Abstract
The rise in incidence of hepatocellular carcinoma (HCC) in the United States has been well documented. The purpose of this analysis was to examine temporal trends in HCC incidence, mortality, and survival within the U.S. population. The Surveillance, Epidemiology, and End Results data were used to examine incidence and incidence-based (IB) mortality in HCC from 1973 to 2011. Secular trends in age-adjusted incidence and IB mortality by sex and cancer stage were characterized using the Joinpoint Regression program. In 1973, HCC incidence was 1.51 cases per 100,000, whereas in 2011, HCC incidence was 6.20 cases per 100,000. Although HCC incidence continues to increase, a slowing of the rate of increase occurs around 2006. In a sensitivity analysis, there was no significant increase in incidence and IB mortality from 2009 to 2011. There was a significant increase in overall median survival from the 1970s to 2000s (2 vs. 8 months; P < 0.001). On multivariable Cox's regression analysis, age, sex, race, tumor grade, stage at diagnosis, lymph/vascular invasion, number of primary tumors, tumor size, and liver transplant were independently associated with mortality.
Conclusion
Our results indicate a deceleration in the incidence of HCC around 2006. Since 2009 and for the first time in four decades, there is no increase in IB mortality and incidence rates for HCC in the U.S. population. The nonsignificant increase in incidence and IB mortality in recent years suggest that the peak of the HCC epidemic may be near. A significant survival improvement in HCC was also noted from 1973 to 2010, which seems to be driven by earlier detection of HCC at a curative stage and greater utilization of curative modalities (especially transplant).
Hepatocellular carcinoma (HCC) is the third-most common cause of cancer-related deaths worldwide.1,2 Although HCC has historically been more common in the developing world, its incidence in developed countries has almost doubled in the last two decades, largely as a result of liver cirrhosis.3,4
Liver transplantation (LT), hepatic resection, and early-stage radiofrequency ablation (RFA) are considered potentially curative. However, several factors limit the utility of these curative modalities. For example, RFA is only curative for small tumors (<3 cm), and though LT has lower recurrence rates, compared to resection and RFA, it is not readily available to everyone. Noncurative treatment options of advanced HCC include new agents, such as sorafenib, systemic chemotherapy, and transarterial chemoembolization.5,6 Though these treatments have shown modest improvement in overall survival in early stage disease, there is no curative treatment for advanced HCC.
Previous studies have examined national survival trends and patterns of therapy for HCC. El-Serag et al. using data from the Surveillance, Epidemiology, and End Results (SEER) database between 1977 and 1996 found a small improvement in survival mostly restricted to the year following diagnosis.7 This finding raised the concern for lead-time bias as the source of the apparent benefit in survival. Recently, Altekruse et al. using the SEER database also found improving HCC survival over the last decade associated with a concomitant increase in the number of cases diagnosed and treated at an early stage.8
We hypothesized that increased utilization and advances in imaging technology, surgical techniques, and adjuvant therapies has improved overall survival of patients with HCC. If these survival benefits are large enough, they should be detectable in a temporal trend analysis of HCC incidence and mortality. Therefore, we examined trends in HCC incidence, incidence-based mortality, and survival of HCC in the U.S. population. As a secondary goal, we have also identified independent predictors of mortality after the diagnosis of HCC among U.S. adults.
Materials and Methods
Data Source
The SEER database is derived from cancer registries representing approximately 28% of the U.S. population and is maintained by the National Cancer Institute (NCI; available at: www.seer.cancer.gov). The SEER population-based cancer registries contain information on cancer incidence and survival in selected geographic areas. Selection of the included geographic areas was based on the quality of their cancer reporting systems and population diversity. Appropriate institutional review board approval was obtained before data abstraction.
