Abstract
Objective(s):
Herbal medicines are promising cancer preventive candidates. It has been shown that Punica granatum L. could inhibit angiogenesis and tumor invasion. In this study, we investigated whether the anti-angiogenic effect of pomegranate peel extract (PPE) is partly attributable to Peroxisome proliferator-activated receptors (PPARs) activation in the Human Umbilical Vein Endothelial Cells (HUVECs).
Materials and Methods:
Ethanol extract from PPE was prepared. HUVECs were treated in four groups (with PPE (10 μg/ml) alone, PPE with or without PPARγ (T0070907) and α (GW6471) antagonists, and control group). The possible effect of PPARs on angiogenic regulation was checked by Matrigel assay. The mRNA expression levels of vascular endothelial growth factor (VEGF) was detected by Quantitative reverse transcription-polymerase chain reaction (QRT-PCR).
Results:
PPE significantly inhibited both tube formation (size, length, and junction of tubes) and VEGF mRNA expression (P<0.05). Our results showed that the anti-angiogenic effects of PPE were significantly reversed by both PPAR antagonists (P<0.05). There was no difference between PPE plus antagonists groups and the control group.
Conclusion:
In summary our results showed that the anti-angiogenic effects of PPE could be mediated in part through PPAR dependent pathway.
Keywords: Angiogenesis, Peroxisome proliferator, activated receptors, (PPARs), Pomegranate, Vascular endothelial, growth factor
Introduction
Angiogenesis is the process of new blood vessel generation and is involved in the pathophysiology of a wide variety of diseases such as cancer (1, 2). Anti-angiogenic therapy represents a new approach to the early intervention and prevention of malignant disease (3). It is highly desirable to find dietary sources of anti-angiogenic molecules that have the ability to reduce cancer risk as well.
Punica granatum L. (pomegranate) has been known for its several beneficial effects including prevention and treatment of several types of cancer through its various metabolites (4).
Pomegranate peels are characterized by large amounts of phenolic compounds, and their great antioxidant potential (5). We previously showed that pomegranate peel extract (PPE) inhibits Human Umbilical Vein Endothelial Cells (HUVECs) angiogenesis, VEFG mRNA expression and secretion (6).
Previous studies have shown that PPE inhibited cell proliferation and angiogenesis marker expression (7, 8).
Recent research demonstrated that some parts of pomegranate inhibit angiogenesis via reduction of vascular endothelial growth factor (9). Also experimental studies have shown that the skin and arils of pomegranate extract inhibit tumor angiogenesis (10).
Although previous studies have proved the effectiveness of anti-angiogenic effect of pomegranate compartments but still the mechanisms underlying its anti-angiogenic activity and specially PPE remain unknown.
Some studies have shown that pomegranate carries out its therapeutic effects by affecting the Peroxisome proliferator-activated receptors (PPARs) pathway (11, 12).
PPARs are ligand-activated transcription factors that perform diverse metabolic functions (13). Three subtypes of PPARs (α, ß or δ, γ) have been recognized, each encoded by distinct genes and expressing in a different way in many parts of the body (14). The PPAR activators seem to inhibit tumor growth by several mechanisms including inhibition of angiogenesis (15).
PPARγ and PPARα have a wide range of events involving the vasculature, including atherosclerotic plaque formation and stability, vascular tone, and angiogenesis (16).
Recent studies showed that the PPAR-γ pathway may be a therapeutic target for numerous diseases in which excessive angiogenesis is implicated, including cancer (17, 18).
Hence, in this study, we investigated whether the anti-angiogenic effect of PPE is partly attributable to PPARs activation in the HUVECs. For this purpose, we used T0070907 as a selective ligand for PPARγ, and GW6471 as a PPAR alpha specific inhibitor(19, 20).
Materials and Methods
Preparation of pomegranate peel ethanol extract
Fresh pomegranate fruit was purchased from Agriculture research center of Isfahan, Iran. Pomegranate peels were dried and powdered and extracted with ethanol 70% containing 1% acetic acid at room temperature for 24 hr. The extract was prepared as described previously (6).
Total anthocyanin content was determined by the pH differential method (21). High-performance thin-layer chromatography method for the determination of ellagic acid as the major component of pomegranate was done at 280 nm using a TLC Scanner 3 (CAMAG, Muttenz, Switzerland) (22).
