Abstract
Aims
Although non-vitamin K antagonist oral anticoagulants are recommended for stroke prevention in patients with non-valvular atrial fibrillation (NVAF) based on clinical trial results, there is a need for safety and efficacy data from unselected patients in everyday clinical practice. XANTUS investigated the safety and efficacy of the Factor Xa inhibitor rivaroxaban in routine clinical use in the NVAF setting.
Methods and results
Consecutive consenting patients with NVAF newly started on rivaroxaban were eligible and were followed up at ∼3-month intervals for 1 year, or for at least 30 days after permanent discontinuation. All adverse events (AEs) were recorded as AEs or serious AEs; major outcomes (including major bleeding, symptomatic thromboembolic events [stroke, systemic embolism, transient ischaemic attack, and myocardial infarction], and all-cause death) were centrally adjudicated. There were 6784 patients treated with rivaroxaban at 311 centres in Europe, Israel, and Canada. Mean patient age was 71.5 years (range 19–99), 41% were female, and 9.4% had documented severe or moderate renal impairment (creatinine clearance <50 mL/min). The mean CHADS2 and CHA2DS2-VASc scores were 2.0 and 3.4, respectively; 859 (12.7%) patients had a CHA2DS2-VASc score of 0 or 1. The mean treatment duration was 329 days. Treatment-emergent major bleeding occurred in 128 patients (2.1 events per 100 patient-years), 118 (1.9 events per 100 patient-years) died, and 43 (0.7 events per 100 patient-years) suffered a stroke.
Conclusion
XANTUS is the first international, prospective, observational study to describe the use of rivaroxaban in a broad NVAF patient population. Rates of stroke and major bleeding were low in patients receiving rivaroxaban in routine clinical practice.
Trial registration number
Clinicaltrials.gov: NCT01606995.
Keywords: Anticoagulants, Atrial fibrillation, Real world, Rivaroxaban, Stroke, Thromboembolism
See page 1154 for the editorial comment on this article (doi:10.1093/eurheartj/ehv532)
Introduction
Atrial fibrillation (AF) affects ∼2% of the European population, and its prevalence is rising due to concomitant conditions and ageing populations.1 Stroke is one serious consequence of AF,2 but oral anticoagulation can prevent most cases of AF-related stroke.3,4 Although the evidence supporting the use of anticoagulation for stroke prevention in AF has been generated with dose-adjusted vitamin K antagonists (VKAs), four non-VKA oral anticoagulants (NOACs) have been found to be at least as effective and safer than VKAs for stroke prevention in patients with non-valvular AF (NVAF).5–8 These NOACs have been approved for use in this indication and are recommended as alternatives to VKAs in international guidelines.3,4 Further information on the effectiveness of NOACs is still accumulating in the form of retrospective registries and additional randomized clinical trials. A high volume of prospectively collected information in large patient groups is still lacking. This was recognized by the European Medicines Agency (EMA), leading to a requirement to conduct observational studies as part of the post-approval plan. Here, outcomes are reported from the XANTUS study, which assessed the safety and efficacy of rivaroxaban in routine, ‘real-world’ clinical practice.
Methods
The design of the international, non-interventional, observational XANTUS study was approved by the EMA and details have been published previously.9
Study population and screening
Eligible patients had a diagnosis of NVAF, were aged ≥18 years, started rivaroxaban therapy to reduce the risk of stroke or systemic embolism (SE), and provided written informed consent. All patients were screened sequentially and documented in an anonymous log file independent of therapy.
Medication and follow-up
Decisions about rivaroxaban prescription were at the discretion of the treating physician, including dose and duration of therapy. Label-recommended rivaroxaban doses for stroke prevention in NVAF are 20 mg once daily (od) for patients with normal renal function or mild impairment (creatinine clearance [CrCl] ≥50 mL/min) and 15 mg od for patients with moderate or severe renal impairment (CrCl 15–49 mL/min; e.g. in Europe). After the screening visit, follow-up data collection was at the time of hospital discharge, if applicable, and approximately every 3 months thereafter. The overall follow-up period was 1 year. For patients who discontinued therapy before the end of the 1-year follow-up, the observation period ended ∼30 days after the last dose of rivaroxaban.
