Abstract
Objective(s):
The study aimed to investigate the effects of adrenomedullin (ADM) and proadrenomedullin N- terminal 20 peptide (PAMP) on angiotensin (AngII)-stimulated proliferation in vascular smooth muscle cells (VSMCs).
Materials and Methods:
Thoracic aorta was obtained from Wistar rats and VSMCs were isolated from aorta tissues and then cultured. In vitro cultured VSMCs were stimulated with Ang II (10-8 mol/l) followed by various doses of PAMP or ADM (10-9, 10-8, or 10-7 mol/l). Cell proliferation as assessed by 3H-TdR incorporation. Protein kinase C (PKC) activity was measured by counting γ-32P radioactivity with liquid scintillation. In a separate cohort, in vitro cultured rat aortic vessels were treated with different doses of Ang II or PAMP (10-9, 10-8, or 10-7 mol/l). Cellular and secreted levels of PAMP, ADM and Ang II were measured using radioimmunoassay in the tissues and intubation mediums, respectively.
Results:
Ang II (10-8 mol/l) treatment significantly increased both 3H-TdR incorporation and PKC activity in VSMCs (by 2.68 and 1.02-fold, respectively; both P<0.01 vs. the control). However, Ang II-induced elevation of 3H-TdR incorporation, and PKC activity was significantly inhibited by various doses of ADM and PAMP (all P<0.01 vs. the Ang II group). In rat aortic vascular tissues or intubation media, Ang II treatments stimulated the expression and secretion of PAMP and ADM in a dose-dependent manner, while PAMP treatments had no significant effects on Ang II levels.
Conclusion:
ADM and PAMP inhibit Ang II-induced VSMCs proliferation. The interaction of Ang II, ADM and PAMP may regulate VSMCs and cardiovascular function.
Keywords: Adrenomedulin, Angiotension II, Proadrenomedullin N-terminal 20 peptide, Proliferation, Vascular smooth muscle-cell
Introduction
Hypertension is a frequently encountered clinical condition that, if left untreated, leads to heart or kidney failure, stroke and even death. Although the underlying molecular mechanisms of hypertension have been extensively studied, the interactions between vasodilators and vasoconstrictors have not been fully revealed.
Adrenomedullin (ADM) has been recognized as a vascular dilating polypeptide that could decrease blood pressure. The ADM precursor comprises 185 amino acid residues that can be digested into four fragments by proteolytic cleavage. The N-terminal fragment is the functional proadrenomedullin N-terminal 20 amino acid peptide, known as PAMP. Increased levels of both ADM and PAMP have been clinically detected in the plasma of hypertensive patients, as well as in the spontaneously hypertensive rat (SHR) model. While it is hypothesized that ADM and PAMP are involved in blood pressure elevation, their precise contributions remain largely undefined (1).
The vasoconstrictor angiotensin II (AngII) is a key regulator of the hypertensive process and plays an important role in atherosclerosis. Mechanistically, AngII stimulates protein kinase C (PKC) activity and promotes the proliferation and hypertrophy of vascular smooth muscle cells (VSMC). AngII can also activate phospholipase C by coupling with G protein, causing hydrolysis of phosphatidyl inositol bisphosphate and generating inositol trisphosphate and diglyceride. The inositol trisphosphate then promotes an increase in intracellular Ca2+ and cell constriction, while the diglyceride increases intracellular protein synthesis and cell body enlargement by activation of protein kinase C, thereby promoting cell proliferation (1). In the normal human circulatory system, vasodilators and vasoconstrictors act in concert to maintain the appropriate blood pressure, and perturbed expression of each may lead to life-threatening events. In this study, we sought to determine whether the vasodilators ADM and PAMP were functionally related to the vasoconstrictor AngII. In addition, the potential effects of ADM and PAMP on AngII-stimulated VSMC proliferation was investigated using in vitro cultured rat aortic VSMCs.
Materials and Methods
Preparation and culture of rat aorta VSMCs
Four male Wistar rats, weighing 180 to 200 g, were obtained from the Animal Center at Peking Medical University, China. All procedures involving animals were conducted in accordance with guidelines for the care and use of laboratory animals published by the National Institute of Health (USA) and with pre-approval by the local ethics committee. Rats were euthanized by decapitation, and aortas were obtained immediately via thoracotomy. Blood was gently rinsed using normal saline chilled to 4 °C before removing the attached extravascular connective tissue. The aorta was then cut into 2-3 mm long slices, divided equally into six tubes and prepared for incubation.