A retrospective cohort study was performed using data from the SEER database, based on the November 2013 submission. Data were examined from 1973 through 2011 from the SEER 9 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah), and for 2000 through 2011 from the SEER 18 registries (SEER 9 plus Los Angeles, San Jose–Monterey, Rural Georgia, Alaska Native Tumor Registry, Greater California, Kentucky, Louisiana, and New Jersey with adjustments for the areas impacted by Hurricanes Katrina and Rita).
The SEER data set includes information on patient demographics, tumor and disease characteristics, and cancer-associated treatments, use of cancer-directed surgery, and survival for individuals with cancer. Surgical intervention is coded in the SEER database as a separate variable that indicates whether an operation was performed and whether or not it was recommended. The actual surgical procedure directed at the primary site is coded as a separate variable. No record of chemotherapy appears in this database.
Study Population
To minimize under-reporting bias of outcome variables during the last years of the reported SEER data, the study population selected for our descriptive analysis (excluding incidence trends) did not include the 2011 cases. The SEER database was queried to identify patients with HCC using the International Classification of Disease (ICD) for Oncology codes (8170 to 8175) and site code C22.0 from 1973 to 2010 (Fig. 1).9,10 Patients with fibrolamellar carcinoma (code 8171) and those diagnosed within 1 month before death (including patients diagnosed at autopsy or by death certificate only) were excluded. Patients with the fibrolamellar variant of HCC were excluded because they differ in clinical course and prognosis, compared to conventional HCC.11-13 Patients who were diagnosed within 1 month before death were included in incidence analyses, but excluded from survival analyses because of SEER calculation of survival time in months and not days, for which these cases would be deemed to have a survival of zero. Similarly, patients who were diagnosed on the basis of a death certificate only or for the first time at autopsy were excluded because of SEER calculation of a survival time of zero (Fig. 1). Patients with another malignant primary tumor diagnosed within 5 years before their HCC diagnosis were excluded to minimize the chance that metastatic disease to the liver was misdiagnosed as HCC. To ensure a uniform cancer staging classification across all study years, we used the SEER historic stage, which provides consistent definitions over time, as opposed to American Joint Committee on Cancer staging, which is more commonly used in the clinical settings, but is not available for many of the years analyzed. The SEER historic stages were: localized (confined to primary site); regional (spread to regional lymph nodes); and distant (cancer had metastasized).
Fig. 1.
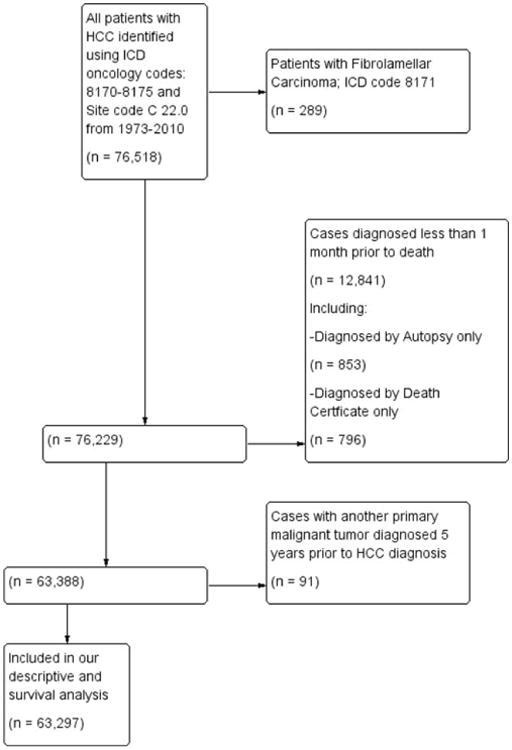
Flow diagram of patient selection out of the total 76,518 patients identified with HCC to arrive at those patients who were included in our descriptive (excluding incidence trends) and survival analysis within the SEER database 1973-2010.
