Abstract
Objective(s):
Sublingual allergen-specific immunotherapy is a safe and effective method for treatment of IgE-mediated respiratory allergies; however, the underlying mechanisms are not fully understood. This study was planned to test whether sublingual immunotherapy (SLIT) can exert epigenetic mechanisms through which the airway allergic responses can be extinguished.
Materials and Methods:
BALB/c mice were sensitized intraperitoneally and challenged intranasally. Then, they received sublingual treatment with recombinant Che a 2 (rChe a 2), a major allergen of Chenopodium album. After SLIT, allergen-specific antibodies in sera, cytokine profiles of spleen cell cultures, mRNA and protein expression of lung-derived IL-33, IL-25, and TSLP (thymic stromal lymphopoietin), and histone modifications of these three genes were assessed.
Results:
Following Immunotherapy, systemic immune responses shifted from Th2 to Th1 profile as demonstrated by significant decrease in IgE and IL-4 and substantial increase in IgG2a and IFN-γ. At local site, mRNA and protein levels of lung-derived pro-inflammatory cytokines IL-33 and TSLP were markedly down-regulated following SLIT that was associated with marked enrichment of trimethylated lysine 27 of histone H3 at promoter regions of these two cytokines.
Conclusion:
In our study, sublingual immunotherapy with recombinant allergen effectively attenuated allergic immune responses, at least partly, by induction of distinct histone modifications at specific loci. Additionally, the lung-derived pro-allergic cytokines IL-33 and TSLP could be promising mucosal candidates for either monitoring allergic conditions or therapeutic approaches.
Keywords: Chenopodium album Histone modifications, IL-25, IL-33, Sublingual mmunotherapy, TSLP
Introduction
Sublingual allergen-specific immunotherapy is currently considered as a safe, valid, and effective approach for treatment of IgE-mediated respiratory allergies (1). Sublingual immunotherapy (SLIT) is known to attenuate allergic responses via immunomodulatory effects (2). Allergens either in native or recombinant forms are used for SLIT (1, 2).
A recent study demonstrated that a single allergen could induce respiratory allergy by exerting epigenetic alterations (3). Epigenetic mechanisms, which cause reversible heritable changes in gene function without changing DNA sequences, may provide explanations for how environmental factors such as allergens can trigger respiratory allergies (4, 5). The epigenetic perturbation of gene expression is now believed to play a key role in the development of allergic diseases (6).
Bronchial epithelial barriers, as direct targets of aeroallergens, play active roles in initiation and amplification of airway allergies, partly, by releasing of pro-inflammatory cytokines including interleukin (IL)-33 (a member of the IL-1 cytokine family), IL-25 (also called IL-17E), and thymic stromal lymphopoietin (TSLP; a member of the hematopoietic cytokine family) (7). These newly-described innate cytokines are now known to orchestrate downstream Th2-type immune responses and subsequent airway pathologies (8). However, a paucity of information exists on the epigenetic alterations of these lung-derived cytokines; particularly following pollen exposure and no study has already evaluated the epigenetic effects of sublingual allergen-specific immunotherapy on the aforementioned cytokines. To do so, we first established a mouse model of pollen allergy. We selected Che a 2, a major allergen of Chenopodium album (9), for induction of respiratory allergy because this weedy plant is a common cause of pollinosis particularly in semi-desert and arid areas worldwide (10), including Iran (11-13). Next, we conducted sublingual pollen-specific immunotherapy by using recombinant Che a 2 (rChe a 2). We used chromatin immunoprecipitation (ChIP) approach to examine possible changes in acetylated lysine 9 of histone H3 (H3K9ac), and trimethylated lysine 4 and lysine 27 of histone H3 (H3K4me3 and H3K27me3), within the promoter regions of the above cytokines following allergy induction and SLIT treatment.
Materials and Methods
Animals
Six- to eight-week-old female BALB/c mice were obtained from the Razi Vaccine and Serum Research Institute (Mashhad, Iran). All mice were adjusted to the environment for seven days before the experiment began. All experiments were carried out according to standard guidelines of animal care and were accepted by the Animal Ethics Committee (No. 910235) of Mashhad University of Medical Sciences, Mashhad, Iran.
Experiment design
Recombinant Che a 2 and the mouse model were previously described by our laboratory (14, 15). Four intraperitoneal injections were administered to mice (n=16) at weekly intervals with 5 μg of rChe a 2 adsorbed in 5 mg Al (OH)3 (Sigma-Aldrich) suspended in 0.2 ml of phosphate-buffered saline (PBS). The sensitization procedure was done by 20-min aerosol challenge of 1% w/v rChe a 2 in PBS on days 28 and 34 after immunization, using an Omron CX3 nebulizer (Omron global, Japan). Control mice (n=5) received PBS plus alum and challenged with PBS, using similar schedule and routes as the experimental mice. Sensitized mice were randomly divided into two groups (n=8) and rested for one week. One group was sublingually treated with 0.1 mg of rChe a 2 (120 μl) every other day for three weeks (the solution was kept under the tongue for 1–2 min and then swallowed). The control (non-sensitized) and PBS (sham-treated) groups received PBS in the same way. Seven days later, mice were challenged with 1% w/v rChe a 2 in PBS on two consecutive days, and sacrificed after 48 hr.
