Abstract
Objective(s):
The application of stem cells holds great promises in cell transplants. Considering the lack of optimal in vitro model for hepatogenic differentiation, this study was designed to examine the effects of laminin matrix on the improvement of in vitro differentiation of human bone marrow mesenchymal stem cells (hBM-MSC) into the more functional hepatocyte-like cells.
Materials and Methods:
Characterization of the hBM-MSCs was performed by immunophenotyping and their differentiation into the mesenchymal-derived lineage. Then, cells were seeded on the laminin-coated or tissue culture polystyrene (TCPS). The differentiation was carried out during two steps. Afterward, the expression of hepatocyte markers such as AFP, ALB, CK-18, and CK-19 as well as the expression of C-MET, the secretion of urea, and the activity of CYP3A4 enzyme were determined. Moreover, the cytoplasmic glycogen storage was examined by periodic acid–Schiff (PAS) staining.
Results:
The results demonstrated that the culture of hBM-MSC on laminin considerably improved hepatogenic differentiation compared to TCP group. A significant elevated level of urea biosynthesis and CYP3A4 enzyme activity was observed in the media of the laminin-coated differentiated cells (P<0.05). Furthermore higher expressions of both AFP and ALB were determined in cells differentiated on laminin matrix. Glycogen accumulation was not detected in the undifferentiated hBM-MSCs, however, both differentiated cells in laminin and TCPS groups demonstrated the intracellular glycogen accumulation on day 21 of hepatogenic differentiation.
Conclusion:
Taken together, these findings may indicate that laminin matrix can improve terminal differentiation of hepatocyte-like cells from hBM-MSCs. Thus, laminin might be considered as a suitable coating in hepatic tissue engineering designs.
Keywords: Bone marrow, Differentiation, Hepatocyte, Laminin, Mesenchymal stem cell
Introduction
End stage liver disease is an irreversible condition with extremely poor prognosis and an increasing incidence worldwide (1). The orthotropic liver transplantation, as the current optimal treatment of liver failure is confronted with two major obstacles, limited availability of donor livers and immunological incompatibilities (2). Accessibility, abundance, and immunosuppressive properties of adult stem cells have attracted attentions for their use in regenerative medicine. Increasing evidence reported the potential of mesenchymal stem cells (MSCs) differentiation into the hepatocyte-like cells in vitro (3). Despite using several protocols and different cytokine cocktails known to play a role during liver development, the in
vitro culture systems still do not recreate all signals present in vivo (4). So far, differentiated hepatocyte-like cells have shown several hepatic functions, however levels of albumin secretion, urea production, glycogen storage, and CYP450 and GST activities are still approximately 5 to 10 folds lower than those of mature hepatocytes (5). The regulatory signals from a niche through their tissue specific extracellular matrix (ECM) are poorly studied and understood during the stem cell differentiation in vitro (6-8).
The ECM is a complex mixture of matrix molecules, including the fibronectins, collagens, laminins, and proteoglycans that assemble into fibrils or other complex macromolecular arrays which can develop basement membranes(BM). In the parenchyma of the normal adult human liver, BM is absent (9). However, during human liver development, some BM component such as type IV collagen and laminin has been detected in the parenchyma (10), suggesting their potential importance during hepatocyte differentiation. Moreover, a recent study demonstrated that cell-deposited ECM which mimics liver’s in vivo microenvironment could promote the differentiation of bone marrow derived mesenchymal stem cells (BM-MSCs) to adult liver fates (11). In the adult liver, the ECM and BM of the bile ducts are mainly composed of laminin and type IV collagen (12). Based on in vivo evidence, laminin is a potent hepatogenesis factor after hemihepatectomy (13), an inducer of liver metastasis (14), and preserves induced damage in liver explants (15). Furthermore, recent studies demonstrated laminin in the subendothelial space of normal liver and suggested that it is produced by the hepatic lipocyte, a perisinusoidal cell (16). Moreover, laminin gene expression has been demonstrated in the hepatic stellate cells and endothelial cells (17, 18). Thus, laminin can be considered as one of the important liver tissue specific ECM components.
In vitro production of human hepatocytes is of increasing importance in basic research, pharmacotoxicology, and biotherapy of liver diseases. Laminin, as a tissue specific ECM component was reported to be in association with the development and regeneration of the liver cells in vivo (19-21). Considering the lack of optimal in vitro model for hepatogenic differentiation, this study was designed to examine the effects of laminin matrix on the improvement of in vitro differentiation of human BM-MSCs (hBM-MSCs) into the more functional hepatocyte-like cells.