Study outcomes
The primary outcomes were related to the safety of rivaroxaban, recorded as adverse events (AEs) or serious AEs (SAEs), and included major bleeding events (defined using International Society on Thrombosis and Haemostasis [ISTH] criteria), all-cause death, and any other AEs and SAEs.9 Secondary outcomes included symptomatic thromboembolic events (stroke, non-central nervous system SE, transient ischaemic attack [TIA], and myocardial infarction [MI]) and non-major bleeding events (defined as any bleeding event not meeting the criteria for a major haemorrhage) across patients with different baseline risk profiles for stroke or bleeding. Intracranial bleeding that met the definition of stroke was included in both stroke and major bleeding endpoints. Haemorrhagic transformations of ischaemic stroke were counted as a major bleeding event regardless of whether symptomatic or not. Other outcomes included treatment persistence, patient satisfaction (reported by patients using standardized questionnaires), healthcare resource use, and details of treatment interruption and interventions such as management of bleeding events and stroke.
Study conduct
XANTUS applied several quality assurance measures. Physicians were specifically requested to document at every visit if bleeding, stroke, SE, TIA, MI, or other AEs had occurred, and this was captured as a ‘yes/no’ response for each event of interest. To detect unreported events, the database was searched for concomitant medications, interventions, other key words, and laboratory findings potentially associated with a bleeding or thromboembolic event. Questionable findings from this search triggered medical queries to the investigator and potentially central adjudication.
An independent Central Adjudication Committee (CAC) of five expert physicians (not directly involved in the care of XANTUS study patients) adjudicated major bleeding, stroke, SE, TIA, MI, and all-cause death. The CAC had access to all patient records. Bleeding events were documented by the investigators as AEs. A verified algorithm was used to search the database for all recorded bleeding AEs that were associated with transfusions, were fatal, occurred at a critical site, were associated with an intervention, or were assessed as ‘major’ by the investigator. The algorithm also identified any recorded haemoglobin decreases of ≥2 g/dL regardless of the documentation of an AE. All cases identified via this algorithm were adjudicated by the CAC. Thromboembolic events were also recorded as AEs. In cases of potential stroke, SE, TIA, or MI from either investigator assessment or a database search, central adjudication was performed. The CAC also adjudicated the type of stroke and occurrence of a haemorrhagic transformation of ischaemic stroke. Clinical cause of death was centrally adjudicated. To ensure reporting standards, quality assessment and source data verification visits were performed at 61 (19.6%) sites between Q4–13 and Q3–14, and documentation related to 581 patients (8.6%) was reviewed.
Study governance
The study complied with the Declaration of Helsinki, was approved by the appropriate Health Authorities, independent Ethics Committees, and Independent Review Boards as required, and was conducted in accordance with Good Pharmacoepidemiological Practice (GPP). An independent academic Steering Committee oversaw the design, execution, and conduct of the study, was responsible for manuscript development, had full access to all data, and approved all versions of the manuscript.
Patients' informed consent included the permission for data collection and analysis. To minimize the risk of loss to follow-up, in countries where this is permitted, patients could voluntarily provide an alternative contact to the investigator/independent patient management team. In compliance with Good Clinical Practice (GCP) standards, data management and statistical analyses were overseen by the sponsor. The lead statistician oversaw programming and validation of the statistical analyses.
Statistical analysis
Events were considered ‘treatment-emergent’ if they occurred from the day of the first dose of rivaroxaban, and up to 2 days after the last dose in the event of permanent discontinuation. Statistical analyses of the events were descriptive, exploratory, and generally limited to frequency tables or summary statistics (e.g. mean ± standard deviation [SD] or median ± quartiles). The primary analysis population was the full safety population, defined as all patients who had taken at least one dose of rivaroxaban. Both raw incidence proportions (patients with events/number of treated patients) and incidence rates (events per 100 patient-years) are presented, with corresponding 95% confidence intervals.