Statistical Analyses
We obtained SEER incidence, incidence-based (IB) mortality, and survival data using SEER*Stat software (version 8.12). All rates were age adjusted to the 2000 U.S. standard population. Standard mortality statistics are not available for HCC because death certificates do not include the histology of the cancer. We obtained IB mortality data by linking the characteristics of the incident cancer (e.g., stage and histology) to the death certificate.14 For a given year, HCC IB mortality rate is the proportion of the total number of deaths in that year attributed to HCC. Attribution to HCC is made when the cause of death on the death certificate is HCC and the deceased is listed in the registry as having been diagnosed with HCC at any earlier time.
The NCI's Joinpoint Regression Analysis program (version 3.5.3) was used to examine trends in HCC incidence and IB mortality.14 Incidence and IB mortality data were modeled in a segmented log-linear form. For each linear segment, annual percentage changes (APCs) in age-adjusted incidence and IB mortality were calculated, and 95% confidence intervals (CIs) were reported. The APC represents the slope or gradient of each linear segment and describes both the direction and the steepness of the segment. To predict the year in which the incidence of HCC is predicted to stabilize (i.e., APC = 0), a simple linear regression model was then fitted to the last line segment.
The SEER-9 (1973-2011) catchment was selected for overall analysis over SEER-18 (2000-2011) because it enabled analysis of trends over nearly 4 times as many years. However, a sensitivity analysis of recent trends (2000-2011) was done using the SEER 18 registries because it covers approximately 28% (vs. 10% for SEER 9) of the U.S. population and includes high HCC incidence areas, such as all of California, Louisiana, and New Jersey.
In our survival analysis, the study population was divided according to decade of diagnosis: 1970s through 2000s (2011 data were not included). Median survival and survival rates were calculated overall and for each decade. Trends in ordinal data were evaluated using the linear-by-linear association test. The linear-by-linear test of trend offers a measure of significance for ordinal variables (decade quartiles ordered from lowest to highest). Unless otherwise specified, the P values reported for an analysis of survival trend refers to all of the four decade quartiles. Subgroup analyses by treatment modality and stage at diagnosis were performed. Cumulative survival rates were calculated using the method of Kaplan and Meier, and survival curves were compared using the log-rank test.15
Cox's proportional hazard regression was used to determine independent predictors of mortality. Covariates with a P value <0.1 on univariate analysis were entered into multivariate analysis. Covariates analyzed included age, gender, ethnicity, tumor characteristics, stage of disease, and treatment modality. All reported P values were two-tailed, and for all tests, P < 0.05 was considered statistically significant. All survival analyses were performed using IBM SPSS (version 20.0; IBM Corp., Armonk, NY).
Results
Patient and Primary Tumor Characteristics
A total of 63,297 patients with HCC met the inclusion criteria for our descriptive and survival analysis (Fig. 1). Demographic and pathological characteristics of the study population are shown in Table 1. The median age of the total cohort was 65 (interquartile range: 64-66) years. Most patients were men (n = 47,346; 74.8%) and white (n = 41,396; 65.4%). Overall, the majority of patients had localized disease (n = 26,268; 41.5%), as classified by the SEER historic stage. Diagnosis at an early and localized stage increased significantly throughout the study period (n = 288; 23.3% to n = 23,237; 46.2%; P < 0.001). Most patients (n = 40,067; 63.3%) had an unknown tumor grade, with a progressive decrease in the proportion of patients with unknown tumor grade (n = 985; 79.8% to n = 30,631; 60.9%; P = 0.01). Overall, 85.6% (n = 54,182) of patients had lymphovascular invasion on final pathological examination. Mean tumor size was 3.0 cm (n = 41,637; standard error of the mean = 0.2), and solitary tumors (n = 54,815; 86.6%) were more common than multiple tumors (n = 8,482; 13.4%).
