Abstract
Objective(s):
Hyperglycemia mediated oxidative stress plays a key role in the pathogenesis of diabetic complications like nephropathy. In the present study, we evaluated the effect of ethanolic extract of Ensete superbum seeds (ESSE) on renal dysfunction and oxidative stress in streptozotocin-induced diabetic rats.
Materials and Methods:
Glucose, HbA1c, total protein, albumin, renal function markers (urea, uric acid and creatinine), and lipid peroxidation levels were evaluated. Renal enzymatic and non-enzymatic antioxidants were examined along with renal histopathological study.
Results:
ESSE (400 mg/kg BW t) administration reduced glucose and HbA1c, and improved serum total protein and albumin in diabetic rats. ESSE in diabetic rats recorded decrement in renal function markers and renal lipid peroxidation products along with significant increment in enzymatic and non-enzymatic antioxidants. Renal morphological abnormalities of diabetic rats were markedly ameliorated by E. superbum.
Conclusion:
These results suggest that the antioxidant effect of E. superbum could ameliorate oxidative stress and delay/prevent the progress of diabetic nephropathy in diabetes mellitus.
Keywords: Antioxidant, Diabetes mellitus, Diabetic nephropathy, Ensete superbum Nephroprotection, Streptozotocin
Introduction
Diabetes Mellitus (DM) is a debilitating and often life-threatening disorder with increasing incidence throughout the world (1). It is a chronic metabolic disease characterized by hyperglycemia, resulting from insufficient or inefficient insulin secretion, with changes in carbohydrate, protein and lipid metabolism (2). Diabetes and diabetes-related complications represent one of the most central health problems worldwide, and according to recent estimations, it is likely to get worse to critical levels in the next decades, with the great concern that this disease is rising rapidly in children and adolescents (3).
Chronic hyperglycemia is a crucial factor in the development of diabetic complications such as kidney diseases, heart diseases, retinopathy and neuropathy. Direct or indirect consequence of hyperglycemia-mediated overproduction of reactive oxygen species (ROS) is the common pathophysiology shared by microvascular complications of diabetes. Microvascular deterioration is avertable either by the inhibition of superoxide accumulation or by modulating the blood glucose levels and among the microvascular complications of diabetes, nephropathy can be improved by antioxidants (4, 5).
The major complication of diabetes is diabetic nephropathy, a leading cause of end-stage renal failure in many developed countries, and a condition that accounts for significant morbidity and mortality. In diabetes, renal dysfunction develops through a number of metabolic pathways, characterized by functional as well as structural abnormalities of the kidneys. It is characterized by a deterioration of the renal function and changes in the glomerular structure, including thickening of basement membrane, glomerular hypertrophy and mesangial expansion (6-8).
Several mechanisms have been postulated for the progression of diabetic nephropathy including advanced glycation end products accumulation that stimulate mesangial cells to produce extracellular matrix (ECM), oxidative stress, acceleration of the polyol pathway, and hemodynamic changes. Markers of oxidative stress and reduced levels of antioxidants have been found in tissues and/or blood, including kidney, in both human and experimental animals in diabetes (9, 10).
Therefore, interventions favoring the ROS scavenging and/or depuration (dietary and pharmacological antioxidants) prevent or attenuate the oxidative stress, thereby ameliorating against the subsequent renal damage (11).
Current conventional therapies of diabetes using blood glucose-lowering medications have restrictions in averting the development of renal diseases. Presently, research to develop drugs that slow the progression of diabetic kidney damage with fewer side effects is being conducted, however, showing no significant outcome (12). This has led to increasing consideration of complementary and alternative medicine from natural sources having potent antidiabetic as well as nephroprotective effect with fewer side effects.
Indian rural and folklore ethnomedicinal practices include usage of numerous relatively unidentified medicinal plants with scientifically non-characterized pharmacological activities. One such less exploited folklore plant is Ensete superbum popular in Western Ghats of India, which is consumed as an anti-diabetic therapeutant by tribes and by the local populace. E. superbum has been reported to have a broad range of therapeutic and nutritional values.