Cell culture
The HUVECs (National cell bank of Iran affiliated to Pasteur Institute, Tehran, Iran) were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 1% antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin), and 10% fetal bovine serum. The HUVECs were grown at 37 °C in humidified air with 5% CO2 incubator. When the cells were 70–80% confluent, they were treated with 0.25% trypsin and passaged to a new culture. The cells were grown at 37 °C in humidified air with 5% CO2 incubator. The experiments were conducted with cells from passages 2 to 6.
Angiogenesis assays
A total of 100 μL Matrigel Basement Membrane Matrix (Invitrogen, USA) was coated for 30 min at 37°C within a 24-well plate. The cells were detached by trypsin- EDTA, and after neutralization of trypsin, cells were resuspended in Medium 200PRF containing 10% FBS; then, HUVECs (1×105) in 4 groups were seeded on the Matrigel.
Each well was treated with different treatments, which included PPE (10 μg/ml), PPE + PPAR-γ antagonist (T0070907; 50 μmol/l), PPE + PPARα antagonist (GW6471; 50 μmol/l), and the control group (DMSO 0.1 %).
After 24 hr of incubation, Calcein AM cell-permeable dye was added to each well and photographed with a fluorescent microscope. Finally, tube length, size, and junction were quantified using the AngioQuant v1.33 software (The MathWorks, Natick, MA, USA).
Quantitative reverse transcriptase–polymerase chain reaction (QRT-PCR)
Total RNA was extracted from HUVEC using an RNeasy Mini plus Kit (Qiagen, Valencia, CA, USA), following the manufacturer’s protocols.
cDNA was synthesized using RevertAidTM Reverse Transcriptase (Fermentas, Vilnius, Lithuania) with oligo-dT primers as described before (6). QRT-PCR was performed using specific primers for VEGFA and GAPDH (as an internal control) mRNAs with the Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Vilnius, Lithuania) and run on the Rotor-gene 6000 (Qiagen, Hilden, Germany). The PCR cycling conditions for the genes included an initial denaturation step at 95 °C for 10 min, followed by 45 amplification cycles consisting of denaturation at 95 °C for 15 sec, annealing at 60 °C for 30 sec and an extension at 72 °C for 30 sec.
Primers for qRT-PCR:
GAPDH: AATGCATCCTGCACCACCAA
GAPDH: GTAGCCATATTCATTGTCATA
VEGF-A: GCAGAATCATCACGAAGTGG
VEGF-A: GCATGGTGATGTTGGACTCC
Statistical analysis
The experiments were performed in duplicate and replicated three times. At last, using SPSS 15 tests in one way ANOVA and LSD as post hoc (Bonferroni) analysis were performed. Values of P<0.05 were considered statistically significant.
Results
Antioxidant assay
Using the pH differential method for freeze dried PPE, its anthocyanin content equivalent to cyaniding-3-glucoside was detected as %1.77±1.80.
Dried extract of the pomegranate peel was standardized to contain %1.57±0.128 (g/100 g) ellagic acid.
Angiogenesis
For the assessment of the anti-angiogenic activity of PPE, HUVECs were added on Matrigel then treatment was started. Dimethyl sulfoxide (DMSO) 0.1% was used as control. The results showed that PPE (10 μg/ml) significantly suppressed the formation of tube-like structures (size, length, junction) compared the control (P<0.05) (Figure 1 and Table 1). PPAR antagonists completely antagonized the formation of the tube-like structures of HUVEC cells. There was no significant difference between the control group and PPE plus antagonists group (P>0.05).
Figure 1.
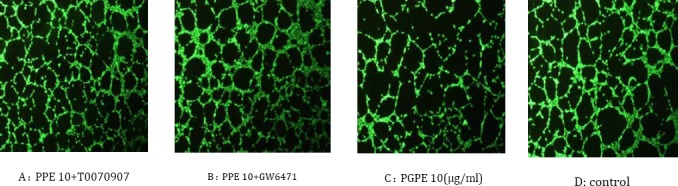
Morphological features of Human Umbilical Vein Endothelial Cells after different treatments. HUVECs were plated on a well coated with 100 μl of Matrigel basement membrane matrix. After they were treated with PPE for 24 hr, the cells were dyed and photographed with a Nikon camera attached to a fluorescent microscope at ×10 magnification. A: HUVECs were treated with PPE +T0070907, B: HUVECs were treated with PPE +GW6471, C: Treatment of the cells with PPE, D: control group
Table 1.