Results
Patient demographics and clinical characteristics
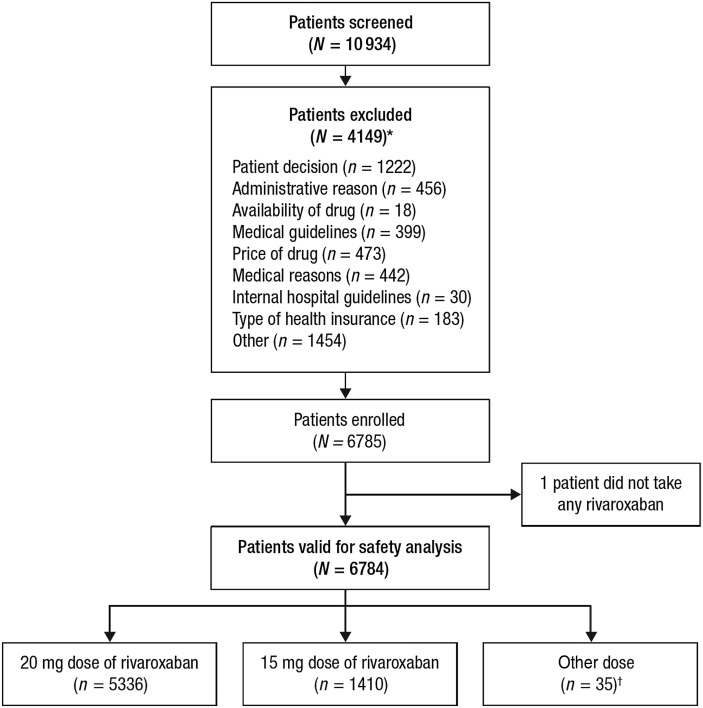
A total of 10 934 patients were screened between June 2012 and December 2013, of whom 6785 were enrolled from 311 centres in Europe, Israel, and Canada. Most patients (5287 [77.9%]) were from Western Europe, Canada, and Israel, with 1497 (22.1%) patients from Eastern Europe. One patient did not take rivaroxaban; therefore, the analysis reported here is based on 6784 patients; of whom, 5336 (78.7%) received rivaroxaban 20 mg od, 1410 (20.8%) received 15 mg od, and 35 (0.5%) received another dose (information on dosing was missing in three patients; Figure 1).
Figure 1.
Patient disposition during the study. *Reasons for not continuing in the study included, but were not limited to, patient decision and administrative and medical reasons. Some patients could have more than one reason for exclusion. †Other dose includes any initial rivaroxaban dose besides 15/20 mg once daily (excluding missing information, n = 3).
The mean observation period was 329 (SD 115, median 366) days. In total, 45.5% of patients had previous VKA use, 54.5% were categorized as VKA naive, 18% had previously used acetylsalicylic acid (excluding combination therapies) for stroke prevention, and 1.0% had received dual antiplatelet therapy alone. The baseline demographics and clinical characteristics of patients are summarized in Table 1. Mean patient age was 71.5 years; 37% of all patients were aged >75 years, and 59% were male. Co-morbidities were common: 74.7% of patients had hypertension; 19.6% had diabetes; 19.0% had experienced a prior stroke, SE, or TIA; and 18.6% had congestive heart failure. The mean CHADS2 score was 2.0 (median 2.0) and the mean CHA2DS2-VASc score was 3.4 (median 3.0). There were 12.7% of patients who had a CHA2DS2-VASc score of either 0 or 1; furthermore, 18.5% of patients were first diagnosed with NVAF, 40.6% with paroxysmal AF, and 40.7% with persistent or permanent AF.
Table 1.
Baseline demographics and clinical characteristics of patients in the XANTUS study
| Rivaroxaban (N = 6784) | |
|---|---|
| Age (years), mean ± SD | 71.5 ± 10.0 |
| Age <65, n (%) | 1478 (21.8) |
| Age ≥65 to ≤75, n (%) | 2782 (41.0) |
| Age >75, n (%) | 2524 (37.2) |
| Gender (male), n (%) | 4016 (59.2) |
| Weight (kg), mean ± SD | 83.0 ± 17.3 |
| BMI (kg/m2), mean ± SD | 28.3 ± 5.0 |
| BMI >30, n (%) | 1701 (25.1) |
| Creatinine clearance (mL/min), n (%) | |
| <15 | 20 (0.3) |
| ≥15 to <30 | 75 (1.1) |
| ≥30 to <50 | 545 (8.0) |
| ≥50 to ≤80 | 2354 (34.7) |
| >80 | 1458 (21.5) |
| Missing | 2332 (34.4) |
| Existing co-morbidities, n (%) | |
| Hypertension | 5065 (74.7) |
| Diabetes mellitus | 1333 (19.6) |
| Prior stroke/non-CNS SE/TIA | 1291 (19.0) |
| Congestive HF | 1265 (18.6) |
| MI | 688 (10.1) |
| Hospitalization at baseline, n (%) | 1226 (18.1) |
| AF, n (%) | |
| First diagnosed | 1253 (18.5) |
| Paroxysmal | 2757 (40.6) |
| Persistent | 923 (13.6) |
| Permanent | 1835 (27.0) |
| Missing | 16 (0.2) |
| CHADS2 score | |
| Mean score ± SD | 2.0 ± 1.3 |
| Score, n (%) | |
| 0 | 703 (10.4) |
| 1 | 2061 (30.4) |
| 2 | 2035 (30.0) |
| 3 | 1111 (16.4) |
| 4 | 618 (9.1) |
| 5 | 222 (3.3) |
| 6 | 34 (0.5) |
| Missing | 0 (0.0) |
| CHA2DS2-VASc score | |
| Mean score ± SD | 3.4 ± 1.7 |
| Score, n (%) | |
| 0 | 174 (2.6) |
| 1 | 685 (10.1) |
| 2 | 1313 (19.4) |
| 3 | 1578 (23.3) |
| 4 | 1405 (20.7) |
| 5 | 837 (12.3) |
| 6–9 | 789 (11.6) |
| Missing | 3 (<0.05) |
| Prior use of antithrombotics, n (%) | |
| VKA | 2767 (40.8) |
| Direct thrombin inhibitor | 208 (3.1) |
| Acetylsalicylic acid (excluding dual antiplatelet therapy) | 1224 (18.0) |
| Dual antiplatelet therapy | 68 (1.0) |
| Factor Xa inhibitor (excluding rivaroxaban) | 10 (0.1) |
| Heparin group | 217 (3.2) |
| Other | 55 (0.8) |
| Multiple | 410 (6.0) |
| VKA | |
| Experienced | 3089 (45.5) |
| Naive | 3695 (54.5) |
CrCl calculated using the Cockcroft–Gault formula.