VSMCs were isolated as previously described (2). Then, cells were subcultured in RPMI-1640 containing 20% fetal bovine serum (FBS) (Sigma-Aldrich Co., USA) according to the method of Hirata (2). Microscopic examination revealed that cells underwent the proper peak and valley kinetic pattern of growth. Fourth generation cultured cells were used for all experiments.
Processing of aortic slices
Six tubes of aortic slices were separately incubated in a solution of 118 mmol/l NaCl, and 4.7 mmol/l each of KCl, CaCl2, MgSO4, KH2PO4, NaHCO3, and D-glucose at 37°C in a dry atmosphere of 95% O2 and 5% CO2. For experiments, AngII, PAMP, or ADM (all from Phoenix Pharmaceuticals, Inc., USA) were added at various concentrations (described below) and incubated for 4 hr at 37 °C in a water bath with tremor oscillations. Internal atmosphere was maintained at 95% O2/5% CO2 (2, 3).
Following incubation, the solution was gently removed from the tissue samples, discarded and replaced with 1 mL acetic acid 1 N. The tissue samples were then boiled for 15 min before weighing. After that, the tissues were homogenized in 2 mol/l acetic acid (1:10 w/v) and centrifuged at 10000 rpm for 20 min. The individual supernatants were collected and filtered through Sep-PakC18 chromatography columns (Perkin-Elmer, Co., USA). Following elution and freeze-drying, samples were stored at -70 °C until further use.
PAMP, ADM and AngII concentrations in the incubation medium and tissues were determined using radioimmunoassay and standard protein quantification. PAMP and ADM radioimmunoassay kits were supplied by Phoenix Pharmaceuticals, Inc. PIESEPCS sensitivity was IC50 30 pg/tube (16.6 pM). There were no cross-responses observed among the ADM-(1-52) antibody and the human ADM-(13-52), rat ADM-(1-52), human calcitonin gene-related peptide (CGRP), human ET-1, alpha human Atrial natriuretic petide (ANP)-(1-28), BNP-32, or CNP-22 antibodies. The methods of measurement were performed according to the PCS instructions. The ranges of standard curves of PAMP and Ang II were 1 to 128 pg/tube and 5 to 160 pg/ml, respectively. The total binding rate was 40.9% and NSB was 8.52%. The mean within-assay variability was 4.1%. No cross-immune responses were found between antibody and ADM.
Treatments with human AngII, PAMP, or ADM
For VSMC experiments, the following reagents and concentrations were added directly into the culture medium: 10-7 mol/l ADM (ADM group); 10-7 mol/l PAMP (PAMP group); 10-8 mol/l Ang II(Ang II group); 10-8 mol/l Ang II with various concentrations (10-9, 10-8, 10-7 mol/l) of ADM (ADM + Ang II group); 10-8 mol/l Ang II with various concentrations (10-9, 10-8, 10-7 mol/l) of PAMP (PAMP + Ang II group). The concentrations of PAMP and ADM used here are similar to the serum levels but different from the tissue levels of PAMP and ADM in vivo, which is more convenient for us to observe the functions of PAMP and ADM. Cells incubated in medium alone were used as controls (4, 5). The assays were repeated in six wells.
For experiments on incubated aortic sections, 10-9, 10-8 or 10-7 mol/l of AngII or PAMP reagent were respectively added to the incubation medium. In the control group, no reagent was added to the incubation medium. The assays were repeated in six wells.
Cell proliferation
VSMC suspensions (5×104 cells/ml) were inoculated in 24-well plates, and incubated for 24 hr, then shifted to low serum (0.5% FBS) RPMI-1640 and incubated for another 12 hr. Various concentrations and combinations of AngII, PAMP or ADM were then added to each well according to the above-mentioned treatment groups. After 6 hr of incubation, 0.5 µ Ci3H-Thymidine (3H-TdR) was added to each well. Reactions were terminated after 15 hr of incubation. The cells were collected using a micropore film (Millipore, USA) and treated with 10% trichloroacetic acid (TCA). Scintillation liquid was added and 3H-radioactivity was measured by a liquid scintillation counting instrument (LKB1209; Beckman, Sweden)(3).