Measurements of rChe a 2-specific Immunoglobulins
After sublingual treatments, blood samples were taken from the tail. Serum allergen-specific antibody levels were determined by enzyme-linked immunosorbent assay (ELISA), as previously described (15). Briefly, the wells of microplates (Nunc, Roskilde, Denmark) were coated with 100 µl of rChe a 2 (20 μg/ml). Mouse sera were diluted 1:10 for IgE, 1:2000 for IgG1, and 1:150 for IgG2a. Biotinylated rat anti-mouse IgE antibody (1:2000; AbD Serotec Inc., Raleigh, NC, USA) and horseradish peroxidase (HRP)-conjugated rat anti-mouse IgG1 and IgG2a antibodies (ebioscience, Inc. San Diego, USA) were used. HRP-streptavidin (1:20000; 1 mg/ml, Bio-Rad, USA) was applied for IgE detection. An ELISA reader (Stat Fax® 2100 Microplate Reader, Awareness Technology Inc., USA) was used to read optical density at 450 nm.
Assessment of cytokines
Spleens cells were homogenized and filtered. Then, red blood cells were lysed. Washed spleen cells were resuspended in complete medium (RPMI 1640 containing 10% fetal calf serum (FCS), 100 U/ml of penicillin and 100 μg/ml of streptomycin), and seeded in duplicate at 1×106 cells per well. Splenocytes were cultured at 37 °C with r Che a 2 (5 μg/ml), phytohemagglutinin (PHA; 1 μg/ml; Sigma), and medium alone. After 48 hr, supernatants were harvested. Thereafter, mouse IFN-γ, IL-4, TGF-β, and IL-10 levels were measured by ELISA according to the manufacturer’s instructions (ebioscience, USA).
Real-time PCR
Total RNA was extracted from 50 mg of lung tissues and cDNA was synthesized (Total RNA extraction kit and Easy cDNA synthesis kit; Pars-Tous biotechnology) using random hexamer primers according to the manufacturer’s instructions. Using Stratagene system (Stratagene Mx-3000P, USA) and the SYBR green 2x Master Mix (Pars-Tous biotechnology), relative mRNA expressions were measured. The expression of IL-33, TSLP, and IL-25 mRNA were normalized to mouse hypoxanthine-guanine phosphoribosyl transferase (mHPRT; a housekeeping gene) and calculated using the Pffafl method (16). Primer sequences are detailed in Table 1.
Table 1.
Primer sequences
| Quantitative PCR | 5’ - 3’ | ChIP | 5’ - 3’ |
|---|---|---|---|
| IL-33 | IL-33 | ||
| F | AAGATATTCACTAAAATCGGGTAC | F | GCCACAGGGAACGATTTTAT |
| R | GGATGCTCAATGTGTCAAC | R | CCTTCAGCATTCCTTTATGGAC |
| TSLP | TSLP | ||
| F | TACTATACTCTCAATCCTATCC | F | ATCTTAACCCAACCCACCAT |
| R | GACTTCTTGTGCCATTTCCT | R | CTAGGGGAGGAACAGCTTCT |
| IL-25 | IL-25 | ||
| F | GGAGTGGCTGAAGTGGAG | F | CTCAAGTCACTCCCTCTA |
| R | ACCCGATTCAAGTCCCTG | R | CACCAGTCACAGGTACTA |
| mHPRT | mHPRT | ||
| F | ATACAGGCCAGACTTTGTTGGR | F | ATTATCTGGGAATCCTCTG |
| R | TTTCCAGTTTCACTAATGACACAA | R | CGGAACTCTTATCTGACTA |
F, Forward R, Reverse TSLP, Thymic stromal lymphopoietin mHPRT, mouse hypoxanthine-guanine phosphoribosyl transferase.