AF, atrial fibrillation; BMI, body mass index; CNS, central nervous system; CrCl, creatinine clearance; HF, heart failure; MI, myocardial infarction; SD, standard deviation; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
Outcomes
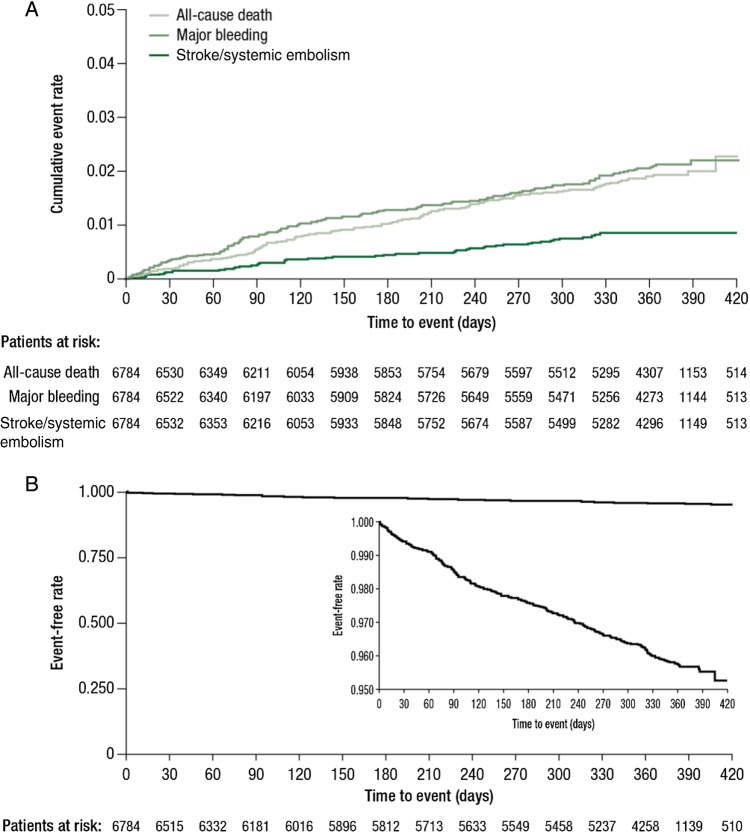
In the cohort of 6784 patients, the overall numbers of major bleeding and thromboembolic events and all-cause deaths were low and increased progressively over time (Figure 2A). Most patients (6522 [96.1%]) did not experience any of the outcomes of treatment-emergent major bleeding, all-cause death, or stroke/SE (freedom from events; Figure 2B).
Figure 2.
(A) Cumulative rates (Kaplan–Meier) for treatment-emergent all-cause death, major bleeding events, and stroke/systemic embolism. (B) Event-free rate (Kaplan–Meier) for treatment-emergent all-cause death, major bleeding events, and stroke/systemic embolism. In total, 6522 (96.1%) patients did not experience any of the outcomes of treatment-emergent all-cause death, major bleeding, or stroke/systemic embolism. Safety analysis set.