Determination of PKC activity
VSMC suspensions (1×106 cells/ml) were inoculated in 24-well plates and shifted to low serum culture media for 24 hr. After that, cells were shifted to low serum (0.5% FBS) RPMI-1640) and incubated for an additional 24 hr. Various concentrations and combinations of AngII, PAMP, or ADM were added according to the above treatment groups and allowed to incubate for one hour. Cells were then washed in triplicate using PBS chilled to 4oC. Cells were collected by centrifugation and resuspended in a reaction solution of 5.2 mmol/l MgCl2, 94 mmol/l KCl, 12.5 mmol/l HEPES, 12.5 mmol/l EGTA, and 8.2 mmol/l CaCl2, (pH 7.4). Then, 0.2 mmol/l PKC substrate peptide and 0.3 μl Streptolysin O were added to make a final volume of 150 μl. After 10 min of incubation at 37 °C, γ-32P-ATP (Sigma-Aldrich) was added at 37 kBq/well (containing 0.2 mmol/l ATP-Na2) to initiate the reaction. After 10 min, 100 μl of stop solution (2.5% TCA dissolved in 2 mol/l acetic acid) was added to terminate the reaction, followed by incubation on ice for 10 min. The cells were collected by centrifugation (30000 rpm for 5 min). Aliquots of 120 μl supernatant were spotted on P81 ion-exchange chromatography filter paper (Whatman, USA) and dried in open-air. Then, the filters were washed two times with the eluent solution (30% acetic acid (v/v) and 1% phosphoric acid (v/v)), and once with anhydrous alcohol. The filter paper was then placed into a scintillation vial following desiccation, and scintillation liquid was added in order to measure the 32P radioactivity using a β liquid scintillation counter (LKB1209; Beckman, Sweden). The control test was performed simultaneously without applying PKC substrate peptide. Relative PKC activity was determined by calculating the difference in radioactivity between the experimental and control samples, and was expressed as pmol Pi/106 cell (3).
Statistical analyses
Each experiment was performed as parallel-well experiments, and was repeated at least six times. The results are expressed as mean () ±SEM SEM. Differences between groups were analyzed by analysis of variance and q-test. A P-value of less than 0.05 was considered statistically significant. The software used was SPSS13.0.
Results
PAMP and ADM inhibited AngII-induced cell proliferation and PKC activation
Since Ang II plays significant role in the development of hypertension through promoting cellular proliferation and PKC activity, we examined the effects of PAMP and ADM on these Ang II-induced features in the VSMCs.
In the control group, the VSMC 3H-TdR incorporation was 2159.64±483.25 cpm/well and the PKC activity was 486.06±126.18 pmol pi/mg protein. The VSMC 3H-TdR incorporation and PKC activity after PAMP or ADM treatment (10-7 mol/l) were not significantly different compared with the control group (both P>0.05) (Table 1).
Table 1.
Effects of adrenomedullin (ADM) and proadrenomedullin N- terminal 20 peptide (PAMP) (10-7 mol/l) on 3H-TdR incorporation and protein kinase C (PKC) activity in cultured rat aorta vascular smooth muscle cells (VSMCs)
| Group | 3H-TdR incorporation (cpm/well) | PKC activity (pmol pi/mg protein) |
|---|---|---|
| Control | 2159.64±483.25 | 486.06±126.18 |
| ADM 10-7mol/l | 2182.40±232.72 | 511.21±112.56 |
| PAMP 10-7mol/l | 2328.99±214.85 | 496.18±115.45 |
Results are presented as x̄ ±SEM n=6 cpm, counts per million
Ang II (10-8 mol/l) increased VSMC 3H-TdR incorporation and PKC activity by 2.68- and 1.02-fold, respectively (P<0.01 vs. control) (Table 2).
Table 2.