Western blotting analysis
Mouse IL-33 and TSLP were cloned and expressed in Escherichia coli for this study. Then, polyclonal antibodies were produced in rabbits immunized with the purified recombinant mouse IL-33 and TSLP (unpublished data). Next, 50 mg of lung tissues were homogenized in lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, and 1% Triton x-100) containing protease inhibitors (1 mM PMSF, 2 µg/ml aprotinin, and 2 µg/ml leupeptin). Samples were incubated on ice for 15 min, sonicated on ice for 2 min (1 sec on/off pulse at 80% amplitude; Sonopuls Bandelin electronic, Germany), and then subjected to centrifugation (12,000 g) for 15 min at 4 °C. Supernatants were collected, and protein concentrations were measured using the bicinchoninic acid (BCA) assay (17). Western blot was performed as described earlier, with slight modifications (18). Briefly, samples (100 μg/lane) were fractionated by 12.5% SDS-PAGE and transferred to polyvinylidene difluoride membrane (PVDF; Millipore Corp., Bedford, USA). Primary antibodies (at 1:1000 dilutions) used in this protocol were as follows: polyclonal rabbit anti-mouse IL-33 and TSLP (produced in this study) and polyclonal rabbit anti-mouse IL-25 (Bioss Antibodies-online, USA). HRP-conjugated polyclonal goat anti-rabbit antibody (Bio-Rad, USA) was used as the secondary antibody (1:60000). Rabbit anti-mouse beta actin (1:2000; Abcam, France) was used as an internal control. Finally, the signals were developed by the chemiluminescent substrates as recommended by the manufacturer (Pars-Tous biotechnology) and documented with a G-Box imaging system (Syngene, UK). Image analysis software (Kodak 1D version 3.6) was used to quantify the relative intensity of the bands based on previously detailed methodology (19).
Chromatin immunoprecipitation (ChIP)
ChIP was performed as detailed earlier with the following modifications (20). In brief, lung tissue (50 mg) was cut into small pieces with a scalpel and fixed with 1% v/v formaldehyde in PBS. Cross-linking was quenched by adding glycine (0.125 M) followed by a centrifugation step (300 g). The pellet was washed in cold PBS and resuspended in 260 µl of lysis buffer (1% SDS, 5% Triton X-100, 5 mM EDTA, 750 mM NaCl, 100 mM HEPES) containing 0.1% bovine serum albumin (BSA; Biosera, France). Chromatin was fragmented using a Sonopuls sonicator (Bandelin electronic, Germany) at 70% amplitude and 1 sec on/off pulses for 90 sec, to reduce DNA size ranging from 200 to 1000 base pairs. Solubilized chromatin was then aliquoted into individual 40 µl volumes. One aliquot was removed for the total input DNA control. Four aliquots were diluted tenfold in ChIP dilution buffer (360 µl/sample) and pre-treated with Protein-A sepharose beads (100 µl/sample; GE Healthcare Life Sciences, USA) for 2 hr at 4 °C. The diluted samples were incubated overnight at 4 °C with (three samples) or without (no antibody sample as negative control) one of the three polyclonal antibodies (2 µg/reaction) including anti-acetyl lysine 9 of histone H3 (H3K9ac; Epigentek Group Inc., USA) and anti-trimethyl lysine 4 and lysine 27 (H3K4me3 and H3K27me3; Epigentek). Immune complexes were precipitated using Protein-A sepharose beads (100 µl/sample). The beads were washed sequentially with 0.8 ml low salt wash buffer, high salt wash buffer, LiCl wash buffer, and TEsolution (10 mM Tris-HCl (pH 8.0), 1 mM EDTA). Protease inhibitors were added to all buffers. Immunoprecipitates were eluted in 0.8 ml of elution buffer, then, incubated overnight at 65 °C under high salt (32 µl of 0.2 M NaCl) condition to reverse DNA-protein cross-linking. DNA was extracted and purified using a column-based kit (Pars-Tous biotechnology) and amplified by SYBR green-based quantitative PCR. Primers were designed on -600 base pair (bp) promoter regions of IL-33 (21) and mHPRT, the proximal promoter region (-200 bp) of TSLP (22), and the transcriptional start site (TSS)-containing promoter region of IL-25, relative to the TSS. Primer sequences are listed in Table 1. All amplifications were performed in duplicate using 2 µl of eluted DNA per reaction. ChIP data was calculated by Percent Input Method described in the Life Technologies website (www.-lifetechnologies.com/en/life-science/epigenetics).
The signal from the antibody of interest was normalized to the total input. The background signal (no antibody control) was measured in comparison with the antibody of interest.
Statistical analysis
All data were analyzed and plotted by Prism 5.0 software (GraphPad Software Inc., San Diego, USA). Data is displayed as means ± standard error of the mean (SEM). Differences among various groups were evaluated using one-way ANOVA and Tukey post hoc test. All P-values <0.05, <0.01, and<0.001 were considered significant.
Results
Systemic responses following SLIT
Induction of type 1 allergy was serologically affirmed via assessment of anti-rChe a 2-specific IgE (data not shown). Serum specific antibody responses and splenocyte-derived cytokine concentrations were measured after SLIT (Table 2). The sensitized (PBS) mice showed significantly increased levels of IgE, IgG1, IgG2a IL-4, and IL-10 (P<0.01), and significantly reduced IFN-γ levels (P<0.05) as compared to non-sensitized mice (Control group; Figure 1), signifying a strong Th2-biased responses. TGF-β production was not significantly different between the two groups (P=0.98; Figure 1g).
Table 2.
