] [PubMed] [Google Scholar]
- 18.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 19.Prevoo ML, van Gestel AM, vanT Hof M et al. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 1996;35:1101–5. 10.1093/rheumatology/35.11.1101 [DOI] [PubMed] [Google Scholar]
- 20.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med 2007;146:406–15. 10.7326/0003-4819-146-6-200703200-00005 [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Tugwell P, Kraag GR et al. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. J Rheumatol 1993;20:557–60. [PubMed] [Google Scholar]
- 22.van der Woude D, Young A, Jayakumar K et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum 2009;60:2262–71. 10.1002/art.24661 [DOI] [PubMed] [Google Scholar]
- 23.van der Helm-van Mil AH, Verpoort KN, Breedveld FC et al. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949–58. 10.1186/ar1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessels JA, van der Kooij SM, le Cessie S et al. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum 2007;56:1765–75. 10.1002/art.22640 [DOI] [PubMed] [Google Scholar]
- 25.van Dongen H, van Aken J, Lard LR et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2007;56:1424–32. 10.1002/art.22525 [DOI] [PubMed] [Google Scholar]
- 26.de Croon EM, Sluiter JK, Nijssen TF et al. Predictive factors of work disability in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2004;63:1362–7. 10.1136/ard.2003.020115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus T, Cutolo M. Clinical trials documenting the efficacy of low-dose glucocorticoids in rheumatoid arthritis. Neuroimmunomodulation 2015;22:46–50. 10.1159/000362734 [DOI] [PubMed] [Google Scholar]
- 28.de Jong PH, Hazes JM, Barendregt PJ et al. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: first results of the tREACH trial. Ann Rheum Dis 2013;72:72–8. 10.1136/annrheumdis-2011-201162 [DOI] [PubMed] [Google Scholar]
- 29.den Uyl D, ter Wee M, Boers M et al. A non-inferiority trial of an attenuated combination strategy (‘COBRA-light’) compared to the original COBRA strategy: clinical results after 26 weeks. Ann Rheum Dis 2014;73:1071–8. 10.1136/annrheumdis-2012-202818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ter Wee MM, den Uyl D, Boers M et al. Intensive combination treatment regimens, including prednisolone, are effective in treating patients with early rheumatoid arthritis regardless of additional etanercept: 1-year results of the COBRA-light open-label, randomised, non-inferiority trial. Ann Rheum Dis 2015;74: 1233–40. 10.1136/annrheumdis-2013-205143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2015-000143supp.pdf (110.5KB, pdf)