Materials and Methods
Culture conditions of human BM-MSCs
The second passages of hBM-MSC were obtained from Royan Cell Bank (Royan Institute, Tehran, Iran). The cells were cultured at 37 °C in a humidified 5% CO2 incubator, in growth medium containing DMEM-high glucose (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS, 2 mM L-glutamine, 100 mg/ml streptomycin, and 100 U/ml penicillin (all from Sigma-Aldrich, St Louis, USA). When cells reached 70-80% confluence, cultures were harvested with 0.25% trypsin-EDTA solution, resuspended in growth medium, and subcultured.
Immunophenotyping of human BM- MSCs by flowcytometry
To characterize the obtained cells, cells at the 3rd passage were harvested, resuspended in phosophate buffered saline (PBS), and centrifuged. After counting, about 2 × 105 cells were centrifuged at 300×g for 5 min, at room temperature (RT). The pellet was suspended in PBS and incubated for 45 min on ice, with appropriate antibodies including fluorescent isothiocyanate (FITC)-conjugated mouse anti-human CD90 and CD45 and phycoerythrin (PE)-conjugated CD44, CD105, and CD34 (all antibodies from Abcam, Cambridge, MA, USA). Then, the labeled cells were washed with PBS and analyzed by FACS-Calibur (Becton Dickinson, USA). An isotype control with FITC or PE-labeled was included in each experiment and specific staining was measured from the cross point of the isotype with the specific antibody graph. Histograms were created with WinMDI 2.8 software (Scripps Institute, La Jolla, CA, USA).
Adipogenic and osteogenic differentiation of BM-MSCs
For adipogenic differentiation, the cells in the 3rd passage were cultured for 2 weeks in the defined adipogenic medium, as previously described (22, 23), containing growth medium supplemented with 200 μM indomethacin (Sigma-Aldrich, St. Louis, USA), 1 μM dexamethasone (Sigma), 1.7 μM insulin, and 500 μM isobutyle methyl zantin. In negative control, MSCs of the 3rd passage were cultured in growth medium for 2 weeks and stained by Oil red.
For osteogenic differentiation, the cells in the 3rd passage were cultured for 2 weeks in the defined osteogenic medium, as previously described (22, 23), containing growth medium supplemented with 10 μM dexamethasone, 10 μM glycerol phosphate (Sigma), and 0.05 g/l ascorbic acid (Sigma). In negative control, MSCs of the 3rd passage were cultured in growth medium for 2 weeks and stained by alizarin red.
Laminin coating
The wells were coated using 2 µg/cm2 of laminin (Sigma L2020) diluted in PBS and incubated overnight at 37 °C. Then, wells were washed with PBS and the cells were seeded.
MTT assay
To determine the viability of MSCs cultured on laminin and polystyrene groups, MTT assay was performed according to the manufacturer guide. Briefly, MTT (5 mg/ml) was added to each well and incubated at 37 °C for 4 hr. Then, formazan crystals were dissolved by addition of DMSO to each well. Finally, the absorbance was measured using a UV-microplate reader (Epson LQ-100) at 570 nm.
Hepatogenic differentiation of human BM-MSCs
Hepatogenic differentiation was performed according to the protocol developed in our lab (22, 23). Briefly, BM-MSCs in the 4th-5th passage were plated at 2.5×103 cells/cm2 on 24-well plates (Nunc, Wiesbaden-Biebrich, Germany) which were coated with 2 µg/cm2 laminin (Sigma) or uncoated TCPS. Hepatic differentiation was induced in two steps. In the first step, differentiation was induced for 7 days by growth medium supplemented with 20 ng/ml hepatocyte growth factor (HGF) (R&D Systems) and 10-7 M dexamethasone (Sigma). The second step was performed by changing the first step differentiation medium to growth medium supplemented with 20 ng/ml HGF, 10-7 M dexamethasone, and 10 ng/ml oncostatin M (R&D Systems). The differentiation media was refreshed every 3 days. Following 21 days, the cultured cells were used in different experiments and evaluations.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from experimental group differentiated cells, undifferentiated MSCs, and hepG2 cell line using RNeasy plus micro kit (Cat NO: 74034, Qiagen). Then, cDNA was synthesized by power cDNA synthesis kit (Intron) and PCR was performed for CK18 and CK19 by maxime PCR premix kit (Intron). PCR condition was optimized as follows: primary denaturation at 94 °C for 2 min, 40 cycles denaturation at 94 °C for 30 sec, annealing at 62 °C for 20 sec, extension at 72 °C for 30 sec, and final extension at 72 °C for 5 min (primer sequence is shown in Table 1).