Table 1. Trends in Baseline Demographic and Pathological Characteristics of the Study Population (1973-2010).
| Variable | Total | 1970s | 1980s | 1990s | 2000s |
|---|---|---|---|---|---|
| No. of patients (n)* | 63,297 | 1,235 | 2,966 | 8,799 | 50,297 |
| Median age (years) | 65 | 66 | 66 | 66 | 62 |
| Gender, n (%)* | |||||
| Women | 15,951 (25.2) | 338 (27.4) | 860 (29.0) | 2,449 (27.8) | 12,122 (24.1) |
| Men | 47,346 (74.8) | 897 (72.6) | 2,106 (71.0) | 6,350 (72.2) | 38,175 (75.9) |
| Race, n (%)* | |||||
| White | 41,396 (65.4) | 823 (66.6) | 1,852 (62.4) | 5,259 (59.8) | 33,699 (67.0) |
| Black | 7,722 (12.2) | 165 (13.4) | 372 (12.5) | 960 (10.9) | 6,287 (12.5) |
| Asian/Pacific Islander | 13,292 (21.0) | 241 (19.5) | 715 (24.1) | 2,479 (28.2) | 9,506 (18.9) |
| American Indian/Alaskan Native | 696 (1.1) | 4 (0.3) | 24 (0.8) | 83 (0.9) | 553 (1.1) |
| SEER historic stage, n (%)* | |||||
| Localized | 26,268 (41.5) | 288 (23.3) | 701 (23.6) | 2,750 (31.3) | 23,237 (46.2) |
| Regional | 16,647 (26.3) | 288 (23.3) | 716 (24.1) | 2,278 (25.9) | 13,429 (26.7) |
| Distant | 11,077 (17.5) | 372 (30.1) | 713 (24.0) | 1,728 (19.6) | 7,997 (15.9) |
| Unstaged | 9,368 (14.8) | 287 (23.2) | 836 (28.2) | 2,043 (23.2) | 5,633 (11.2) |
| Grade, n (%)* | |||||
| Well differentiated | 8,355 (13.2) | 76 (6.2) | 294 (9.9) | 1,152 (13.1) | 6,891 (13.7) |
| Moderately differentiated | 8,798 (13.9) | 55 (4.5) | 152 (5.1) | 936 (10.6) | 7,947 (15.8) |
| Poorly differentiated | 5,443 (8.6) | 84 (6.8) | 194 (6.5) | 803 (9.1) | 4,376 (8.7) |
| Undifferentiated | 696 (1.1) | 35 (2.8) | 38 (1.3) | 129 (1.5) | 453 (0.9) |
| Unknown | 40,067 (63.3) | 985 (79.8) | 2,288 (77.1) | 5,779 (65.7) | 30,631 (60.9) |
Significant at P < 0.05.
Overall Incidence and Mortality Trends
Overall, the incidence of HCC is on the increase (Fig. 2). In 1973, HCC incidence was 1.5 cases per 100,000, whereas in 2011, it was 6.2 cases per 100,000. Although HCC incidence continues to be on a rising trend, the slope of the rise decreased around 2007. From 2002 to 2007 the APC (i.e., the slope of increase in incidence) was 6.0% per year (95% confidence interval [CI]: 3.3-8.7; P < 0.05), whereas from 2007 to 2011, the change was 2.7% per year (95% CI: 0.6-4.9; P < 0.05). HCC incidence-based mortality follows a similar pattern of a continued rise, with a deceleration occurring around 1994 (Fig. 3). From 1988 to 1994, the APC was 4.9% (95% CI: 2.2-7.7; P < 0.05), whereas from 1994 to 2011, the change was 3.6% per year (95% CI: 3.3-3.9; P < 0.05).
Fig. 2.