E. superbum (Roxb.) Cheesm., (Wild/Rock Banana) belongs to the family Musaceae, commonly known as ‘cliff banana’. Indigenous communities consume its flowers, fruits and stem as a vegetable (13-15). In Ayurvedic system of medicine, the pseudostem and seeds of E. superbum were used for the treatment of various human ailments like diabetes, kidney stone (16), leucorrhea (17), measles (18), and stomach ache (19). However, there were no records of systematic pharmacological studies that support this claim. Though there is no scientific evidence for the antidiabetic and nephroprotective effects of E. superbum, tribal people have been using this plant in the management of DM. Preliminary in vitro studies revealed the antioxidant potential of E. superbum seeds. The aim of the present study is to ascertain the scientific basis for the use of E. superbum in the management of diabetes. Keeping in view the protective effect of E. superbum in DM, the present study was undertaken to explore the nephroprotective activity of E. superbum seeds in streptozotocin-induced diabetic rats.
Materials and Methods
Plant material and extraction
Seeds of E. superbum were collected from Wayanad, Kerala, India and authentically identified by Dr Valsaladevi (Curator, Department of Botany, University of Kerala, Kerala, India). The seeds were dried and then powdered by a mechanical grinder. The hard outer coverings of seeds were removed. The weighed seed powder was extracted with ethanol. The whole extract (ESSE) was collected, filtered and further concentrated in vacuum under pressure using rotary flash evaporator. The dried extract was suspended in distilled water and used for experimental study.
Chemicals
All chemicals and biochemicals used in this study were of analytical grade and obtained from Sigma-Aldrich (St. Louis, MO, USA), Merck chemical company (Darmstadt, Germany) and Sisco Research Laboratories (Mumbai, India).
Experimental animals
Normal healthy male Wistar albino rats (220–240 g) were used for this study. The animals were housed in polypropylene cages in a room with temperature maintained at 25 ±2 °C and a 12:12 hr light and dark cycle. Animals were fed with laboratory chow (Hindustan Lever Limited Lab diet) and water ad libitum. The protocol of this study was approved by Institutional Animal Ethics Committee.
Experimental induction of diabetes
Diabetes was induced in overnight fasted rats by a single intraperitoneal injection of freshly prepared Streptozotocin (STZ, 40 mg/kg body weight) in 0.1 M citrate buffer of pH 4.5 (20). Rats were given 5% of glucose in drinking water for the first 24 hr to encounter any initial hypoglycemia. Animals were allowed free access to feed and water after the injection. Hyperglycemia was allowed to develop over a minimum period of 48 hr. After 48 hr, animals showing marked hyperglycemia (RBG>250 mg/dl) were selected for the study.
Experimental design
Rats were divided into four groups comprising six rats in each group. Group I was normal control rats; group II consisted of normal rats treated with E. superbum seed extract at a dose of 400 mg/kg body weight; group III consisted of STZ-induced diabetic rats; group IV consisted of STZ-induced diabetic rats treated with ESSE at a dose of 400 mg/kg body weight. On the fourth day after STZ injection, ESSE treatment was started and this was considered as the first day of treatment. Intragastric administration of ESSE was continued for 60 days. At the end of the experimental period, the rats were fasted overnight and sacrificed. Blood was collected in clean, dry test tubes and centrifuged at 3000 rpm for about 10 min to obtain blood serum. Serum samples were separated immediately and used for the analysis of serum biochemical parameters. Kidneys were collected in ice-cold containers for analysis of various biochemical parameters.
Analytical methods
Biochemical parameters
Blood Glucose, total protein, albumin and renal function markers such as urea, uric acid, creatinine and blood urea nitrogen (BUN) were determined spectrophotometrically using commercial diagnostic kits under the instructions provided by the manufacturer (Erba diagnostics, India). Glycosylated hemoglobin (HbA1c) was estimated by commercial diagnostic kit based on ion exchange method (21).
Enzymatic and non-enzymatic Antioxidants
The antioxidant enzymes in kidney homogenate were evaluated by standard procedures (22-26). Superoxide dismutase (SOD) was determined by the method of Kakkar et al, catalase (CAT) by the method of Maehly and Chance, Glutathione peroxidase (GPx) by the method of Agerguard and Jense and Glutathione reductase (GRd) activity was determined by the procedure of David and Richard. Renal glutathione content (GSH) was determined according to the method of Patterson and Lazarow.
Lipid peroxidation
The concentration of Thiobarbituric Acid Reactive substances (TBARs) in renal tissue was estimated by the method of Ohkawa et al (27). Hydroperoxides (HP) and conjugated dienes (CD) were estimated by the method of John and Steven (28).