Table 1.
Primers and annealing temperatures used for reverse transcription polymerase chain reaction (RT-PCR)
| Gene | Sequences | Product Size | tm |
|---|---|---|---|
| GAPDH | F: AGAAGGCTGGGGCTCATTTGC R: TGCAGGAGGCATTGCTGATG |
141bp | 60 |
| CK18 | F: CCACGAAGAGGAAGTAAAAGG R:TCCTCAATCTGCTGTGAGACCAG |
182bp | 62 |
| CK19 | F: CGAACCAAGTTTGAGACGGAAC R: CGTACTGATTTCCTCCTCATGG |
180bp | 62 |
Immunocytochemistry
Following 21 days cultivation in differentiation medium, the cells in the experimental groups including differentiated cells on laminin-coated and TCPS, as well as positive and negative controls were assessed for the expression of hepatocyte markers. The undifferentiated MSCs were considered as negative control and HepG2 cell line as positive control in different experiments. For immunofluorescent staining, the cells were washed twice with PBS, fixed with 4% paraformaldehyde (PFA) for 20 min, and permeabilized using 0.1% Triton X-100 for 5 min at RT. After washing with PBS and blocking with 5% goat serum for 45 min at RT, the cells were incubated overnight at 4 °C with appropriate primary antibodies including monoclonal anti-human albumin IgG (ALB, R&D Systems), monoclonal anti-human cytokeratin 18 (Santa Cruz, Biotechnology), and monoclonal anti-human alpha-fetoprotein (AFP, R&D System). Subsequently, the cells were washed with PBS/Tween 0.1% for three times and incubated for 3 hr at RT with appropriate FITC-conjugated secondary antibodies, including goat anti-mouse (Santa Cruz, Biotechnology, Dallas, TX, USA), and nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole; 1:1000) for 2 min at RT. Then, cells were washed with PBS, rinsed in deionized water, and mounted. For negative controls, normal mouse serum IgG was used instead of primary antibody. Finally, the immunoreactivity of the cells was determined using a fluorescence microscope (Nikon, Japan).
C-MET flowcytometry
Differentiated cells in the experimental groups were detached on day 21, and 104 cells/ml were resuspended in PBS and centrifuged for 5 min at 300×g. Then, the cells were stained by incubating them for 45 min at 4 °C with anti-hHGF R/C-MET fluorescein-conjugated mouse IGg1 (R&D system). Then, labeled cells were washed with PBS and analyzed by FACSCalibur (Becton Dickinson, USA). An isotype control with FITC or PE-labeled was included in each experiment and specific staining was measured from the cross point of the isotype with the specific antibody graph. Histograms were created with WinMDI 2.8 software (Scripps Institute, La Jolla, CA, USA).
Urea production
On day 21 of differentiation, urea level was measured in the experimental groups using urea assay kit (Zistshimi, Iran). The assay was based on the reduction of ammonia produced via urea hydrolysis. At first, ammonium was produced by urease, and then glutamate dehydrogenase converted ammonium and NADH to NAD. Afterward, the decrease in NADH concentration at 340 nm showed a relationship with enzyme activity and urea level of the cultured cells in our experimental groups and undifferentiated MSCs, as the negative control. In this method, the samples and standard were mixed with the solution of the kit. Then, following 30 and 60 seconds, respectively the primary (A1) and the secondary (A2) absorptions of the samples and standard were read at 340 nm. Finally, the urea concentration was calculated according to the following formula:
Urea (mg/dl) = (A2-A1) sample / (A2-A1) standard × standard concentration
CYP3A4 activity
The P450 CYP3A4 screening system kit with Luciferin-IPA (cat no: V9001, Promega) was used to evaluate the CYP3A4 activity in the cultured cells of control and experimental groups according to the manufacture. Briefly, the cells on the 3rd week of differentiation (day 21) were incubated at 37 °C for 1hr in DMED containing 3 µM Luciferin-IPA. An equal volume of Luciferin detection reagent was added to each well and mixed gently by tapping or swirling the plate to form a lysate. 100 μl of lysate from each well was transferred to luminometer vessels and incubated for 30-40 min at RT. To exclude the potential background luminescence, Luciferin-IPA in medium was added to an empty well and measured. Finally, luminescence was read using a luminometer (Berthold).