A total of 2709 patients (39.9%) had a treatment-emergent AE and 1200 (17.7%) had a treatment-emergent SAE. There were 142 treatment-emergent major bleeding events in 128 patients (2.1 events per 100 patient-years). The incidence rate of fatal bleeding was 0.2 events per 100 patient-years; critical organ bleeding occurred at a rate of 0.7 events per 100 patient-years, including intracranial haemorrhage (0.4 events per 100 patient-years). The incidence of major gastrointestinal bleeding was 0.9 events per 100 patient-years (Table 2). Stroke occurred in 43 (0.7 events per 100 patient-years) patients, with SE occurring in a further 8 patients (0.1 events per 100 patient-years). Eleven patients (0.2%) had a haemorrhagic stroke and 32 (0.5%) an ischaemic stroke. Left atrial thrombus was recorded in six patients (0.1 events per 100 patient-years). All-cause death occurred in 118 patients (1.9 events per 100 patient-years) within the study treatment period, with the adjudicated cause of death due primarily to cardiovascular causes, mainly heart failure, followed by cancer (Table 3).
Table 2.
Adjudicated treatment-emergent thromboembolic and bleeding events and all-cause death
| Rivaroxaban (N = 6784) |
||
|---|---|---|
| Incidence proportion, n (%) | Incidence rate, events per 100 patient-years (95% CI) | |
| All-cause death | 118 (1.7) | 1.9 (1.6–2.3) |
| Thromboembolic events (stroke, SE, TIA, and MI) | 108 (1.6) | 1.8 (1.5–2.1) |
| Stroke/SE | 51 (0.8) | 0.8 (0.6–1.1) |
| Stroke | 43 (0.6) | 0.7 (0.5–0.9) |
| Primary haemorrhagic | 11 (0.2) | |
| Primary ischaemic | 32 (0.5) | |
| Haemorrhagic transformation | 3 (<0.05) | |
| No haemorrhagic transformation | 29 (0.4) | |
| Uncertain | 0 | |
| SE | 8 (0.1) | 0.1 (0.1–0.3) |
| TIA | 32 (0.5) | 0.5 (0.4–0.7) |
| MI | 27 (0.4) | 0.4 (0.3–0.6) |
| Major bleeding | 128 (1.9) | 2.1 (1.8–2.5) |
| Fatal | 12 (0.2) | 0.2 (0.1–0.3) |
| Critical organ bleeding | 43 (0.6) | 0.7 (0.5–0.9) |
| Intracranial haemorrhage | 26 (0.4) | 0.4 (0.3–0.6) |
| Intraparenchymal | 6 (0.1) | |
| Subarachnoid | 5 (0.1) | |
| Intraventricular | 6 (0.1) | |
| Subdural haematoma | 6 (0.1) | |
| Epidural haematoma | 1 (<0.05) | |
| Haemorrhagic transformation of ischaemic stroke | 3 (<0.05) | |
| Missing | 2 (<0.05) | |
| Mucosal bleedinga | 60 (0.9) | 1.0 (0.7–1.3) |
| Gastrointestinal | 52 (0.8) | 0.9 (0.6–1.1) |
| Haemoglobin decrease ≥2 g/dLb | 52 (0.8) | 0.9 (0.6–1.1) |
| Transfusion of ≥2 units of packed red blood cells or whole bloodb | 53 (0.8) | 0.9 (0.6–1.1) |
| Non-major bleeding events | 878 (12.9) | 15.4 (14.4–16.5) |
CI, confidence interval; MI, myocardial infarction; SE, systemic embolism; TIA, transient ischaemic attack.
aThe numbers shown here are for major mucosal and gastrointestinal bleeding events. Mucosal bleeding events include gingival, epistaxis, gastrointestinal, rectal, macroscopic haematuria, and increased or prolonged menstrual or abnormal vaginal bleeding.
bRepresents major bleeding.
Table 3.
Causes of treatment-emergent adjudicated death
| Adjudicated causes of death | Number of patients (N = 118a), n (%) |
|---|---|
| Cardiovascular | 49 (41.5) |
| Cardiac decompensation, heart failure | 24 (20.3) |
| Sudden or unwitnessed death | 14 (11.9) |
| MI | 6 (5.1) |
| Non-haemorrhagic stroke | 4 (3.4) |
| Dysrhythmia | 1 (0.8) |
| Venous thromboembolism | 0 |
| Other vascular event | 0 |
| Cancer | 23 (19.5) |
| Other | 16 (13.6) |
| Bleeding | 12 (10.2) |
| Extracranial haemorrhage | 5 (4.2) |
| Intracranial bleeding | 7 (5.9) |
| Infectious disease | 10 (8.5) |
| Unexplained | 9 (7.6) |
MI, myocardial infarction.
aMultiple reasons were recorded for the cause of treatment-emergent adjudicated death of some patients.