Histopathological analysis
After the experimental period, rats were anesthetized and the kidneys were removed and preserved in 10% formalin. Dehydration and clearing of the tissues were performed. 5 µm thick sections were prepared and stained with hematoxylin and eosin (H/E). Stained sections were quantitatively evaluated using a digital microscope
(Labomed, iVu 3000, USA). The images were analyzed using the Digipro software (Germany).
Statistical analysis
The results were analyzed using SPSS (version 17). The values were presented as mean±SD. Data were evaluated by analysis of variance (ANOVA). Duncan’s post hoc multiple comparison tests were used to determine significant differences among groups. P<0.05 was considered as statistically significant.
Results
Ratio of kidney weights to body weight of various groups of rats was calculated to assess the renal hypertrophy. Table 1 shows that in diabetic rats, there was a significant increase in the ratio, which indicates kidney enlargement. Moreover, ESSE treatment led to significant reduction in kidney enlargement.
Table 1.
Effect of ESSE (Ensete superbum seeds extract) on renal hypertrophy, HbA1c, TP and albumin
| Experimental Groups | Renal Hypertrophy (*10-3) | HbA1c(%) | Total protein (g/dl) | Albumin (g/dl) |
|---|---|---|---|---|
| I | 5.78±0.52 | 4.92±0.45 | 6.74±0.62 | 4.00±0.36 |
| II | 5.68±0.52 | 4.72±0.43 | 6.85±0.62 | 4.41±0.40 |
| III | 15.93±1.46a | 9.23±0.84a | 4.51±0.41a | 2.39±0.22a |
| IV | 12.77±1.16b | 5.67±0.62b | 5.95±0.54b | 3.56±0.32b |
Values are expressed as mean±SD of six rats.
indicates values are significantly different from Group I.
indicates values are significantly different from Group III, P<0.05.
The blood glucose levels in the diabetic group were significantly increased in comparison to the controls. Administration of ESSE significantly (P<0.05) reduced blood glucose in diabetic rats (Figure 1). Diabetic rats showed a significant increase in the level of glycosylated hemoglobin (HbA1c) as compared to normal rats. Administration of ESSE to diabetic rats significantly reduced the HbA1c level (Table 1). Total protein and albumin levels were found to be significantly decreased in the STZ-diabetic group as compared with the normal control group. The administration of ESSE increased the total protein and albumin levels in diabetic rats.
Figure 1.
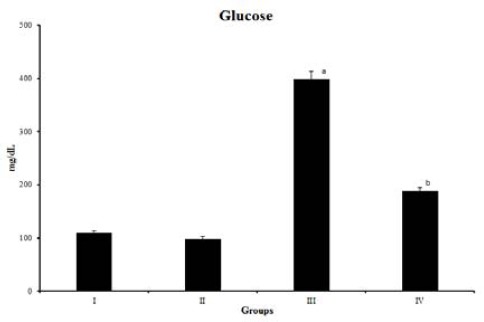
Blood glucose levels in normal and diabetic rats. Values are expressed as mean±SD of six rats, P<0.05. ‘a’ indicates values are significantly different from Group I. ‘b’ indicates values are significantly different from Group III. Group I- Normal, Group II- Normal + ESSE, Group III- diabetic control, Group IV- diabetic +ESSE ESSE (Ensete superbum seeds extract)
significantly as compared with the STZ induced diabetic group (Table 1).
The serum levels of renal function markers, i.e. urea, uric acid, creatinine, and BUN were significantly increased in diabetic rats as compared to the control rats. Diabetic rats treated with ESSE showed markedly decreased levels of these markers (P<0.05) (Table 2).
Table 2.
Effect of ESSE (Ensete superbum seed extract) on renal function markers
| Experimental Groups | Urea (mg/dl) | BUN (mg/dl) | Creatinine (mg/dl) | Uric acid (mg/dl) |
|---|---|---|---|---|
| I | 34.50±3.15 | 15.64±1.43 | 0.88±0.08 | 3.45±0.31 |
| II | 31.27±2.85 | 14.61±1.33 | 0.79±0.07 | 3.27±0.30 |
| III | 49.54±4.52a | 21.25±1.94a | 2.95±0.27a | 7.47±0.68a |
| IV | 41.39±3.78b | 17.33±1.77b | 1.91±0.18b | 6.22±0.57b |
Values are expressed as mean±SD of six rats.
indicates values are significantly different from Group I.
indicates values are significantly different from Group III, P<0.05
In the diabetic group, the activities of renal antioxidant enzymes (CAT, SOD, GPx, and GRd) were lower, suggesting that these rats suffered from oxidative stress. Treatment with ESSE significantly (P<0.05) enhanced activities of these enzymes in diabetic rats (Table 3). The concentration of renal GSH was significantly decreased in diabetic rats when compared to the control group. Administration of ESSE to diabetic rats tends to increase the GSH level.