Periodic ascid Schiff (PAS) staining
Differentiated cells in the experimental groups were washed twice with PBS on day 21 and fixed with 4% PFA for 20 min at RT. The fixed cells were oxidized in 1% PAS for 5 min at RT, rinsed with dH2O, and treated with Schiff reagent for 5 min at RT. After rinsing with dH2O2, the nuclei were stained with Mayer’s hematoxylin for 1 min. Finally, glycogen pigments were observed with invert microscope.
Statistics
The differences between groups were evaluated using ANOVA with Scheffe’s test and results were considered statistically significant if P≤0.05.
Results
Characterization of isolated human BM-MSCs
The results of immunophenotyping profile of the obtained cells indicated that the cells were positive for CD90 (96.30), CD105 (96.08%), and CD44 (90.97), as the markers for human MSCs, while they were negative for hematopoitic lineage marker CD45 (0.35%), and the human leukocyte marker CD34 (0.18%). The results of flowcytometry are shown in Figure 1a-e.
Figure 1.
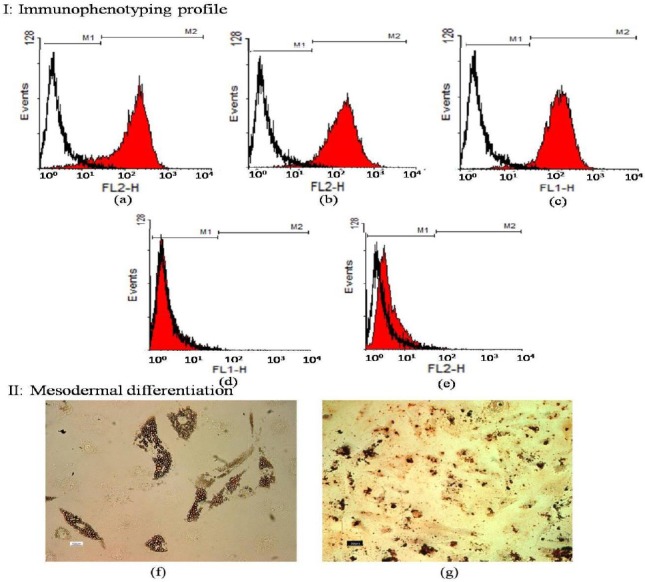
I: Immunophenotyping of human bone marrow derived mesenchymal stem cells (hBM-MSCs) using flowcytometry. MSCs were positive for CD44, CD90, and CD105 (a-c). These cells were negative for CD34 and CD45 (d-e). The white area shows the profile of the negative control
II: Adipogenic and osteogenic differentiation of bone marrow mesenchymal stem cells. Differentiated hBM-MSCs to adipogenic and osteogenic lineages were positive for Oil red (O-staining) (f) and alizarin red staining (g)
Mesodermal differentiation following Oil red and alizarin red stainings confirmed the presence of lipid droplets and calcium deposits in adipogenic and osteogenic induced differentiated cells, respectively. Undifferentiated hBM-MSCs were negative in both staining procedures (Figure 1f-g). These results indicated that the cultured cells had the basic properties of hBM-MSCs.
MTT assay
MTT assay during the 1st week of cultivation showed proper proliferation of cells on both polystyrene and laminin, with no statistically significant difference (P<0.05) between them (Figure 2).
Figure 2.
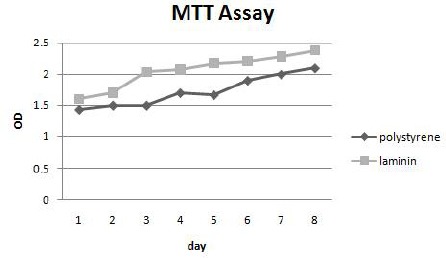
MTT assay in mesenchymal stem cells cultured on laminin and polystyrene
Morphological studies
The morphological features during hepatogenic differentiation showed that before differentiation (day 0), the hBM-MSCs had a spindle-shaped and fibroblast-like morphology (Figure 3a). Upon hepatogenic differentiation (day 7), the fibroblastic feature of MSCs gradually changed toward the polygonal morphology of hepatocyte-like cells and binucleated cells appeared (Figure 3b). By the end of the 2-step differentiation period (end of day 21), less fibroblastic cells were observed in both TCPS and laminin-coated groups. The cells exhibited a polygonal shape with cytoplasmic granules similar to that of the mature hepatocyte-like cells (Figure 3c). In our observations, the number of differentiated cells in the laminin-coated group was relatively higher compared with TCPS group, however to determine the difference, further statistical analyses are needed (data not shown).