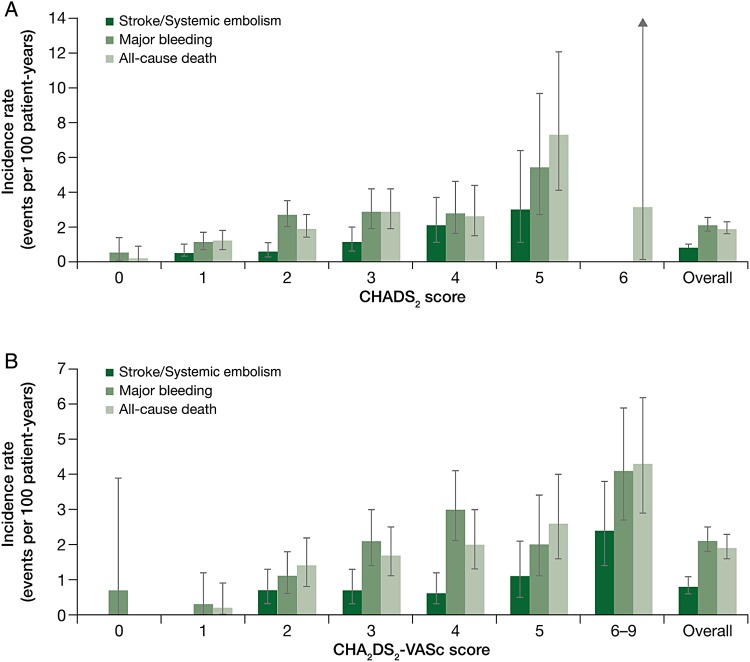
The incidence of major bleeding events increased with age and occurred at a rate of 0.9 events per 100 patient-years in patients aged <65 years, 1.7 events per 100 patient-years in patients aged ≥65 to ≤75 years, and 3.2 events per 100 patient-years in those aged >75 years. The corresponding rates for symptomatic thromboembolic events (stroke/SE, TIA, and MI) were 0.8, 1.8, and 2.3 events per 100 patient-years, respectively. Outcome analysis according to CHADS2 and CHA2DS2-VASc risk scores showed that stroke/SE, major bleeding, and all-cause death generally increased with higher risk scores (Figure 3).
Figure 3.
Outcomes as a function of (A) CHADS2 and (B) CHA2DS2-VASc scores.
Creatinine clearance values were reported in 4452 (65.6%) patients. Of these, 14.4% had CrCl <50 mL/min and 85.6% had CrCl ≥50 mL/min. As expected, rates of major bleeding were highest in patients with documented reduced renal function (3.4% in patients with CrCl <50 mL/min). The lowest incidence proportion for major bleeding (0.6%) was observed in patients for whom no CrCl test results were recorded. Of 3812 patients with a documented CrCl of ≥50 mL/min, 15% received the lower rivaroxaban dose of 15 mg od; conversely, the 20 mg od dose was received by 36% of the 640 patients who had moderate or severe renal impairment documented at any time during the study. Outcomes of major bleeding, all-cause death, or thromboembolic events (stroke, SE, TIA, and MI combined) showed numerically higher incidence rates for the 15 mg od dose compared with the 20 mg od dose: 3.1 vs. 1.8 events per 100 patient-years for major bleeding, 3.7 vs. 1.4 events per 100 patient-years for all-cause death, and 2.3 vs. 1.6 events per 100 patient-years for thromboembolic events, respectively.
Additional outcomes
In total, 598 patients (8.8%) had at least one interruption of rivaroxaban therapy, which was most commonly because of a need for surgery, or because of bleeding or another AE. The median treatment interruption period was 4 days (Q1–Q3; 2–12 days). Among all patients with treatment interruption, major bleeding was recorded in 5.2% during the interruption period or within 2 days of the end of this period; thromboembolic events occurred in 2.0% of patients. Interventions for stroke were rare: among 32 patients with ischaemic stroke, no thrombectomies were performed and two patients underwent thrombolysis. Among 27 patients with MI, no thrombolysis was performed, but percutaneous intervention and coronary artery bypass grafting were performed in 11 patients and two patients, respectively. Major bleeding was mostly treated using conservative methods and non-specific reversal agents were rarely used; the use of prothrombin complex concentrate was documented in two patients, tranexamic acid in three patients, and etamsylate in one patient. Treatment persistence remained high over the 1-year study period, with a discontinuation rate at the end of the observation period of 20.1%. This finding coincided with 5096 (75.1%) patients reporting to their physicians that they were ‘very satisfied’ or ‘satisfied’ with their treatment. The main reason for premature discontinuation (7.9% of all patients) was the occurrence of an AE.