Endothelial tube size, length, and junction after treatment in different groups on Matrigel
| Size | Length | Junction | |
|---|---|---|---|
| Control | 32860±3404 | 8546±209 | 153±9 |
| PPE (10 μg/ml) | 18934±1800# | 4743±1214# | 47±24# |
| PPE+T0070907 | 43074±815* | 8767±695* | 214±52* |
| PPE+GW6471 | 35708±3297 | 8592±1382 | 215±23* |
| T0070907 | 27460±815 | 8830±695 | 214±52 |
| GW6471 | 19149±990 | 6650±835 | 82±40 |
Significant decrease compared to the control group (P<0.05);
Significant increase compared to the PPE group (P<0.05)
Effects of PPAR-γ and α antagonists on tube formation
To examine whether PPE inhibits angiogenesis through PPARs, we used PPAR antagonists. For this purpose, we used T0070907 as PPAR-γ antagonist and GW6471 as PPARα antagonist. Interestingly results have shown that treatment of the endothelial cells with PPE plus antagonists has led to significantly increased tube formation (Figure 1, Table 1).
PPE inhibits expression of VEGF mRNA in HUVECs but this action is suppressed by PPAR antagonists
After optimization of the qRT-PCR, expression of VEGFA and GAPDH genes was determined in the treated and control cells. Relative expression of VEGF gene was determined by dividing its expression amount to that of the GAPDH gene. PPE (10 μg/ml) significantly inhibits VEGF mRNA expression (P<0.05). Interestingly the cells that were treated by both of PPARs antagonists suppressed the PPE activity and there was significant increase in VEGF mRNA expression compared to control and PPE groups (P<0.05). Interestingly, these results confirm the results of Matrigel assay.
Discussion
The present study demonstrates that when HUVECs were treated simultaneously by PPE and PPARα- or PPARγ-antagonist, the anti-angiogenic effect of PPE suppressed and EC tube formation increased. Our qRT-PCR results also corroborate the results of Matrigel assay. After treatment by PPARs antagonists and PPE, increased VEGF mRNA expression raises the possibility that anti-angiogenic effect of pomegranate may be suppressed through the PPAR pathway.
We have recently demonstrated that PPE can suppress the formation of tube-like structures in (200, 300, and 400 μg/ml) doses(6), therefore, we examined the PPE (10 μg/ml) to find the minimal effective dose and for assessment of PPE anti-angiogenic potential in lower doses and the second probable mechanism.
Pomegranate peel is rich in estrogenic flavonoids that have been shown to be anti-angiogenic.
Previous studies indicate that pomegranate compartments, inhibit angiogenesis in cancer and human umbilical vein endothelial cell lines via downregulation of vascular endothelial growth factor (23).
Several in vitro and in vivo studies proposed that selective activation of PPARα and PPARγ promotes a vigorous angiogenic process by a mechanism dependent on VEGF stimulation (24, 25).
We defined the role of PPAR antagonist in suppressing the anti-angiogenic effect of PPE. The treatment of endothelial cells with PPE simultaneous PPAR antagonists showed that anti-angiogenic effects of PPE were inhibited. Further PPE anti-angiogenic properties may be involved by the PPARs-dependent mechanism.
PPARα and PPARγ, which are both expressed in endothelial cells, have several well-characterized roles in endothelial cells, including anti-inflammatory, antiproliferative, and anti-angiogenic effects (26, 27).
Figure 2.