Figure 3.
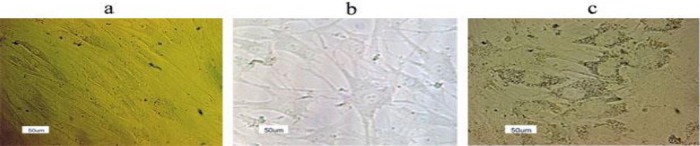
Morphology of the cells on day 0 (a), day 7- the first (b), and day 21- second (c) stages of differentiation
RT-PCR analysis
The results of RT-PCR showed the expression of cytokeratin 18 (CK18) as an epithelial cell marker, and GAPDH as a housekeeping gene, but not cytokeratin 19 (CK19) as a biliary marker, in both differentiated groups. The expression of CK18, CK19, and GAPDH was observed in HepG2 cell line, which had been considered as the positive control group. The result of RT-PCR experiment is shown in Figure 4.
Figure 4.
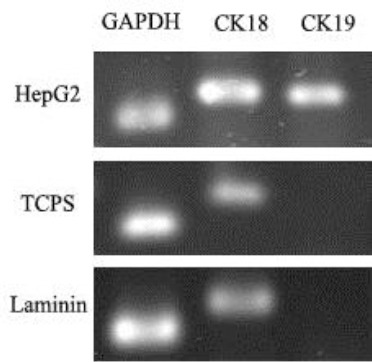
RT-PCR analysis of cytokeratin 18 and 19 in hepG2, differentiated cell on polystyrene and laminin
Immunocytochemical staining
At a protein level, the hepatogenic differentiation of hBM-MSCs was determined using some important hepatocyte markers, including albumin (the most abundant protein synthesized by functional hepatocytes), AFP (a protein specific to hepatocytes), and CK18 (epithelial marker) (Figure 5). The results indicated that these markers were expressed in both TCPS and laminin-coated groups. The number of positive cells in three different plates and in the three random fields of each plate were counted and averaged. The expression of albumin and AFP was higher in the differentiated cells on laminin-coated group (53% ALB, >90% AFP) when compared to that of the polystyrene group (30% ALB, 10% AFP). Indicated markers were not expressed in the undifferentiated MSCs, which were considered as the negative control (data not shown).
Figure 5.
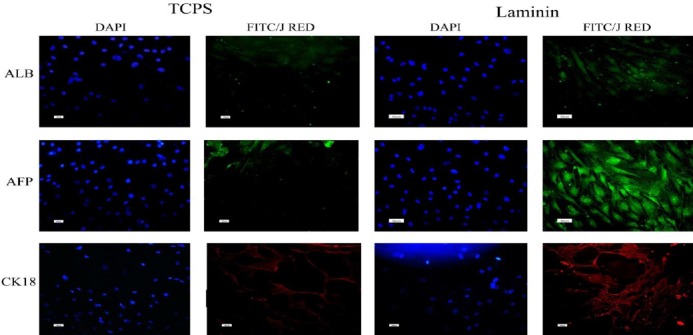
Immunofluorescent staining of albumin, alpha-fetoprotein and cytokeratin 18 in differentiated cells on polystyrene and laminin. Nuclei were stained with DAPI (4’, 6-diamidino-2-phenylindole; 1:1000) in the same cells
C-MET flowcytometry
Flowcytometric analysis on day 0, 10, and 21 of differentiation showed that the undifferentiated MSCs (day 0) and differentiated cells on day 21, on both laminin and TCPS groups were positive for the expression of C-MET, but they were negative on day 10. The expression profile of C-MET on day 0, 10, and 21 of differentiation could explain the developmental procedure of hBM-MSCs in to the hepatocyte-like cells in both laminin-coated and TCPS groups (Figure 6). However, significant differences were not detected between the indicated experimental groups.
Figure 6.