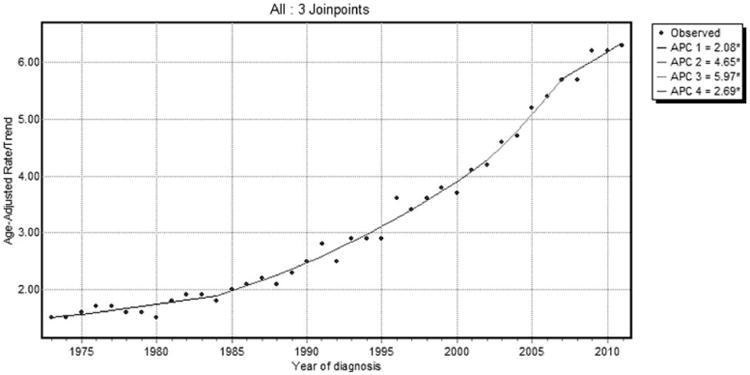
SEER 9 HCC incidence trends overall 1973-2011.
Fig. 3.
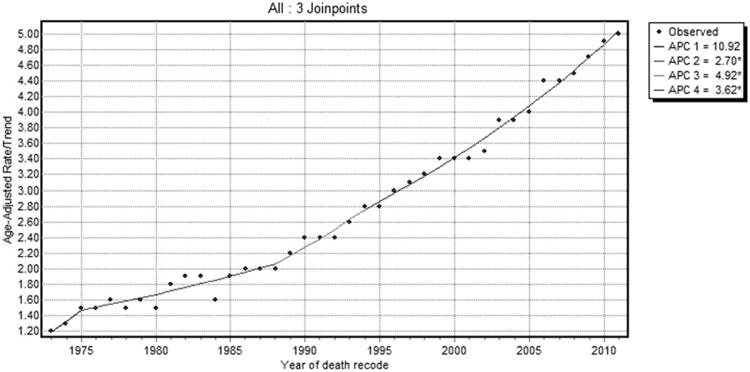
SEER 9 HCC IB mortality trends overall 1973-2011.
In a sensitivity analysis using SEER 18 data, there was an initial increase in the incidence of HCC at a rate of 5.8% per year (95% CI: 3.9-7.8; P < 0.05) from 2000 to 2005, followed by a deceleration from 2005 to 2009 at an annual rate of 4.3%. Interestingly, after 2009, there was no significant increase in HCC incidence (APC, 0.9%; 95% CI: −4.7 to 6.8; P > 0.05; Fig. 4). HCC IB mortality follows a similar pattern, with a deceleration observed from 2002 to 2009 at an annual rate of 4.8%, followed by no significant increase in HCC IB mortality thereafter (APC, 1.3%; 95% CI: −6.1 to 9.3; P > 0.05; Fig. 5).
Fig. 4.
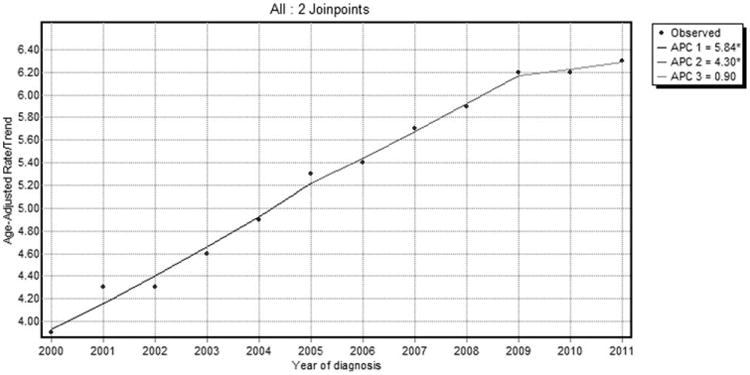
SEER 18 HCC incidence trends overall 2000-2011.
Fig. 5.