Discussion
Studies such as XANTUS complement the outcomes of pivotal trials through the use of unselected real-world populations and conditions. XANTUS is the first international, prospective, non-interventional study describing the use of a NOAC for stroke prevention in a broad NVAF patient population. Whereas patients in the phase III ROCKET AF trial had a mean CHADS2 score of 3.5, with 55% having experienced prior stroke/SE or TIA,7 patients studied in XANTUS had a lower risk of stroke, with a mean CHADS2 score of 2.0 and 19.0% experiencing prior stroke/TIA or SE. The baseline stroke risk of patients in XANTUS is, therefore, similar to that of other NOAC trials, such as RE-LY and ARISTOTLE, in which the mean CHADS2 score was 2.1, and the percentages of patients with a prior stroke were 20.0 and 19.4%, respectively.5,6 XANTUS included patients at a slightly lower baseline stroke risk, with a lower percentage of patients with prior stroke than ENGAGE AF-TIMI 48, for which values were 2.8 and 28.3%, respectively.8
With the distribution of stroke risk scores in XANTUS, real-world stroke incidence was low in patients receiving anticoagulation, with an annual stroke rate of 0.7% (vs. 1.7 events per 100 patient-years in the ROCKET AF on-treatment population). The incidence rates of other thromboembolic events and for all-cause death were also low in this unselected patient population. The incidence rate of major bleeding was 2.1 events per 100 patient-years, which is lower than in ROCKET AF (3.6 events per 100 patient-years)7 and similar to that seen in a large US study of electronic medical records of 27 467 patients (2.9 events per 100 patient-years), although this study used a different bleeding definition.10 The major bleeding rate was also similar to published data from the smaller, real-world, observational Dresden NOAC Registry involving 1200 AF patients treated with rivaroxaban (3.1 events per 100 patient-years).11 The incidence rates of fatal bleeding, critical organ bleeding, and intracranial haemorrhage were similar to those observed in ROCKET AF (XANTUS vs. ROCKET AF 0.2 vs. 0.2 events per 100 patient-years, 0.7 vs. 0.8 events per 100 patient-years, and 0.4 vs. 0.5 events per 100 patient-years, respectively), whereas major gastrointestinal bleeding occurred less frequently (0.9 events per 100 patient-years) than that seen in ROCKET AF (2.0 events per 100 patient-years).12
Throughout the study, the use of non-specific reversal agents (such as prothrombin complex concentrate) was low. This finding is in line with outcomes from ROCKET AF and the Dresden NOAC Registry,11,13 and suggests that these agents are rarely used in clinical practice. The lowest incidence proportion of major bleeding (0.6%) was observed in patients for whom no CrCl test results were recorded throughout the study, suggesting that laboratory testing may have been reserved for patients at higher risk, and clinical assessment may have been judged appropriate in patients with overall acceptable health. Because this was an observational study, it is also possible that CrCl tests may have been performed but not documented. The Executive Steering Committee specifically asked all investigators in a letter to document renal function; however, it cannot be excluded that measured CrCl has not been documented and this would contribute to the missing data. In addition, major bleeding, all-cause death, and thromboembolic events (stroke, SE, TIA, and MI combined) occurred at higher incidence rates for the 15 mg od vs. the 20 mg od dose, which indicated that dosing decisions might have been based on other clinical considerations besides impaired renal function.
Drug persistence is a major concern in stroke prevention because anticoagulant discontinuation potentially leaves patients unprotected from the risk of stroke. Recent data obtained with VKAs showed discontinuation rates of 32 and 38% at 6 and 12 months, respectively.14,15 Available data on NOACs suggest higher persistence rates. Persistence with rivaroxaban in XANTUS was 80% at 1 year, which is higher than recent US studies14,15 but in line with that observed in other real-world studies such as the Dresden NOAC Registry, in which discontinuations of ∼15% were recorded in the first year.16
There are some limitations to this real-world study. XANTUS was a single-arm study and, as with any open-label study, the study design can introduce bias related to knowledge about treatment. In addition, patients agreeing to participate in the study may, to some extent, have self-selected for risk of stroke or bleeding, and conscientious participation, and a selection bias based around intact cognitive function could have arisen with the investigator. Owing to the observational design, interference with patient management, such as reinforcement of laboratory and other investigations, was not allowed. This led, for example, to a large number of patients with unknown CrCl values. Although it was possible to assess persistence, there was no possibility to assess drug adherence in a standardized fashion in an observational study. Finally, outcomes per rivaroxaban dose were not adjusted for baseline risk factors for this analysis.
Strengths of this study include its meaningful sample size and a prospective design allowing for greater completeness of data and potentially better data quality compared with retrospective designs. The independent endpoint adjudication is expected to have reduced reporting bias.