Serum specific antibody responses and splenocyte-derived cytokine concentrations from the respective groups after SLIT
| Groups | IgE | IgG1 | IgG2a | IFN-γ | IL-4 | IFN-γ/IL-4 | TGF-β | IL-10 |
|---|---|---|---|---|---|---|---|---|
| Control | .07±.007 | .16±.02 | .25±.02 | 445±44 | 16±3 | 30±9 | 364±8 | 167±4 |
| PBS | 1.37±.1# | .59±.07# | .77±.06# | 81±7# | 74±8# | 1±.2# | 330±10 | 319±15# |
| rChe a 2 | .55±.05* | .44±.01 | 1.09±.01* | 689±97* | 38±4* | 19±5 | 1036±56* | 223±35 |
Results are means optical density (OD; for antibodies) or mean concentration (pg/ml; for cytokines) ± SEM. P<.05 vs PBS group,
P <.05 vs control group
Figure 1.
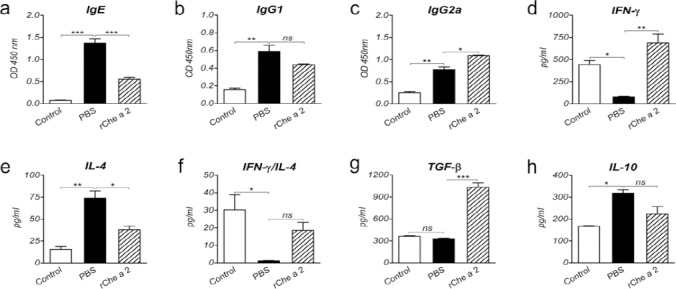
Systemic responses after sublingual immunotherapy. a-c, Specific IgE (a), IgG1 (b), and IgG2a (c) serum levels in control (non-sensitized), PBS-treated (sham treated), and rChe a 2-treated groups. d-h, IFN-γ (d), IL-4 (e), TGF-β (g), and IL-10 (h) levels and IFN-γ/IL-4 (f) in the supernatants of splenocytes stimulated with rChe a 2 from the respective groups. Bars indicate the average OD of antibody levels or cytokine concentrations in pg/ml ± SEM. ns, not significant. * P<0.05, ** P<0.01, and ***P<0.001
Sublingual immunotherapy was performed using rChe a 2 as allergen-specific immunotherapy. IgE (P=0.001) and IL-4 (P=0.05) production, signatures of Th2 response, in rChe a 2-treated mice was significantly lower than that in PBS-treated mice (Figures 1a and 1e). On the contrary, IgG2a (P=0.03) and IFN-γ (P=0.002), signatures of Th1 response, were significantly higher in the SLIT-mice than in the mice that received PBS (Figures 1c and 1d), all indicating a shift toward Th1 responses after rChe a 2 treatment. IgG1 levels (P=0.3) and IFN-γ/IL-4 ratio (P=0.2) were not significantly different between SLIT-treated and PBS-treated mice (Figures 1b and 1f).
To investigate the regulatory effects of SLIT, the production of TGF-β and IL-10 was assessed. TGF-β was significantly greater in the SLIT-treated than the PBS-treated mice (P<0.001, Figure 1g). However, No significant differences were detected between serum level of IL-10 in rChe a 2-treated and that in the PBS-treated mice (P=0.1, Figure 1h).
Expression analysis of lung-derived pro-allergic cytokines following SLIT (local responses)
We anticipated that expression of lung-derived IL-33, TSLP, and IL-25 would be up-regulated following airway allergy induction, and down-regulated following treatment. Expressions of these cytokines under these conditions were evaluated by quantitative real-time PCR and western blotting.
IL-33 mRNA expression was not markedly increased in lung tissues of the PBS-treated as compared to the control mice (P=0.06; Figure 2a). SLIT with rChe a 2 (P=0.03) substantially reduced IL-33 mRNA expression comparing to the PBS-treated mice. Western blots demonstrated the presence of 30 and ~20 kDa bands regarding to full-length (f) and cleaved (c)-IL-33, respectively (Figure 2d). These two forms are now considered as biologically active proteins (23). Expression of both full-length and cleaved IL-33 protein was markedly lower in the controls and the rChe a 2-treated than in the PBS-treated mice (P<0.05; Figures 2e and 2f). In all experimental groups, the level of full-length IL-33 was greater than the cleaved form. Regarding the full-length to cleaved IL-33 ratio, no significant differences were observed among the groups (Figure 2g).
Figure 2.