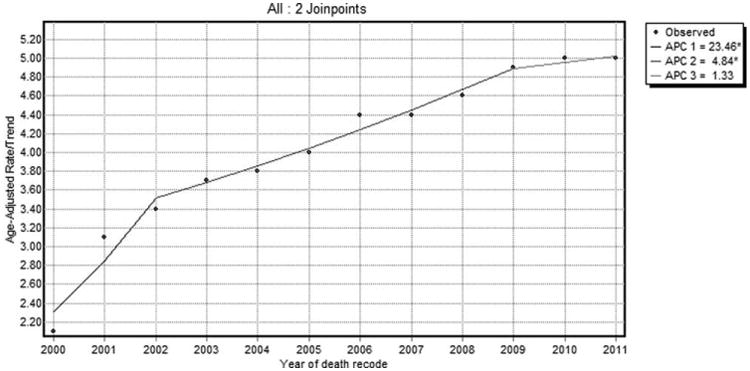
SEER 18 HCC IB mortality trends overall 2000-2011.
In a simple linear regression analysis using the SEER 18 data and assuming a stable rate of deceleration in HCC incidence after 2005, an APC of 0% was projected in 2017.
Trends by Sex
When sex-specific trends were explored, we found a continued increase in HCC incidence in men from 1973 to 2006, followed by a deceleration thereafter at an APC of 3.0%. HCC incidence in females follows a similar pattern, with a decrease in the APC at a rate of −2.0% after 2009 to 2011 at a rate of −2.2% (Supporting Fig. 1A,B). In both men and women, there was an initial increase in IB mortality rates, followed by a deceleration after 1995 (Supporting Fig. 2A,B).
Trends by Stage
The trends by cancer stage showed an overall increase incidence of localized and distant HCC (Supporting Fig. 3A,B) across the study period, with a deceleration occurring around 2008. There was a concomitant initial increase in IB mortality for all stages of cancer, followed by a deceleration in mortality in recent years. For localized HCC, IB mortality initially increased at an APC of 8.2% (95% CI: 7.3-9.3) from 1988 to 2003 and then slowed to an annual increase of 5.1% (95% CI: 3.9-6.4) thereafter. For distant HCC IB mortality, the initial APC increased at a rate of 2.3% (95% CI: 1.8-2.8) from 1985 to 2009, but decreased from 2009 to 2011 at a rate of −2.2% (Supporting Fig. 4A,B).
Trends in Cancer-Directed Surgical Treatment
Of the 63,297 patients included, 14,431 (22.8%) underwent cancer-directed surgery (LT and resection). In the overwhelming majority of cases (n = 10,332; 71.6%), surgery was done at a local stage of disease. Use of surgical treatment overall, increased significantly over the study period (11.1%-25.6%; P < 0.01). The proportion of patients who had surgery at an early stage increased over time (47.4%-73.4%; P < 0.01).
Trends in First-Course Hepatic Therapy
Data for first-course hepatic therapy (local tumor destruction, resection, and transplant) was available in the SEER database from 1998 to 2010 (n = 49,538). Approximately 75% of patients (n = 36,658) did not have any reported intervention. There was a temporal increase in the use of local tumor destruction and transplant during the study period (21.9%-38.3% and 20.5%-28.8%; P <0.05, respectively). Data on chemotherapy were not available.
Long-Term Survival Outcomes
Overall median survival was 6 months (95% CI: 5.9-6.1), with 1-, 5-, and 10- year survival rates of 31.3%, 5.1%, and 0.8%, respectively. There was a statistically significant increase in overall median survival from the 1970s to 2000s (2 vs. 8 months; P < 0.001). Survival improvement was predominantly noted in patients with localized disease (3-18 months; Fig. 6) and to a lesser degree in patients with regional spread (3-6 months; Supporting Fig. 5A). Metastatic disease had a small, but statistically significant, increase in median survival (2-3 months; Supporting Fig. 5B).
Fig. 6.