Table 3.
Effect of ESSE (Ensete superbum seed extract) on enzymatic and non-enzymatic antioxidants
| parameters | Experimental groups | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Catalase | 6.68±0.61 | 7.62±0.70 | 2.09±0.19a | 4.32±0.39b |
| SOD | 2.23±0.21 | 2.60±0.24 | 0.55±0.05a | 1.61±0.15b |
| GPx | 14.96±1.36 | 17.88±1.63 | 6.53±0.59a | 11.09±0.59b |
| GRd | 25.65±2.34 | 27.33±2.43 | 11.60±1.05a | 20.31±1.68b |
| GSH content | 215.82±19.70 | 226.53±20.58 | 138.81±12.67a | 194.37±17.73b |
Values are expressed as mean±SD of six rats.
indicates values are significantly different from Group I.
indicates values are significantly different from Group III, P<0.05
CAT: Catalase expressed as *10-3 units/mg protein; SOD: Superoxide dismutase; GPx: Glutathione Peroxidase and GRd: Glutathione Reductase are expressed as units/mg protein.; GSH content is expressed as mM/100 g tissue
Lipid peroxidation products such as TBARS, HP and CD levels in the kidneys are presented in Table 4. A significant increase in lipid peroxidation levels was observed in the kidney of diabetic rats as compared to those of the control. Supplementation with ESSE induced a significant decrease (P<0.05) in lipid peroxidation levels in the kidney of diabetic rats compared to the untreated diabetic group.
Table 4.
Effect of ESSE (Ensete superbum seed extract) on lipid peroxidation products
| parameters | Experimental groups | |||
|---|---|---|---|---|
| I | II | III | IV | |
| TBARs | 0.91±0.08 | 0.89±0.08 | 1.33±0.10a | 0.97±0.09b |
| HP | 20.58±1.62 | 17.52±1.51 | 43.61±2.14a | 35.56±1.88b |
| CD | 35.58±3.25 | 36.14±3.30 | 70.39±6.42a | 56.98±5.21b |
Values are expressed as mean±SD of six rats.
indicates values are significantly different from Group I.
indicates values are significantly different from Group III, P<0.05. Concentrations of TBARs, HP and CD are expressed as mM/100 g tissue. TBARs: Thiobarbituric Acid Reactive substances; HP: Hydroperoxides; CD: conjugated dienes
Histopathological findings of the kidneys (Figure 2) revealed that STZ induced diabetic rats have degenerated renal corpuscles, glomerular hypertrophy, mesangial expansion, and hypercellularity. The tubules are dilated and atrophic. Mild interstitial edema is noted in STZ induced diabetic rats. While the kidneys of normal rats and normal rats treated with extract showed normal kidney morphology, kidneys of diabetic rats treated with E. superbum showed normal glomerulus and tubulointerstitial area.
Figure 2.

Photomicrographs of kidney stained with Hematoxylin-Eosin (10x) of control, diabetic and treated rats: Group I – Normal control; Group II – Normal + ESSE; Group III – Diabetic control- showed mesangial expansion, dilated-atrophic tubules, degenerated renal corpuscles, and mild interstitial edema; Group IV – Diabetic + ESSE (Ensete superbum seed extract).
Discussion
Diabetes Mellitus (DM) is probably world’s largest growing metabolic disease with micro and macro vascular complications, and results in significant morbidity and mortality. As diabetes is a multifactorial disease which leads to complications, it demands a multiple therapeutic approach. Since hyperglycemia-mediated oxidative stress seems to be the main cause of diabetic complications (29, 30), an ideal antidiabetic drug should combine both hypoglycemic and antioxidant properties. Drug regimens currently available for management of DM have certain drawbacks and therefore, there is a requirement for safer and more effective antidiabetic drugs, which are products that naturally contain compounds conferring health benefits. Hence, the aim of our study was to find out the scientific evidence for the safe use of the E. superbum seeds to treat and manage DM and diabetic nephropathy.