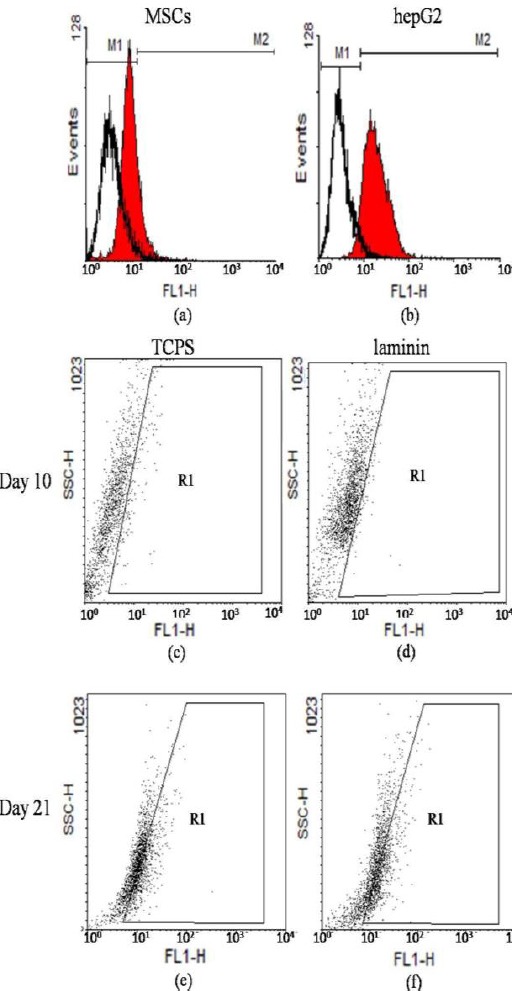
Flowcytometric analysis of C-MET expression with WinMDI 2.8 software. C-MET expression in human bMSCs (day 0) (12.39%) (panel a), hepG2 (93.35%) (panel b), 10th day of differentiation in cells on polystyrene (3.5%) (panel c) and on laminin (3.8%) (panel d), and 21th day of differentiation in cells on polystyrene (34.6%) (panel e) and on laminin (36.20%) (panel f)
Urea production
The levels of urea secretion in culture media of differentiated cells in both laminin-coated and TCPS groups were detected at the 21th day of differentiation and showed a concentration of 8.67 mg/dl and 6.56 mg/dl, respectively. The concentration of urea was 1.67 mg/dl in undifferentiated MSCs as the negative control, and 6.78mg/dl in hepG2 cell line as the positive control.
The results indicated that urea production by the laminin-coated differentiated cells was significantly more than that of TCPS group (P<0.05) (Figure 7a).
Figure 7.
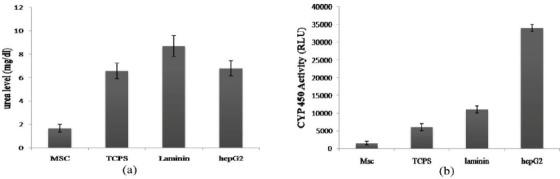
Urea levels and cytochrome P450 activity in differentiated cells on laminin and polystyrene. Significant differences was shown between urea levels (a) and enzyme activity (b) of differentiated cells on laminin and polystyrene (P<0.05). MSCs and hepG2 were used as positive and negative control, respectively. To reproduce the results, these experiments were performed in triplicate
CYP3A4 enzyme activity
The activity of cytochrome P450 was analyzed at the 21th day of differentiation in both laminin-coated and TCPS groups as well as in the undifferentiated hBM-MSCs and hepG2 cell line, as the negative and positive controls, respectively. The results showed that enzyme activity in the laminin-coated group was approximately 2-fold more than that of the TCPS, which was statistically significant (P<0.05) (Figure 7b). In undifferentiated hBM-MSCs, no enzyme activity was observed, while positive enzyme activity was detected in hegG2 cell line.
Glycogen storage
PAS staining on day 21 of hepatogenic differentiation in both differentiated cells on laminin and TCPS demonstrated the intracellular glycogen accumulation in both experimental groups (Figure 8). However no difference was observed between two groups. Glycogen accumulation was not detected in the undifferentiated hBM-MSCs, as the negative control.
Figure 8.