Effects of various doses of adrenomedullin (ADM) on angiotensin II (AngII)-induced 3H-TdR incorporation and protein kinase C (PKC) activityin vascular smooth muscle cells (VSMCs)
| Group | 3H-TdR incorporation (cpm/well) | PKC activity (pmol pi/mg protein) |
|---|---|---|
| Control | 2159.64±483.25 | 486.±126.17 |
| AngII10-8mol/l | 7946.26±464.27# | 980.25±142.15# |
| Ang II 10-8mol/l+ADM 10-9mol/l | 4107.00±259.70* | 923.25±112.45* |
| Ang II 10-8mol/l+ADM 10-8mol/l | 3768.47±317.40* | 736.75±128.51* |
| Ang II 10-8mol/l+ADM 10-7mol/l | 3285.37±264.22* | 631.25±110.04* |
Results are presented as x̄ ±SE n=6
P<0.01 vs. Control
P<0.01vs. AngII 10-8mol/l group cpm, counts per million
ADM and PAMP inhibited Ang II-induced VSMC 3H-TdR incorporation and PKC activation in a dose-dependent manner. ADM treatment at 10-9, 10-8, and 10-7 mol/l suppressed the Ang II-induced VSMC 3H-TDR incorporation by 48%, 52%, and 59%, respectively (all P<0.05 vs. the Ang II group). Similarly, Ang II-induced PKC activation was inhibited after ADM treatment by 8%, 25%, and 27%, respectively (all P<0.05 vs. the Ang II group). (Table 2). Meanwhile, PAMP treatment at 10-9, 10-8, and 10-7 mol/l led to significant inhibition of the VSMC 3H-TDR incorporation (by 52%, 58%, and 62%, respectively) and PKC activity (by 16%, 25%, and 36%, respectively) (P<0.01) (Table 3).
Table 3.
Effects of various doses of proadrenomedullin N- terminal 20 peptide (PAMP) on angiotensin II (AngII)-induced 3H-TdR incorporation and protein kinase C (PKC) activity in vascular smooth muscle cells (VSMCs)
| Group | 3H-TdR incorporation (cpm/well) | PKC activity (pmol pi/mg protein) |
|---|---|---|
| Control | 2159.64±193.30 | 486±50.47 |
| AngII10-8mol/l | 7946.26 ±185.71# | 980.25±56.86# |
| AngII10-8mol/l+ PAMP10-9mol/l | 3802.06±133.43* | 897.5 ±25.22* |
| AngII10-8mol/l+PAMP 10-8mol/l | 3356.17±134.3* | 789.00±44.4* |
| AngII10-8mol/l+PAMP 10-7mol/l | 3041.69±88.6* | 717.00±33.56* |
Results are presented as x̄±SEM, n=6
P<0.01 vs. Control
P<0.01vs. AngII 10-8mol/l group
cpm, counts per million
AngII increased the levels of secreted and intracellular PAMP and ADM
The effect of Ang II on PAMP and ADM secretion from rat aorta tissues was examined in the incubation medium and the tissue, respectively.
In the control group, PAMP and ADM concentrations detected by radioimmunoassay in the incubation medium of the rat aorta slices were 0.048±0.006 and 0.052±0.011 fmol/mg protein tissue, respectively. PAMP/ADM ratio was 0.95±0.03. AngII treatment led to a concentration-dependent increase in secreted PAMP and ADM from rat aortic slices, as compared to the control group. Specifically, the PAMP concentration increased by 0.63-, 1.04-, and 1.46-fold in response to AngII at concentrations of 10-9, 10-8 and 10-7 mol/l, respectively (all P<0.05 vs. control). The release of ADM was significantly higher than that of the control group, increasing by 0.58-, 1.35-, and 1.92-fold, respectively (all P<0.05 vs. control) (Figure 1A). In addition, secreted PAMP/ADM values also decreased gradually in response to the increasing levels of AngII treatment (P<0.01 vs. control). There were significant differences among all three concentrations of agents (all P<0.05) (Figure 1C).
Figure 1.