In our study, STZ was used to induce Diabetes mellitus in rats. It is the widely accepted animal model and reported to resemble human hyperglycemic DM, and is often associated with kidney hypertrophy that may contribute to end-stage renal damage, hepatotoxicity, oxidative stress, and hypercholesterolemia (31, 32). Our data revealed that there was a marked elevation in the kidney weight of the diabetic rats compared to that of the control. The degree of renal hypertrophy was expressed as the ratio of the weight of the two kidneys to the total body weight. The ratio was found to be elevated in diabetic rats (33). This may be due to enlargement of tubular cell lining, fatty infiltration, large hemorrhagic area, and lymphocyte infiltration in hyperglycemic rats (34). Administration of ethanolic extract of seeds of E. superbum at 400 mg/kg BWt decreased the kidney / b.wt. ratio to the near normal value, thus proving the ameliorative activity of ESSE in diabetic rats by maintaining the renal cell histoarchitecture, and may also be due to the improvement in glycemic control.
The fasting blood glucose level is an important basal parameter for monitoring diabetes (35). It has shown that supplementation of ESSE to diabetic rats causes the reduction in blood glucose level. The significant decrease in the level of fasting blood glucose in the diabetic group might be explained by the stimulation of the residual pancreatic mechanism, partial regeneration or protection of pancreatic cells, potentiating insulin secretion from protected β-cells of the islets of Langerhans (36), and probably by increasing peripheral utilization of glucose (37).
HbA1c is a marker for estimating the severity of DM and the degree of protein glycation in DM. HbA1c was found to be increased in patients with DM and the level is directly proportional to the blood glucose level. During DM, the excess glucose existing in the blood reacts with hemoglobin to form HbA1c. Administration of ESSE to diabetic rats reduced the glycosylation of hemoglobin by virtue of its hypoglycemic activity and thus decreased the levels of glycosylated hemoglobin in diabetic rats. This normalization of glycosylated hemoglobin indicates decreased glycation of proteins.
Associated with the progression of diabetes, a state of decreased total protein and albumin concentration is evidenced, which may have resulted from either amplified protein catabolism and/or hyperfiltration induced diabetic nephropathy. Reduction in total protein and albumin levels were noticed in diabetic rats and this is consistent with the previous reports (38). The decline in protein and albumin may be due to microalbuminuria and proteinuria, which are important clinical markers of diabetic nephropathy (33, 39). The results of the present study demonstrated that the treatment of diabetic rats with the ESSE caused a noticeable elevation in the serum total protein and albumin levels.
STZ administration elevated renal function markers, i.e. the serum urea, BUN, uric acid, and creatinine levels indicating progressive renal damage, which is taken as an index of altered Glomerular Filtration Rate (GFR) in diabetic nephropathy (33, 40). DM causes renal damage also due to abnormal glucose regulation, with elevated glucose and glycosylated protein levels, hemodynamic changes within the kidney tissue, and increased oxidative stress (41). The STZ-induced diabetic rats exhibited significantly higher serum urea, BUN, uric acid and creatinine levels compared to the control. However, the ESSE supplementation lowered these values to a control range. Thus, it would appear that the ESSE supplementation for 60 days could lower the serum urea, BUN, uric acid, and creatinine levels, and thus by enhancing the renal function that is generally impaired in diabetic rats.
Experimental research established the role of oxidative stress, a central factor in onset and progression of diabetic nephropathy. Oxidative stress influences the pathogenesis of DN not only through overproduction of ROS but also through the reduction of antioxidant enzyme activities, the formation of lipid peroxides, and non-enzymatic protein glycosylation. An imbalance between antioxidants system and the production of ROS is believed to be involved in diabetes-induced renal failure (42). There have been reports that the formation of reactive oxygen intermediates is associated with decreased Glomerular Filtration Rate (43).
Elevation in lipid peroxidation is attributed to the enhanced production of ROS. Induction of diabetes caused a significant elevation of lipid peroxidation products such as TBARs, HP and CD. In the present study, a significant elevation in lipid peroxidation products such as TBARs, HP and CD, was observed in diabetic rats. Renal TBARs, HP and CD levels were significantly lower in the ESSE treated diabetic group compared to diabetic control rats. The above result proposes that the ESSE may exert antioxidant efficacy and protect the tissues from lipid peroxidation.