Conclusion
XANTUS is the first large, international, prospective study describing the use of rivaroxaban for stroke prevention in a broad NVAF patient population. The rates of major bleeding and stroke with rivaroxaban were found to be low in routine clinical practice.
Authors’ contributions
S.K.: performed statistical analysis; S.Ha., M.v.E., A.J.C., P.K., S.He., A.G.G.T., and P.A.: conceived and designed the research; A.J.C., P.K., and S.He.: drafted the manuscript; S.Ha., A.G.G.T., P.A., and M.v.E.: made critical revision of the manuscript for key intellectual content.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was supported by Bayer HealthCare Pharmaceuticals and Janssen Research & Development, LLC. Funding to pay the Open Access publication charges for this article was provided by Chameleon Communications International.
Conflict of interest: A.J.C. has served as a consultant for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, Sanofi, Aryx, and Johnson & Johnson. P.A. has served as a consultant for Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, AstraZeneca, Sanofi, Boston Scientific, Edwards, Lundbeck, Merck, and Kowa Pharmaceutical. S.Ha. has served as a consultant for Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, and Sanofi. P.K. has received consulting fees and honoraria from 3M Medica, MEDA Pharma, AstraZeneca, Bayer HealthCare, Biosense Webster, Boehringer Ingelheim, Daiichi Sankyo, German Cardiac Society, Medtronic, Merck, MSD, Otsuka Pharma, Pfizer/Bristol-Myers Squibb, Sanofi, Servier, Siemens, and Takeda. M.v.E., S.He., and S.K. are employees of Bayer HealthCare Pharmaceuticals. A.G.G.T. has been a consultant for Bayer HealthCare, Janssen Pharmaceutical Research & Development, Astellas, Portola, and Takeda.
Supplementary Material
Acknowledgements
The XANTUS Steering Committee thanks all patients, caregivers, and families who participated in the study as well as the XANTUS investigators (see Supplementary material online, Appendix) and their associated teams. The authors thank Birgit Schmidt for Global Project Management and David Whitford for editorial assistance in the preparation of the manuscript, with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
References
- 1. Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc 2015;4:e001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J; CRYSTAL AF Investigators Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 3. Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al Attar N, Alfieri O, Angelini A, Blomstrom-Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbuchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FWA. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 5. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 7. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM, ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 8. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 9. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, van Eickels M, Turpie AGG. XANTUS: rationale and design of a noninterventional study of rivaroxaban for the prevention of stroke in patients with atrial fibrillation. Vasc Health Risk Manag 2014;10:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamayo S, Frank PW, Patel M, Sicignano N, Hopf KP, Fields LE, Sarich T, Wu S, Yannicelli D, Yuan Z. Characterizing major bleeding in patients with nonvalvular atrial fibrillation: a pharmacovigilance study of 27 467 patients taking rivaroxaban. Clin Cardiol 2015;38:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beyer-Westendorf J, Förster K, Pannach S, Ebertz F, Gelbricht V, Thieme C, Michalski F, Kohler C, Werth S, Sahin K, Tittl L, Hänsel U, Weiss N. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC Registry. Blood 2014;124:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nessel C, Mahaffey K, Piccini J, Pan G, Patel MR, Becker R, Singer D, Halperin J, Hankey G, Berkowitz SD, Fox K, Califf R. Incidence and outcomes of gastrointestinal hemorrhage in patients with atrial fibrillation treated with rivaroxaban or warfarin: results from the ROCKET AF trial. Chest 2012;142:84A. [Google Scholar]
- 13. Piccini JP, Garg J, Patel MR, Lokhnygina Y, Goodman SG, Becker RC, Berkowitz SD, Breithardt G, Hacke W, Halperin JL, Hankey GJ, Nessel CC, Mahaffey KW, Singer DE, Califf RM, Fox KAA, ROCKET AF Investigators. Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J 2014;35:1873–1880. [DOI] [PubMed] [Google Scholar]
- 14. Laliberté F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, Damaraju CV, Schein JR, Lefebvre P. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin 2014;30:1317–1325. [DOI] [PubMed] [Google Scholar]
- 15. Nelson WW, Song X, Coleman CI, Thomson E, Smith DM, Damaraju CV, Schein JR. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin 2014;30:2461–2469. [DOI] [PubMed] [Google Scholar]
- 16. Beyer-Westendorf J, Förster K, Ebertz F, Gelbricht V, Schreier T, Göbelt M, Michalski F, Endig H, Sahin K, Tittl L, Weiss N. Drug persistence with rivaroxaban therapy in atrial fibrillation patients-results from the Dresden non-interventional oral anticoagulation registry. Europace 2015;17:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.