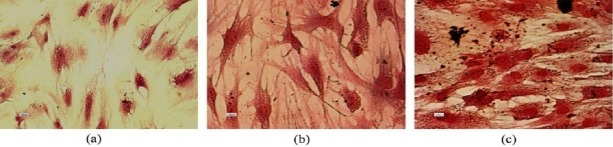
Periodic acid-Schiff staining assay in hepatogenic differentiated cells. PAS staining in undifferentiated MSCs (a), glycogen uptake by the cells differentiated on polystyrene (b) and laminin (c)
Discussion
Extensive studies have been undertaken to improve the hepatogenic differentiation of stem cells using different natural and synthetic matrices. In tissue engineering researches, the application of natural or synthetic matrices to stimulate cell adhesion and particularly cell functions are of vital importance. Some ECM components have ubiquitous distribution in the developing embryo. Restricted distribution of laminin was reported to be localized in the liver BM (24), which might suggest a specific of the most important components of liver ECM may lead to the activation of a large number of integrins on the cell surface (9). Integrins are a family of cell adhesion receptors, linking the ECM to stimulate and control several intracellular signaling pathways (10-12). Thus, it seems that laminin coating improves survival of hBM-MSCs during differentiation period. Takayama et al (25) showed that the type III laminin had positive effects on the maintenance and expansion of hepatoblast-like cells derived from ES/iPS cells, which confirmes our results of cell survival improvement due to laminin treatment. However, to the best of our knowledge, no data was available to address the effects of laminin on improving hepatogenic differentiation of MSCs. Considering the fact that during the initiation of the regenerative process in damaged liver, the hepatic progenitors acquire an ability to attach to laminin (24), prompted us to examine the possibility that laminin as a liver specific ECM component might role for laminin in hepatogenic differentiation. Moreover, the adhesive properties of laminin as one improve the terminal hepatogenic differentiation. To address this, hepatic differentiation of hBM-MSCs was studied in the presence and the absence of laminin matrix coating during the in vitro differentiation process.
Following characterization of obtained MSCs with the inclusion of the expression of MSCs markers, such as CD44, CD90, and CD105, as well as the exclusion of the expression of hematopoietic and leukocyte lineages markers such as CD34, and CD45; differentiation potential of the isolated hBM-MSCs to osteogenic and adipogenic lineages was examined. Our results indicated their multipotency, as shown in Figure 1. Then, according to our previously established method (22, 23), with brief modifications, differentiation process with HGF, OSM, and dexamethasone was carried out by cultivation of the cells on the laminin-coated as well as the polystyrene wells. At first step, the expression of the hepatocyte markers such as albumin, AFP, CK18, and CK19 which were generally used in the previous studies to confirm the hepatogenic differentiation of stem cells (13-15) was examined. The results of the immunocytochemistry studies revealed that albumin as one of the specific markers of final hepatic differentiation was expressed comparatively higher in the differentiated cells on laminin-coated group (53%) than those on the polystyrene group (30%), while no immunoreactivity was observed in the control MSCs and the negative control (Figure 5). Consistently, the number of AFP positive cells was higher in laminin-coated hBM-MSCs compared to that of the polystyrene group (Figure 5). No staining was observed in the control MSCs and the negative control. The expression of CK18 (as an epithelial marker) was observed in both laminin-coated and polystyrene groups (Figure 4) and no expression was observed in the negative control group.
In the next step, the expression of C-MET as one of the epithelial cell markers, including hepatocytes was evaluated. The result of flowcytometry on day 10 was negative in both groups while the expression of C-MET on day 21 was 34% and 36% on polystyrene and laminin-coated groups, respectively (Figure 6). Further studies are required to determine the developmental expression pattern of C-MET in the stem cell differentiation process into the fully developed hepatocyte-like cells. Moreover, we examined the cytoplasmic glycogen storage by PAS staining. As shown in Figure 8, the intracellular glycogen accumulation wasn’t detectable in undifferentiated hBM-MSCs while glycogen storage was observed in both experimental groups with no statistically significant difference. Finally, in order to evaluate the functionality of the differentiated cells, the secretion of urea in culture media and the detoxification capacity of the cells in our experimental groups were examined. The activity of CYP3A4 as one of the most vital drug metabolizing enzymes in the hepatic detoxification has been reported as one of the functional properties of the fully differentiated hepatocytes (16). Our findings revealed the activity of CYP3A4 in both laminin and polystyrene groups. The statistical analysis showed that the activity of CYP3A4 was two times higher in the cells differentiated on laminin-coated vessels compared to that of the polystyrene group which was statistically significant (P<0.05) (shown in Figure 7b). Thus, it can be concluded that laminin matrix could improve terminal differentiation of hBM-MSCs in to the hepatocyte-like cells. The urea secretion in culture media of differentiated cells on both laminin and polystyrene groups were detected on day 21 of differentiation at the concentration of 8.67 mg/dl and 6.56 mg/dl, respectively. As shown in Figure 7a, the level of urea secretion which is considered as one of the most vital functionality assays in the differentiated cells was significantly higher (P<0.05) in laminin-coated group compared to other groups. Based on these data, it can be concluded that laminin may provide an environment resembling a natural ECM, which would enhance the biological activity of growth factors and cytokines for improving the terminal hepatic differentiation of hBM-MSCs.