Antioxidant enzymes are the defense system that protect against cellular and tissue injury. CAT is responsible for the scavenging or detoxification of H2O2, whereas GPx scavenges H2O2 and lipid peroxides. SOD, a principal antioxidant enzyme for the elimination of superoxide anion, dismutates superoxide into molecular oxygen and hydrogen peroxide. The product hydrogen peroxide is further detoxified to water by the enzymes CAT or GPx. In addition, GPx is engaged in diminution of highly cytotoxic products like lipid-peroxides and other organic hydroperoxides. Reduced glutathione (GSH), is an essential cosubstrate for the activity of GPx. GPx oxidizes the GSH into oxidized glutathione. GRd recycled oxidized glutathione back to glutathione, through an NADPH-consuming process (44). Hence, activities of these key enzymatic antioxidants i.e., CAT, SOD, GPx, and GRd were diminished during oxidative stress. Since diabetes state increases oxidative stress, activities of these enzymatic antioxidants were found to be decreased in diabetic rats. However, treatment with ESSE significantly enhanced the activities of enzymatic antioxidants, which are evident from the lower lipid peroxidation level in the kidney of diabetic rats. In this context, various studies have reported the diminished activities of these enzymatic antioxidants in the diabetic kidney and several compounds with antioxidant activity have been shown to improve the activity of these enzymes.
GSH is a major endogenous antioxidant that helps to counterbalance free radical-mediated damage. Some studies reported that the GSH level in diabetic kidney was found to be significantly reduced, suggesting that the GSH may play a role in the development of diabetic complications (45, 46). GSH functions as a direct scavenger of free radicals, a co-substrate for GPx activity and also as a cofactor for many enzymes (47). GSH protects normal cell structure and function by keeping the redox homeostasis, participating in detoxification reactions, and quenching of free radicals. The reduction in tissue GSH could be the result of increased degradation of GSH by oxidative stress or decreased synthesis in diabetes (48). Studies have shown that in STZ-induced diabetic rats, the tissue GSH concentrations are significantly lower when compared with the control rats (49). In the present study, elevation in renal GSH levels was observed in ESSE treated diabetic rats. This indicates that ESSE can either increase the GSH biosynthesis or diminish the oxidative stress that leads to less degradation of GSH or has both effects. Treatment with E. superbum amplified the activity of antioxidant enzymes and thus may help to avoid increased formation of lipid peroxidation products during diabetes.
The biochemical parameters were correlated with the renal histopathological studies. STZ induced diabetes triggered a significant damage in renal structure showing glomeruli and tubular damages, mesangial expansion due probably to the generation of reactive radicals and to subsequent lipid peroxidation. The major morphological abnormalities observed in our study were tubular cell swelling, glomerular hypertrophy, hypercellularity, mesangial expansion, dilated-atrophic tubules, degenerated renal corpuscles, and mild interstitial edema. The administration of ESSE to diabetic rats improved the histological alterations induced by STZ, which could be attributed to its antioxidant/antiradical effects.
Conclusion
The present study reveals hypoglycemic and nephroprotective nature of E. superbum seeds in streptozotocin-induced diabetic rats. The protective effects are, possibly, due to the decline in the free radical generation. ESSE treatment in streptozotocin-induced diabetic rats exhibited a prominent ameliorative potential, probably by mitigating hyperglycemia-mediated oxidative stress, thereby assuaging the basic alterations in kidneys. Further detailed studies are in progress to elucidate the detailed mechanism by which ESSE exerts its nephroprotective potential.