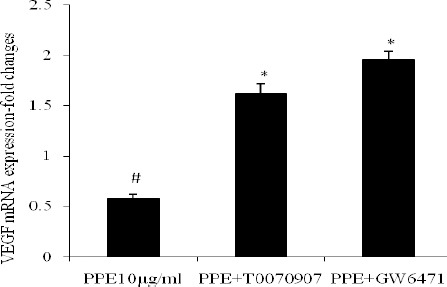
Quantitative analysis of mRNA expression of vascular endothelial growth factor in Human Umbilical Vein Endothelial Cells by real-time RT-PCR. After treatment of HUVECs by PPE alone or PPE with PPAR antagonists, total RNA was extracted. The relative expression of VEGF was normalized to GAPDH levels measured in the same RNA preparation. Data shown are from three independent experiments analyzed in duplicate. # Significant decrease compared to the control group. *Significant increase compared to the control group
PPARα has been shown to regulate the expression of the VEGF. Biscetti et al have indicated that WY14643 (PPARα agonist) promotes corneal angiogenesis in vivo and enhances endothelial tubulogenesis in vitro (28).
PPARα ligands might act as potent anti- angiogenic factors, for example, endothelial cells proliferation was avoided by fenofibrate through inhibiting cyclooxygenase-2 expression (29, 30).
PPARα agonists were shown to inhibit endothelial VEGFR2 expression by inhibiting Sp1-dependent promoter binding and transactivation (31). Finally, we have shown that PPARα and γ agonists inhibit endothelial tube formation (32).
Inhibition of VEGF, and VEGF receptor expression and mitogen-activated protein kinase-dependent activation are probable mechanisms of anti-angiogenic actions of PPARγ (33, 34). However, the anti-angiogenic mechanism of PPARγ activation in the endothelial cells remains currently unclear.
Hontecillas et al in their studies demonstrate that punicic acid, which is a conjugated linolenic acid isomer frequently found in pomegranate, binds and robustly activates PPARγ, increases PPARγ-responsive gene expression (12), furthermore, other pomegranate compartments such as flowers, have been shown to activate PPARs.
These findings indicate for the first time that PPAR-γ and α might be the molecular targets for PPE extract and provide a better understanding of the potential mechanism of the anti-angiogenic action of PPE.
Further in vivo and in vitro studies are needed to shed more light on the underlying mechanisms of PPE beneficial effects in cancer prevention and treatment.
Conclusion
It seems that the anti-angiogenic effects of PPE could be mediated in part through PPAR dependent pathway.
Acknowledgment
This study was supported by Isfahan University of Medical sciences (grant no.290110), Isfahan, Iran.
Conflicts interests
The authors declare that they have no competing interests
References
- 1.Mund JA, Shannon H, Sinn AL, Cai S, Wang H, Pradhan KR, et al. Human proangiogenic circulating hematopoietic stem and progenitor cells promote tumor growth in an orthotopic melanoma xenograft model. Angiogenesis. 2013;16:953–962. doi: 10.1007/s10456-013-9368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo SA, Kalluri R. Angiogenesis is controlled by miR-27b associated with endothelial tip cells. Blood. 2012;119:2439–2440. doi: 10.1182/blood-2012-01-403642. [DOI] [PubMed] [Google Scholar]
- 3.Li WW, Li VW, Hutnik M, Chiou AS. Tumor angiogenesis as a target for dietary cancer prevention. J Oncol 2012. 2012 doi: 10.1155/2012/879623. 879623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarfeshany A, Asgary S, Javanmard SH. Potent health effects of pomegranate. Adv Biomed Res. 2014;3:100. doi: 10.4103/2277-9175.129371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasoubi M, Barzegar MA, Sahari MHA. Total phenolic contents and antioxidant activity of Pomegranate (Punica granatum L.) Peel Extracts. J Agric Sci Technol. 2007;9:35–42. [Google Scholar]
- 6.Dana N, Haghjooy Javanmard Sh RL. Inhibition of vascular endothelial growth factor-induced angiogenesis by black Pomegranate peel extract and antiproliferative effect in melanoma cell line. Res Pharm Sci. 2015;10:117–124. [PMC free article] [PubMed] [Google Scholar]
- 7.Jeune MAL, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J Med Food. 2005;8:469–475. doi: 10.1089/jmf.2005.8.469. [DOI] [PubMed] [Google Scholar]
- 8.Khan GN, Gorin MA, Rosenthal D, Pan Q, Bao LW, Wu ZF, et al. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integr Cancer Ther. 2009;8:242–253. doi: 10.1177/1534735409341405. [DOI] [PubMed] [Google Scholar]
- 9.Jurenka JS. Therapeutic applications of pomegra-nate (Punica granatum L.) a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 10.Sartippour MR, Seeram NP, Rao JY, Moro A, Harris DM, Henning SM, et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int J Oncol. 2008;32:475–480. [PubMed] [Google Scholar]