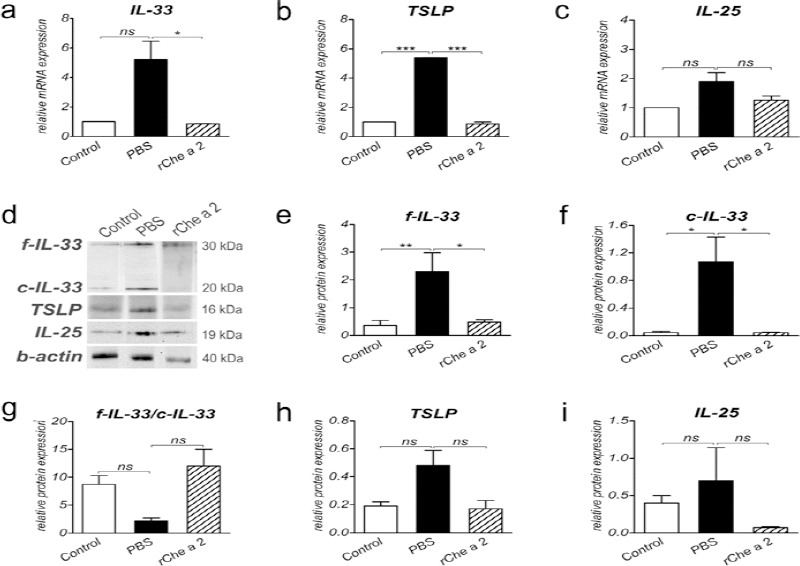
Analysis of the expression of lung-derived cytokines following sublingual immunotherapy. a-c, The relative mRNA expressions of IL-33 (a), TSLP (b), and IL-25 (c) from lung tissues of the respective treated groups were evaluated by CYBR green-based real-time PCR (n = 5 to 8 mice per group). Expression level was normalized to mouse HPRT mRNA. d, Western blot analysis of above-mentioned cytokines. e-i, The relative protein band intensity of full-length IL-33 (e), cleaved IL-33 (f), TSLP (h), and IL-25 (i). g, Full-length to cleaved IL-33 ratio. Expression level was normalized to mouse beta-actin protein. Data are expressed as mean ± SEM. ns, not significant. * P<0.05, ** P<0.01, and ***P<0.001
TSLP mRNA was markedly up-regulated in PBS-treated as compared to control mice (P<0.001; Figure 2b); however, TSLP protein (MW = 16 kDa; Figure 2d)
(P=0.057) expression was not significantly different between the two groups (Figure 2h). Following SLIT, mRNA expression of lung TSLP was significantly reduced in the rChe a 2- treated mice as compared to the PBS-treated mice (P<0.001, Figure 2b); however, no significant differences in TSLP protein expression were found among groups (P=0.058, Figure 2h).
IL-25 mRNA (P=0.4) and protein (MW = 19 kDa; Figure 2d) (P=0.9) expressions were not markedly elevated in sensitized (PBS) mice as compared to non-sensitized (control) animals (Figures 2c and 2i). Lung IL-25 mRNA (P=0.7) or protein (P=0.6) expression in rChe a 2-treated mice was not significantly lower than those of PBS-treated mice (Figures 2c and 2i).
Histone modifications of lung-derived cytokines after SLIT
To elucidate whether alterations in IL-33, TSLP, and IL-25 expression following sensitization and treatment were associated with changes in histone codes, we assessed the levels of H3k9ac and H3K4me3, the markers for active chromatin (24), and H3k27me3, a marker of inactive chromatin (25), in lung-extracted chromatin from sublingually-treated mice using ChIP-qPCR. Mouse HPRT and no-antibody samples were used as positive and negative controls, respectively.
Although following sensitization (PBS group) an increasing trend was observed for the levels of H3K9ac and H3K4me3 within IL-33, TSLP and IL-25 genes, it was not significant (Figure 3). Therefore, regarding these two active markers, the results indicated that up-regulation of these three cytokines upon sensitization is poorly dependent on H3K9ac and H3K4me3. Moreover, a non-significant decrease in H3K27me3 levels at promoters of all three cytokines, was observed (Figure 3).
Figure 3.