Acknowledgment
This work was supported by funding from the Kerala State Council for Science Technology and Environment (KSCSTE), Government of Kerala, India, in the form of junior research fellowship.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- 1.World Health Organization. Diabetes mellitus: Report of a WHO study group, WHO Technical Report Series. 1985:727. [PubMed] [Google Scholar]
- 2.Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of aglucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. [Google Scholar]
- 3.Vivian EM. Type 2 children and adolescence-the next epidemic? Curr Med Res Opin. 2006;22:297–306. doi: 10.1185/030079906X80495. [DOI] [PubMed] [Google Scholar]
- 4.Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol. 2003;95:134–143. doi: 10.1159/000074840. [DOI] [PubMed] [Google Scholar]
- 5.Yildirim O, Buyukbingol Z. In vivo effect of vitamin C with cobalt on oxidative stress in experimental diabetic rat kidney. Diabetes Nutr Metab. 2003;16:208–213. [PubMed] [Google Scholar]
- 6.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II Early renal structural changes in type 1 diabetes. Diabetes. 2002;51:1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 7.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol. 2000;11:1667–1673. doi: 10.1681/ASN.V1191667. [DOI] [PubMed] [Google Scholar]
- 8.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson PJ, Carrington AL, Frost GS, Boulton AJ. Neurovascular disease, antioxidants and glycation in diabetes. Diabetes Metab Res Rev. 2002;18:260–272. doi: 10.1002/dmrr.305. [DOI] [PubMed] [Google Scholar]
- 10.Odetti P, Pesce C, Traverso N. Comparative trial of Nacetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes. 2003;52:499–505. doi: 10.2337/diabetes.52.2.499. [DOI] [PubMed] [Google Scholar]
- 11.Devinder Singh, Rajnendrapal Kaur, Vikas Chander, Kanwaljit Chopra. Antioxidants in the Prevention of Renal Disease. J Med Food. 2006;9:443–450. doi: 10.1089/jmf.2006.9.443. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS. Nondiabetic kidney disease. N Engl J Med. 2002;347:1505–1511. doi: 10.1056/NEJMcp013462. [DOI] [PubMed] [Google Scholar]
- 13.Arinathan V, Mohan VR, John De Britto A, Murugan C. Wild edibles used by the palliyars of the western ghats, Thamil nadu. Indian J Traditional knowledge. 2007;6:163–168. [Google Scholar]
- 14.Angami A, Gajurel PR, Rethy P, Singh B, Kalita SK. Status and potential of wild edible plants of Arunachal Pradesh. Indian J traditional knowledge. 2006;5:541–550. [Google Scholar]
- 15.Saroj kumar V, Jaishanker R, Annamalai A, Iyer CSP. Ensete superbum (Roxb.) Cheesman: A rare medicinal plant in urgent need of conservation. Curr Sci. 2010;98:602–603. [Google Scholar]
- 16.Yesodharan K, Sujana KA. Ethnomedicinal knowledge among Malamalasar tribe of Parambikulam Wildlife Sanctuary, Kerala. Indian J Traditional knowledge. 2007;6:481–485. [Google Scholar]
- 17.Udayan PS. Some common plants used by Kurichiar tribes of Tiruneli Forest, Wayanad District, Kerala in medicine and other traditional uses. Indian J Traditional knowledge. 2008;7:250–255. [Google Scholar]
- 18.Patil HM, Bhaskar VV. Medicinal knowledge system of tribals of Nandurbar dist. Maharastra. Indian J Traditional knowledge. 2006;5:327–330. [Google Scholar]
- 19.Jagtap SD. Ethnomedicobotanical uses of endemic and RET plants utilised by the KORKU Tribe of Amaravati District, Maharastra. Indian J Traditional knowledge. 2008;7:284–287. [Google Scholar]
- 20.Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562–566. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- 21.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 23.Maehly AC, Chance B. The assay of catalases and peroxidases. In: Glick D, editor. The methods of biochemical analysis. New York: Interscience press; 1954. pp. 357–408. [DOI] [PubMed] [Google Scholar]
- 24.Agerguard N, Jense PJ. Procedure for blood glutathione peroxidase determination in cattle and swine. Acta Vet Scand. 1982;23:515–527. doi: 10.1186/BF03546770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David MG, Richard JS. Glutathione reductase. In: Bergmeyer HU, editor. Method of enzymatic analysis. Germany: Weinheim Verlag Chemie; 1983. pp. 258–265. [Google Scholar]