- 11.Huang THW, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, et al. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol. 2005;207:160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Hontecillas R, O'Shea M, Einerhand A, Diguardo M, Bassaganya-Riera J. Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J Am Coll Nutr. 2009;28:184–195. doi: 10.1080/07315724.2009.10719770. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, et al. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem Pharmacol. 2011;82:464–475. doi: 10.1016/j.bcp.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Biscetti F, Straface G, Pitocco D, Zaccardi F, Ghirlanda G FA. Peroxisome proliferator-activated receptors and angiogenesis. Nutr Metab Cardiovasc Dis. 2009;19:751–759. doi: 10.1016/j.numecd.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 17.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 18.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee G, Elwood F, McNally J, Weiszmann J, Lindstrom M, Amaral K, et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem. 2015;277:19649–19657. doi: 10.1074/jbc.M200743200. [DOI] [PubMed] [Google Scholar]
- 20.Ding L, Liang X-G, Lou Y-J. Time-dependence of cardiomyocyte differentiation disturbed by peroxisome proliferator-activated receptor alpha inhibitor GW6471 in murine embryonic stem cells in vitro. Acta Pharmacol Sin. 2015;28:634–642. doi: 10.1111/j.1745-7254.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 21.Giusti MM, Rodríguez-Saona LE, Wrolstad RE. Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. J Agric Food Chem. 1999;47:4631–4637. doi: 10.1021/jf981271k. [DOI] [PubMed] [Google Scholar]
- 22.Jeganathan NS KK. HPTLC method for estimation of ellagic acid and gallic acid in Triphala churanam formulations. Res J Phytochem. 2008;2:1–9. [Google Scholar]
- 23.Toi M, Bando H, Ramachandran C, Melnick SJ, Imai A, Fife RS, et al. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesi. 2003;6:121–128. doi: 10.1023/B:AGEN.0000011802.81320.e4. [DOI] [PubMed] [Google Scholar]
- 24.Namba T, Oida H, Sugimoto Y, Kakizuka A, Negishi M, Ichikawa A, et al. cDNA cloning of a mouse prostacyclin receptor. Multiple signaling pathways and expression in thymic medulla. J Biol Chem. 1994;269:9986–9992. [PubMed] [Google Scholar]
- 25.Pola R, Gaetani E, Flex A, Aprahamian TR, Bosch-Marcé M, Losordo DW, et al. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: a possible role for peroxisome proliferator-activated receptors. J Mol Cell Cardiol. 2004;36:363–370. doi: 10.1016/j.yjmcc.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 27.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 28.Biscetti F, Gaetani E, Flex A, Aprahamian T, Hopkins T, Straface G, et al. Selective activation of peroxisome proliferator-activated receptor (PPAR)alpha and PPAR gamma induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes. 2008;57:1394–1404. doi: 10.2337/db07-0765. [DOI] [PubMed] [Google Scholar]
- 29.Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnés CM, Fannon M, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varet J, Vincent L, Mirshahi P, Pille J V, Legrand E, Opolon P, et al. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell Mol Life Sci. 2003;60:810–819. doi: 10.1007/s00018-003-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meissner M, Stein M, Urbich C, Reisinger K, Suske G, Staels B, et al. PPARalpha activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ Res. 2004;94:324–332. doi: 10.1161/01.RES.0000113781.08139.81. [DOI] [PubMed] [Google Scholar]
- 32.Dana N, Javanmard SH, Fazilati M, Pilehvarian AA. A comparison of peroxisome proliferator-activated receptor-α agonist and antagonist on human umbilical vein endothelial cells angiogenesis. Adv Biomed Res. 2013;2:54. doi: 10.4103/2277-9175.115792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goetze S, Bungenstock A, Czupalla C, Eilers F, Stawowy P, Kintscher U, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension. 2002;40:748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Campbell SC, Bedford DF, Nelius T, Veliceasa D, Shroff EH, et al. Peroxisome proliferator-activated receptor gamma ligands improve the antitumor efficacy of thrombospondin peptide ABT510. Mol Cancer Res. 2004;2:541–550. [PubMed] [Google Scholar]