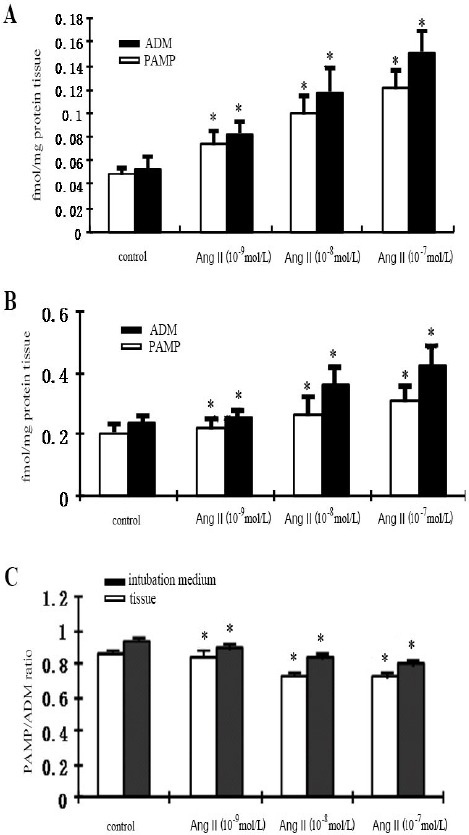
A. The effect of different doses of angiotensin II (Ang II) on secreted proadrenomedullin N- terminal 20 peptide (PAMP) levels in the intubation medium. B. The effect of Ang II on the tissue concentrations of PAMP and adrenomedullin (ADM). C. The effect of AngII on the tissue and incubation concentrations of PAMP and ADM (PAMP/ADM). Data were presented as mean ± SEM; n=6. *P<0.01 vs. control
In the control group, radioimmunoassay-detected intracellular PAMP and ADM levels were 0.203±0.027 and 0.235±0.025 fmol/mg protein tissue, respectively. The PAMP/ADM ratio was 0.86±0.02. AngII treatment led to a dose-dependent increase in both PAMP and ADM peptide levels, in which PAMP was increased by 0.15-, 0.3-, and 0.49-fold, respectively (all P<0.01 vs. control). The levels of ADM were significantly increased by 0.09-, 0.55-, and 0.81-fold, respectively (all P<0.01 vs. control) (Figure 1B). PAMP/ADM ratio was also significantly decreased along with the increase of concentrations (P<0.01 vs. control) (Figure 1C).
PAMP did not affect secreted levels of AngII
To determine whether PAMP could affect the levels of secreted AngII, aorta tissues were treated with 10-9, 10-8 or 10-7 mol/l of PAMP and the incubation medium and tissues were then analyzed by radioimmunoassay. The AngII concentration detected in the control group was 9.935±1.32 fmol/mg protein tissue. Following the PAMP treatment, AngII levels in the culture medium were not significantly increased regardless of the PAMP concentration (all P>0.05 vs. control). Similar pattern was found in the tissues. PAMP treatment (10-9, 10-8 and 10-7 mol/l) did not lead to a dose-dependent increase in secreted AngII (all P>0.05 vs. control) (Figure 2).
Figure 2.
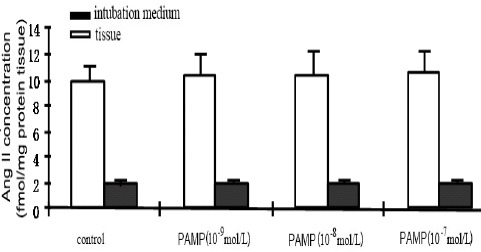
The effect of proadrenomedullin N- terminal 20 peptide (PAMP) on the tissue and incubation concentrations of angiotensin II (Ang II). Data were presented as mean ± SEM; n=6. *P<0.05 vs. control
Discussion
In the present study, we used 3H-TdR incorporation as an indicator of DNA synthesis, and thus were able to monitor cell proliferation in cultured rat VSMCs in response to various treatments. We found that AngII could stimulate proliferation and PKC activity. In contrast, PAMP or ADM alone had no effect on either VSMC 3H-TdR incorporation or PKC activity, suggesting that neither peptides contributed to the proliferation of quiescent VSMCs. However, both ADM and PAMP were able to inhibit AngII-stimulated VSMC proliferation and PKC activity in dose-dependent manners. The same concentrations of either PAMP or ADM produced similar inhibitory effects on AngII-induced VSMC 3H-TdR incorporation and PKC activity.