- 26.Patterson JW, Lazarow A. Determination of glutathione. In: Glick D, editor. Methods of biochemical analysis. Newyork: Interscience; 1955. pp. 259–279. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa H, Ohishi Ν, Yagi Κ. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.John AB, Steven DA. Microsample lipid peroxidation. In: Fleischer S, Packer L, editors. Methods in Enzymology. New York: Academic Press; 1978. pp. 302–310. [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Stevens MJ. Redox-based mechanisms in diabetes. Antioxid Redox Signal. 2005;7:1483–1485. doi: 10.1089/ars.2005.7.1483. [DOI] [PubMed] [Google Scholar]
- 31.Heidland A, Ling H, Vamvakas S, Paczek L. Impaired proteolytic activity as a potential cause of progressive renal disease. Miner Electrolyte Metab. 1996;22:157–161. [PubMed] [Google Scholar]
- 32.Rabkin R, Schechter P, Shi JD, Boner G. Protein turnover in the hypertrophy in kidney. Miner Electrolyte Metab. 1996;22:153–156. [PubMed] [Google Scholar]
- 33.Tanwar RS, Sharma SB, Singh UR, Prabhu KM. Attenuation of renal dysfunction by anti-hyperglycemic compound isolated from fruit pulp of Eugenia jambolana in streptozotocin-induced diabetic rats. Indian J Biochem Biophys. 2010;47:83–89. [PubMed] [Google Scholar]
- 34.Evan AP, Mong SA, Connors BA, Aronoff GR, Luft FC. The effect of alloxan, and alloxan induced diabetes on the kidney. Anat Rec. 1984;208:33–47. doi: 10.1002/ar.1092080105. [DOI] [PubMed] [Google Scholar]
- 35.Rajkumar M, Uttam Kumar D, Ghosh D. Attenuation of hyperglycemia and hyperlipidemia in streptozotocin-induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol Pharm Bull. 2005;28:1172–1176. doi: 10.1248/bpb.28.1172. [DOI] [PubMed] [Google Scholar]
- 36.Suba V, Murugesan T, Bhaskara Rao R, Ghosh L, Pal M, Mandal SC. Antidiabetic potential of Barleria lupulina extract in rats. Fitoterapia. 2004;75:1–4. doi: 10.1016/s0367-326x(03)00163-1. [DOI] [PubMed] [Google Scholar]
- 37.Erah PO, Osmde GE, Omogbai EKI. Hypoglycemic effect of the extract of Solenostemon monostachys leaves. J West Afr Pharm. 1996;10:21–27. [Google Scholar]
- 38.Tuvemo T, Ewald U, Kobboh M, Proos LA. Serum magnesium and protein concentrations during the first five years of insulin dependent diabetes in children. Acta Paediatr. 1997;418:7–10. doi: 10.1111/j.1651-2227.1997.tb18297.x. [DOI] [PubMed] [Google Scholar]
- 39.Almdal JP, Vilstrup H. Strict insulin therapy normalizes organ nitrogen contents and the capacity of urea nitrogen synthesis in experimental diabetes in rats. Diabetologia. 1988;31:114–118. doi: 10.1007/BF00395558. [DOI] [PubMed] [Google Scholar]
- 40.Mansour HA, Newairy AA. Amelioration of impaired renal function associated with diabetes by Balanites aegyptiaca fruits in streptozotocin-induced diabetic rats. J Med Res Inst. 2000;21:115–125. [Google Scholar]
- 41.Craven PA, Melhem MF, DeRubertis FR. Thromboxane in the pathogenesis of glomerular injury in diabetes. Kidney Int. 1992;42:937–946. doi: 10.1038/ki.1992.370. [DOI] [PubMed] [Google Scholar]
- 42.Aurell M, Bjorck S. Determination of progressive renal disease in diabetes mellitus. Kidney Int. 1992;41:38–42. [PubMed] [Google Scholar]
- 43.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14:250–253. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 44.Negre-Salvayre A, Salvayre R, Auge N, Pamplona R, Portero-Otin M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 45.Obrosova IG, Fathallah L, Liu E, Nourooz-Zadeh J. Early oxidative stress in diabetic kidney: effect of DL-a-lipoic acid. Free Radic Biol Med. 2003;34:186–195. doi: 10.1016/s0891-5849(02)01195-4. [DOI] [PubMed] [Google Scholar]
- 46.Lee YM, Kim H, Hong EK, Kang BH, Kim SJ. Water extract of 1:1 mixture of Phellodendron cortex and Aralia cortex has inhibitory effects on oxidative stress in kidney of diabetic rats. J Ethnopharmacol. 2000;73:429–436. doi: 10.1016/s0378-8741(00)00302-0. [DOI] [PubMed] [Google Scholar]
- 47.Gregus Z, Fekete T, Halaszi E, Klaassen CD. Lipoic acid impairs glycine conjugation of benzoic acid and renal excretion of benzoylglycine. Drug Metab Dispos. 1996;24:682–688. [PubMed] [Google Scholar]
- 48.Loven D, Schedl H, Wilson H, Daabees TT, Stegink LD, Diekus M. Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin induced diabetes. Diabetes. 1986;35:503–507. doi: 10.2337/diab.35.5.503. [DOI] [PubMed] [Google Scholar]
- 49.Ewis SA, Abdel-Rahman MS. Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. Appl Toxicol. 1995;15:387–390. doi: 10.1002/jat.2550150508. [DOI] [PubMed] [Google Scholar]