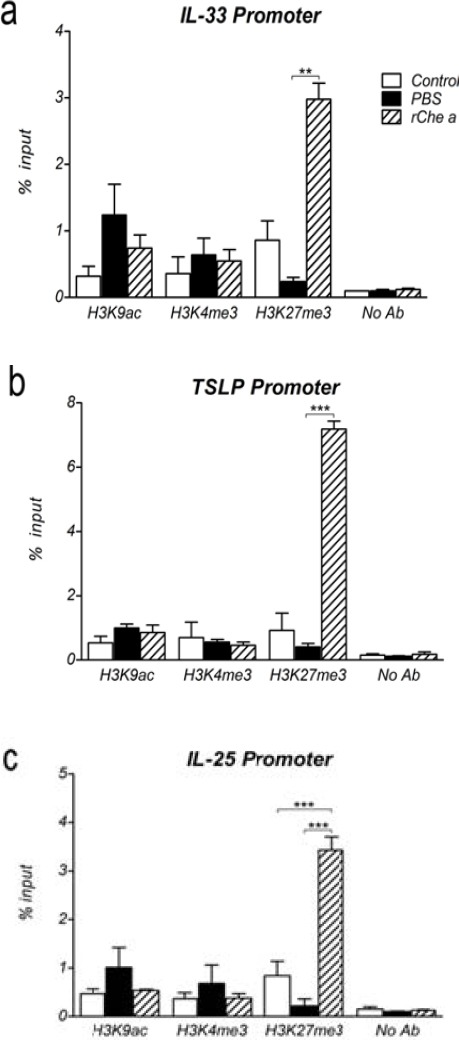
Histone modifications within the promoters of lung-derived cytokines after sublingual immunotherapy. a-c, Chromatins were extracted from lung tissues of sublingual-treated mice and then, H3K9ac, H3K4me3, and H3K27me3 levels within the IL-33 (a), TSLP (b), and IL-25 (c) promoters were determined by means of ChIP-real time PCR in respective treated groups (n=5 to 8 mice per group). No antibody sample was used as negative control. Data are mean ± SEM. **P<0.01 and ***P<0.001
Immunotherapy with rChe a 2, in comparison with PBS treatment, did not significantly affect H3k9ac or H3K4me3 levels at promoters of the three lung-derived cytokines. On the contrary, trimethylated lysine 27 of histone H3, which labels inactive chromatin, was highly enriched on IL-33 (P=0.01), TSLP (P<0.001), and IL-25 (P<0.001) genes, suggesting a role for H3K27me3 in down-regulation of these pro-inflammatory cytokines following rChe a 2 treatment (Figures 3a, 3b, and 3c).
Discussion
This study showed that epigenetic regulation of lung-derived cytokines expression could be a mechanism underlying sublingual treatments of pollen allergies. We observed marked increases in H3K27 trimethylation at the promoters of lung-derived innate cytokines IL-33, TSLP, and IL-25, which were negatively associated with expression levels. To assess histone modifications induced by sublingual administration of recombinant allergen, we established a murine model of Chenopodium album (C. album) pollen allergy.
A recent study demonstrated an inter-relationship between epithelial-derived IL-33, TSLP, and IL-25, as Th2-related allergic responses were promoted by co-expression and joint biological activities of these cytokines (26). In our study, we observed that lung IL-33 and TSLP, but not IL-25, were up-regulated following sensitization and down-regulated following SLIT. Nakanishi et al also showed that IL-33, but not IL-25, was important for development of house dust mite-induced allergic rhinitis (27). Elevated expression of IL-33 and TSLP upon sensitization was linked to Th2-biased responses as indicated by marked production of IgE, IgG1, and IL-4, and significant reduction in IFN-γ level. Genetic association studies and expression analysis of IL-33 and TSLP in human samples, also demonstrated a link between these two cytokines and respiratory type 1 allergies (28). These findings indicate that pulmonary IL-33 and TSLP, as upstream immune responses, could be used to monitor airway allergic responses and that modulation of allergic responses at mucosal sites might be achieved by single or combined targeting of these lung-derived cytokines.
Another study indicated that either full-length or cleaved forms of IL-33 are biologically active especially in clinical conditions (23) and that processing of IL-33 upon inflammatory conditions may increase pathologic functions of IL-33. Both full-length (f) and cleaved (c) forms of IL-33 were detected after sensitization and treatment. Because f-IL-33 level was higher than c-IL-33 in all sensitization or treatment procedures, we assumed that the full-length form might play the main role in C. album allergy. Cleaved IL-33 was at a considerable level only in PBS-treated (sensitized) mice. Accordingly, we speculate that following sensitization, neutrophil enzymes process IL-33 into highly active forms; (23) however, following SLIT, enzymes from other cells may process IL-33 into less active forms. This data could bring new insights into understanding of regulation of IL-33 activity in allergic conditions.
Recombinant allergens are currently adminis-tered for pollen-specific immunotherapy, especially sublingually (29). In our study, SLIT with rChe a 2 effectively suppressed Th2-related responses, as indicated by marked reductions in IL-4 and specific IgE. Immunotherapy also significantly reduced both mRNA and protein levels of pro-inflammatory cytokines IL-33 and TSLP. These findings, together with increased production of Th1 signature cytokines and antibodies (i.e. IFN-γ and IgG2a) indicate a shift from Th2 to Th1 responses upon allergen-specific sublingual therapy. Intriguingly, rChe a 2-treated mice prominently produced TGF-β, the signature cytokine of regulatory T cells. However, suppression of allergic responses was not related to IL-10 production, assuming that distinct subset of regulatory T cells, Th3, might be involved. Similar to this data, Wiedermann and colleagues demonstrated that attenuation of the allergic airway responses following intranasal or oral administration of recombinant Bet v 1, the major birch pollen allergen, was closely correlated with increased expression of TGF-β, but not IL-10 (30). Different from our study, SLIT with Rye grass extracts showed no significant difference in specific IgG and IgE, but significant increase in TGF-β and IL-10 (31). The difference may result from differences in the content of what was used for SLIT, i.e. recombinant allergen versus plant extract. These results provide further evidence for the local and systemic immuno-regulatory mechanisms of allergen-specific SLIT, as reviewed by Allam and Novak (32).