ADM and PAMP, clinically detectable in plasma, are secreted into the bloodstream by vascular endothelial cells through paracrine mechanisms (6). Previous studies reported that both ADM and PAMP mediated dose-dependent antihypertensive effects when administrated separately via intravenous injections in conscious rats. Although PAMP caused less reflex tachycardia compared with ADM, the effects of PAMP were 100 times weaker than those of ADM (4, 5). It has been determined that ADM and PAMP employ different molecular mechanisms to achieve vasodilation. To date, three distinct pathways have been characterized for ADM-induced relaxation of blood vessels: (1) binding of ADM to specific receptors on the VSMC and involving the cAMP signaling pathway; (2) binding of ADM to specific receptors on the endothelial cell surface and involving the nitric oxide-cGMP signaling pathway; (3) ADM binding the CGRP receptor, leading to increased cAMP through the G protein signaling pathway. In contrast, PAMP exerts its blood pressure lowering functions through G protein-mediated inhibition of the N-type Ca2+ ion channels, thus inhibiting the release of renin from peripheral sympathetic nerve terminals (7, 8). Intriguingly, both hypertensive rats and hypertensive patients, suggesting that both are important in the pathophysiology of hypertension despite their distinct mechanisms of action. It has been proposed that ADM and PAMP can serve as clinical indicators of the severity of hypertension and its associated complications (9). Yet, the complete scope of ADM and PAMP in hypertension remains to be understood, and no studies have reported the effects of ADM or PAMP on proliferation of VSMCs and vascular remodeling.
AngII is the strongest biologically active molecule of the renin-angiotensin system. It not only has a strong vasoconstrictory effect, but also has important modulatory effects on the proliferation and differentiation of VSMCs and vascular remodeling. It has been reported that ADM antagonized the vasoconstrictory effects of AngII. In the present study, ADM and PAMP were found to inhibit AngII-stimulated VSMC proliferation. Upon binding with the angiotensin II type 1 (AT1) specific receptor on the cell membrane, AngII activates phospholipase C by coupling with a G protein, thus causing the hydrolysis of phosphatidyl inositol bisphosphate and generating inositol trisphosphate and diglyceride. Inositol trisphosphate is then able to promote an increase in intracellular Ca2+ and cell constriction, while diglyceride causes increases in intracellular protein synthesis and cell body enlargement by activation of protein kinase C, thereby promoting cell proliferation (10). ADM and PAMP were also able to inhibit AngII-induced activation of PKC activity, indicating that the action of ADM and PAMP in the cellular signaling process located upstream of PKC (11). In this respect, both peptides are similar in intensity. However, whether the precise mode of action and mechanisms are similar requires further study.
It was reported that the expression of the ADM gene may rely on PKC activity and cAMP feedback (12). Gene expression of PAMP is not yet fully understood. It is known, however, that AngII can induce the expression of many diverse genes by acting on the AT1 receptor of vascular endothelial cell or smooth muscle cells. In particular, AngII/AT1 binding induces G protein coupling, which then leads to increases in the intracellular Ca2+ concentration and stimulates PKC activity. Therefore, induction of synthesis and release of ADM/PAMP by AngII in vascular endothelial cells and smooth muscle cells may be related to changes in intracellular Ca2+ concentrations (13), PKC activity or other signaling messengers. As previously stated, the vasodilatory effect of ADM is 100-fold more intense than that of PAMP. Thus, if ADM is produced and released more rapidly in the tissue, the resistance effect to vasocontraction of AngII would be more effective. Although PAMP and ADM share the same precursor and are synergetic in physical function, they still can mutually regulate the production of each other and release under the same stimulus. Such an event of mutual regulation among different segments of the same molecule is known as intramolecular regulation. It is believed that this type of regulation is common in cardiovascular peptides and plays important roles in their physiological and pathophysiological effects. In this study, PAMP had no significant effect on AngII release. However, ADM has been previously demonstrated to reduce the release of AngII in vitro (14). It is likely that the pressure control mechanisms are different between PAMP and ADM.
The regulatory mechanisms of the cardiovascular system are very complicated, with mutual effects of the vasoactive substances playing important roles. Cooperation and antagonism of the vasoactive peptides are not only mediated by biological effects, but also by secretion and metabolism of other paracrine and non-paracrine factors. The pathogenesis of primary hypertension is primarily related to changes in the renin-angiotensin system and, particularly, elevation of AngII. The results of this study have led us to speculate that an increase in PAMP/ADM may be caused by general systemic processes leading towards hypertension (15) and by vasoactive substances such as AngII that promote the synthesis and release of PAMP/ADM. As a result, the combination of PAMP, ADM, and AngII may play an important role in the pathophysiology of hypertension.