Conclusion
Liver transplant as the major therapeutic option for liver diseases is confronted with several complications, thus the potential of adult human MSCs to differentiate into hepatocytes has generated much excitement in therapeutic applications.
Acquisition of the capacity for the production of albumin, accumulation of glycogen, secreation of urea, expression of cytokeratin and C-MET, and the detoxification of the differentiated cells indicated that laminin coating improved the in vitro differentiation of the hBM-MSC into the more mature hepatocyte-like cells. Our findings therefore may contribute to stem cell based liver tissue engineering, bioartificial liver development, toxicology studies, drug discovery, and clinical stem cell therapies to treat chronic liver damage.
Acknowledgment
This study was financially supported by National Institute of Genetic Engineering and Biotechnology. The results described in this paper were part of the student thesis.
References
- 1.Li J, Li M, Niu B, Gong J. Therapeutic potential of stem cell in liver regeneration. Front Med. 2011;5:26–32. doi: 10.1007/s11684-011-0107-0. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–1776. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Kosmacheva S, Seviaryn I, Goncharova N, Petyovka N, Potapnev M. Hepatogenic potential of human bone marrow and umbilical cord blood mesenchymal stem cells. Bull Exp Biol Med. 2011;151:142–149. doi: 10.1007/s10517-011-1276-1. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Lechón MJ, Jover R, Donato T, Ponsoda X, Rodriguez C, Stenzel KG, et al. Long-term expression of differentiated functions in hepatocytes cultured in three-dimensional collagen matrix. J Cell Physiol. 1998;177:553–562. doi: 10.1002/(SICI)1097-4652(199812)177:4<553::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Vestentoft PS. Development and molecular composition of the hepatic progenitor cell niche. Dan Med J. 2013;60:B4640. [PubMed] [Google Scholar]
- 6.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 8.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 9.Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. [PubMed] [Google Scholar]
- 10.Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21:63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He H, Liu X, Peng L, Gao Z, Ye Y, Su Y, et al. Promotion of hepatic differentiation of bone marrow mesenchymal stem cells on decellularized cell-deposited extracellular matrix. Biomed Res Int 2013. 2013 doi: 10.1155/2013/406871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T, Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology. 1994;25:143–150. doi: 10.1111/j.1365-2559.1994.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Hernandez A, Delgado FM, Amenta P. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest. 1991;64:157–166. [PubMed] [Google Scholar]
- 14.Wewer UM, Albrechtsen R. Carcinoma-associated perisinusoidal laminin may signal tumour cell metastasis to the liver. Virchows Arch A Pathol Anat Histopathol. 1992;421:87–93. doi: 10.1007/BF01607040. [DOI] [PubMed] [Google Scholar]
- 15.Quondamatteo F, Scharif K, Herken R. Changes in laminin immunoreactivity as a marker for the state of liver preservation. Histochem J. 1994;26:827–832. doi: 10.1007/BF00162927. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knittel T, Janneck T, Muller L, Fellmer P, Ramadori G. Transforming growth factor β1-regulated gene expression of Ito cells. Hepatology. 1996;24:352–360. doi: 10.1053/jhep.1996.v24.pm0008690404. [DOI] [PubMed] [Google Scholar]
- 18.Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19–25. doi: 10.1016/j.tcb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate MC, García AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC. Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 22.Kazemnejad S, Allameh A, Soleimani M, Gharehbaghian A, Mohammadi Y, Amirizadeh N, et al. Biochemical and molecular characterization of hepatocyte-like cells derived from human bone marrow mesenchymal stem cells on a novel three-dimensional biocompatible nanofibrous scaffold. J Gastroenterol Hepatol. 2009;24:278–287. doi: 10.1111/j.1440-1746.2008.05530.x. [DOI] [PubMed] [Google Scholar]
- 23.Ayatollahi M, Soleimani M, Tabei SZ, Salmani MK. Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J Stem Cells. 2011;3:113–121. doi: 10.4252/wjsc.v3.i12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallis YN, Robson AJ, Fallowfield JA, Thomas HC, Alison MR, Wright NA, et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60:525–533. doi: 10.1136/gut.2010.224436. [DOI] [PubMed] [Google Scholar]
- 25.Takayama K, Nagamoto Y, Mimura N, Tashiro K, Sakurai F, Tachibana M, et al. Long-Term Self-Renewal of Human ES/iPS-Derived Hepatoblast-like Cells on Human Laminin 111-Coated Dishes. Stem Cell Reports. 2013;1:322–335. doi: 10.1016/j.stemcr.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