Cheng et al showed that epigenetic disruption of gene expression in the lung could be induced by a specific allergen to induce airway inflammation (3). Therefore, we assessed the epigenetic consequences of rChe a 2 exposure for lung-derived cytokines. We analyzed three histone codes including two transcriptional activators, H3K9 acetylation and H3K4 trimethylation, and one transcriptional repressor, H3K27 trimethylation. Alterations in H3K9ac and H3K4me3 levels at the promoter of lung IL-33, TSLP, and IL-25 genes, were neither significant nor linked to expression levels of the three cytokines. This might be addressed by the facts that H3K9 acetylation and H3K4 trimethylation might occur within other regulatory sites of the three genes including intergenic regions, distal promoters, or 5’ untranslated regions (UTRs) (33), or that other histone codes might be implicated in transcriptional activation of these three cytokines. Interestingly, rChe a 2 treatment led to elevated levels of H3K27me3 at promoter regions of IL-33, TSLP, and IL-25 genes. H3K27 trimethylation at IL-33 and TSLP genes was associated with reduced expression of these cytokines in lung tissue. Therefore, enrichment of H3K27me3 at the IL33 and TSLP genes might be a mechanism underlying specific treatment of type 1 pollen allergy. These finding may impart that epigenetic targets could be considered as novel therapeutic approaches for respiratory allergic diseases (34). However, due to the limitations of the model, we are indispensable for considering the involvement of epigenetic alterations of other immuno-regulatory molecules in the multifaceted process of respiratory allergy development or treatments. The heterogeneity in the lung tissues may also have affected our data. Thus, epigenetic changes induced by rChe a 2 in the lung may involve regulation of gene expression in all or a subset of lung cell types.
Conclusion
This study showed that allergen-specific immunotherapy could attenuate allergic immune responses, at least partly, by induction of distinct histone modifications at specific loci. Therefore, recombinant pollens can be considered as a promising sublingual treatment for pollen allergies. Additionally, since the lung-derived pro-allergic cytokines IL-33 and TSLP can influence downstream allergic responses, they could be promising mucosal candidates for either monitoring allergic inflammatory conditions or therapeutic approaches. Data observed in animal models are now the basis to be translated into the human situation.
Acknowledgment
The results described in this paper were part of PhD thesis of the first author supported by the Research Administration Department of Mashhad University of Medical Sciences, Mashhad, Iran. The study was performed in the Immuno-biochemistry lab, Bu-Ali Research Institute, Mashhad University of Medical Sciences. We thank Dr James McCoy (University of Virginia, USA) for English editing and critical reading of the manuscript.
Conflict of interest
All authors declare they have no actual or potential conflicts of interest including financial, personal, or other relationships with other people or organizations.
References
- 1.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody FM, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130:215–224. e7. doi: 10.1016/j.jaci.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng RY, Shang Y, Limjunyawong N, Dao T, Das S, Rabold R, et al. Alterations of the lung methylome in allergic airway hyper-responsiveness. Environ Mol Mutagen. 2014;55:244–255. doi: 10.1002/em.21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand S, Kesper DA, Teich R, Kilic-Niebergall E, Pinkenburg O, Bothur E, et al. DNA methylation of TH1/TH2 cytokine genes affects sensitization and progress of experimental asthma. J Allergy Clin Immunol. 2012;129:1602–1610. e6. doi: 10.1016/j.jaci.2011.12.963. [DOI] [PubMed] [Google Scholar]
- 5.Shang Y, Das S, Rabold R, Sham JS, Mitzner W, Tang WY. Epigenetic alterations by DNA methylation in house dust mite-induced airway hyperrespon-siveness. Am J Respir Cell Mol Biol. 2013;49:279–287. doi: 10.1165/rcmb.2012-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harb H, Renz H. Update on epigenetics in allergic disease. J Allergy Clin Immunol. 2015;135:15–24. doi: 10.1016/j.jaci.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14:686–698. doi: 10.1038/nri3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barderas R, Villalba M, Pascual CY, Batanero E, Rodriguez R. Profilin (Che a 2) and polcalcin (Che a 3) are relevant allergens of Chenopodium album pollen: isolation, amino acid sequences, and immunologic properties. J Allergy Clin Immunol. 2004;113:1192–1198. doi: 10.1016/j.jaci.2003.12.587. [DOI] [PubMed] [Google Scholar]