Conclusion
ADM and PAMP inhibit the stimulatory effect of AngII on the proliferation of VSMCs. It is likely that PAMP, ADM and their interaction with AngII coordinate vascular tone and proliferation of smooth muscle cells in order to maintain circulatory homeostasis.
Acknowledgment
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
References
- 1.Nishikimi T, Kuwahara K, Nakagawa Y, Kangawa K, Nakao K. Adrenomedullin in cardiovascular diseases: a useful biomarker, its pathological roles and therapeutic application. Curr Protein Pept Sci. 2013;14:256–67. doi: 10.2174/13892037113149990045. [DOI] [PubMed] [Google Scholar]
- 2.Hirata Y, Takagi Y, Takata S, Fukuda Y, Yoshimi H, Fujita T. Calcitonin gene-related peptide receptor in cultured vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1988;151:1113–1121. doi: 10.1016/s0006-291x(88)80481-9. [DOI] [PubMed] [Google Scholar]
- 3.Haller H, Baur E, Quass P, Behrend M, Lindschau C, Distler A, et al. High glucose concentrations and protein kinase C isoforms in vascular smooth muscle cells. Kidney Int. 1995;47:1057–1067. doi: 10.1038/ki.1995.152. [DOI] [PubMed] [Google Scholar]
- 4.Shimosawa T, Fujita T. Hypotensive effect of a newly identified peptide, proadrenomedullin N-terminal 20 peptide. Hypertension. 1996;28:325–329. doi: 10.1161/01.hyp.28.3.325. [DOI] [PubMed] [Google Scholar]
- 5.Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, et al. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1166. doi: 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- 6.Eto T. A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides. 2001;22:1693–711. doi: 10.1016/s0196-9781(01)00513-7. [DOI] [PubMed] [Google Scholar]
- 7.Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, et al. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+mobilization, in bovine aortic endothelial cells. J Biol Chem. 1995;270:4412–4417. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- 8.Takano K, Yamashita N, Fujita T. Proadrenomedu-llin NH2-terminal 20 peptide inhibits the voltage-gated Ca2+channel current through a pertussis toxin-sensitive G protein in rat pheochromocytoma-derived PC 12 cells. J Clin Invest. 1996;98:14–17. doi: 10.1172/JCI118758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inatsu H, Sakata J, Shimokubo T, Kitani M, Nishizono M, Washimine H, et al. Distribution and characterization of rat immunoreactive proadreno-medullin N-terminal 20 peptide (PAMP) and the augmented cardiac PAMP in spontaneously hypertensive rat. Biochem Mol Biol Int. 1996;38:365–372. [PubMed] [Google Scholar]
- 10.Sims C, Ashby K, Douglas JG. Angiotensin II-induced changes in guanine nucleotide binding and regulatory proteins. Hypertension. 1992;19:146–152. doi: 10.1161/01.hyp.19.2.146. [DOI] [PubMed] [Google Scholar]
- 11.Andreis PG, Markowska A, Champion HC, Mazzocchi G, Malendowicz LK, Nussdorfer GG. Adrenomedullin enhances cell proliferation and deoxyribonucleic acid synthesis in rat adrenal zona glomerulosa: receptor subtype involved and signaling mechanism. Endocrinology. 2000;141:2098–2104. doi: 10.1210/endo.141.6.7508. [DOI] [PubMed] [Google Scholar]
- 12.Goichberg P, Kalinkovich A, Borodovsky N, Tesio M, Petit I, Nagler A, et al. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+hematopoietic progenitors. Blood. 2006;107:870–879. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- 13.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 14.Rossi F, Bertone C, Petricca S, Santiemma V. Adrenomedullin antagonizes angiotensin II-stimulated proliferation of human aortic smooth muscle cells. Peptides. 2006;27:2935–41. doi: 10.1016/j.peptides.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Hamid SA, Baxter GF. Adrenomedullin: regulator of systemic and cardiac homeostasis in acute myocardial infarction. Pharmacol Ther. 2005;105:95–112. doi: 10.1016/j.pharmthera.2004.08.012. [DOI] [PubMed] [Google Scholar]