- 10.Villalba M, Barderas R, Mas S, Colas C, Batanero E, Rodriguez R. Amaranthaceae pollens: review of an emerging allergy in the mediterranean area. J Investig Allergol Clin Immunol. 2014;24:371–381. [PubMed] [Google Scholar]
- 11.Fereidouni M, Hossini RF, Azad FJ, Assarehzadegan MA, Varasteh A. Skin prick test reactivity to common aeroallergens among allergic rhinitis patients in Iran. Allergol Immunopathol (Madr) 2009;37:73–79. doi: 10.1016/s0301-0546(09)71108-5. [DOI] [PubMed] [Google Scholar]
- 12.Assarehzadegan MA, Shakurnia A, Amini A. The most common aeroallergens in a tropical region in Southwestern Iran. World Allergy Organ J. 2013;6:7. doi: 10.1186/1939-4551-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadiafshar A, Sepehri S, Mousavinasab S, Torabi SZ. Recognition and frequency of common allergens in allergic patients of zanjan by skin prick test. Zanjan Univ Med Sci J. 2008;16:45–53. [Google Scholar]
- 14.Amini A, sankian M, Assarehzadegan MA, Vahedi F, Varasteh A. Chenopodium album pollen profilin (Che a 2): homology modeling and evaluation of cross-reactivity with allergenic profilins based on predicted potential IgE epitopes and IgE reactivity analysis. Mol Biol Rep. 2011;38:2579–2587. doi: 10.1007/s11033-010-0398-2. Epub 2010/11/19. [DOI] [PubMed] [Google Scholar]
- 15.Nouri HR, Sankian M, Afsharzadeh D, Varasteh A. Immunotherapy with a recombinant hybrid molecule alleviates allergic responses more efficiently than an allergenic cocktail or pollen extract in a model of Chenopodium album allergy. Int Arch Allergy Immunol. 2013;161:325–332. doi: 10.1159/000347136. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nuclic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith P, Krohn RI, Hermanson G, Mallia A, Gartner F, Provenzano M, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 18.Nouri HR, Varasteh A, Vahedi F, Chamani J, Afsharzadeh D, Sankian M. Constructing a hybrid molecule with low capacity of IgE binding from Chenopodium album pollen allergens. Immunol lett. 2012;144:67–77. doi: 10.1016/j.imlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SC, Berkelman T, Yadav G, Hammond M. A defined methodology for reliable quantification of Western blot data. Mol Biotechnol. 2013;55:217–226. doi: 10.1007/s12033-013-9672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sailaja BS, Takizawa T, Meshorer E. Chromatin immunoprecipitation in mouse hippocampal cells and tissues. Methods Mol Biol. 2012;809:353–64. doi: 10.1007/978-1-61779-376-9_24. [DOI] [PubMed] [Google Scholar]
- 21.Negishi H, Miki S, Sarashina H, Taguchi-Atarashi N, Nakajima A, Matsuki K, et al. Essential contribution of IRF3 to intestinal homeostasis and microbiota-mediated Tslp gene induction. Proc Natl Acad Sci U S A. 2012;109:21016–21021. doi: 10.1073/pnas.1219482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Zhang LJ, Guha G, Li S, Kyrylkova K, Kioussi C, et al. Selective ablation of Ctip2/Bcl11b in epidermal keratinocytes triggers atopic dermatitis-like skin inflammatory responses in adult mice. PLoS One. 2012;7:e51262. doi: 10.1371/journal.pone.0051262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard J-P, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakanishi W, Yamaguchi S, Matsuda A, Suzukawa M, Shibui A, Nambu A, et al. IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One. 2013;8:e78099. doi: 10.1371/journal.pone.0078099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byers DE. Defining the roles of IL-33, thymic stromal lymphopoietin, and IL-25 in human asthma. Am J Respir Crit Care Med. 2014;190:715–716. doi: 10.1164/rccm.201408-1539ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallner M, Pichler U, Ferreira F. Recombinant allergens for pollen immunotherapy. Immunotherapy. 2013;5:1323–1338. doi: 10.2217/imt.13.114. [DOI] [PubMed] [Google Scholar]
- 30.Wiedermann U, Jahn-Schmid B, Bohle B, Renz H, Kraft D, Ebner C. Suppression of antigen-specific T-and B-cell responses by intranasal or oral administration of recombinant bet v 1, the major birch pollen allergen, in a murine model of type I allergy. J Allergy Clin Immunol. 1999;103:1202–1210. doi: 10.1016/s0091-6749(99)70200-9. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadiafshar A, Taymourzadeh B, Shaikhi A, Mazloomzadeh S, Torabi Z. Evaluation of IL10, TGF-B and Specific IgE and IgG Levels during Sublingual Rye Grass Immunotherapy. J Allergy Ther. 2013;4:132. [Google Scholar]
- 32.Allam J-P, Novak N. Immunological mechanisms of sublingual immunotherapy. Curr Opin Allergy Clin Immunol. 2014;14:564–569. doi: 10.1097/ACI.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 33.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comer BS, Ba M, Singer CA, Gerthoffer WT. Epigenetic targets for novel therapies of lung diseases. Pharmacol Ther. 2015;147:91–110. doi: 10.1016/j.pharmthera.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