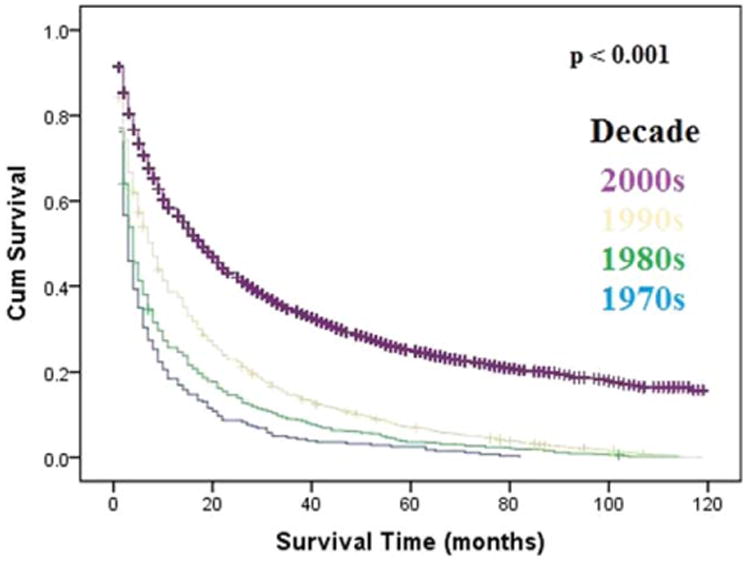
Kaplan-Meier's analysis: localized HCC. Graph shows increasing survival from the 1970s to 2000s, with the largest improvement in survival noted from the 1990s to 2000s. The P values reported for trend analysis refers to comparison among all of the four decade quartiles. Abbreviation: Cum, cumulative.
On multivariable Cox's regression analysis with adjustment for patient clinicodemographics, tumor characteristics, and treatment, age (> 55 years), sex (male), race (black), tumor grade (poorly differentiated), stage at diagnosis (regional and metastatic), lymph/vascular invasion, number of primary tumors (>1), tumor size (≥5 cm), hepatic resection, and LT were independently associated with mortality (Table 2).
Table 2. Multivariate Cox's Proportional Hazards Model Assessing Factors Associated With Mortality After Diagnosis of HCC.
| Risk Factor | HR* | 95% CI | P Value | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Age, years | ||||
| <55 | Referent | |||
| 55-75 | 1.11 | 1.09 | 1.14 | <0.001 |
| >75 | 1.35 | 1.30 | 1.39 | <0.001 |
| Gender | ||||
| Female | Referent | |||
| Male | 1.07 | 1.04 | 1.09 | <0.001 |
| Race | ||||
| Other | Referent | |||
| Asian or Pacific Islander | 0.96 | 0.87 | 1.05 | 0.31 |
| White | 1.13 | 1.04 | 1.24 | 0.01 |
| Black | 1.24 | 1.13 | 1.36 | <0.001 |
| Grade | ||||
| Well differentiated | Referent | |||
| Poorly differentiated | 1.13 | 1.09 | 1.17 | <0.001 |
| Lymph/vascular invasion | ||||
| No | Referent | |||
| Yes | 1.08 | 1.05 | 1.11 | <0.001 |
| Only one primary tumor | ||||
| No | 1.12 | 1.09 | 1.16 | <0.001 |
| Yes | Referent | |||
| Tumor size, cm | ||||
| <5 | Referent | |||
| ≥5 | 1.14 | 1.11 | 1.17 | <0.001 |
| Stage | ||||
| Localized | Referent | |||
| Regional | 1.12 | 1.09 | 1.16 | <0.001 |
| Metastatic | 1.53 | 1.48 | 1.58 | <0.001 |
| Treatment modality | ||||
| Other | Referent | |||
| Resection | 0.75 | 0.69 | 0.82 | <0.001 |
| Transplant | 0.39 | 0.38 | 0.40 | <0.001 |
HRs greater than 1.0 indicate a higher risk of death.
Specifically, receipt of LT (hazard ratio [HR] = 0.39; 95% CI: 0.38-0.40; P < 0.001) or hepatic resection (HR = 0.75; 95% CI: 0.69-0.82; P < 0.001) versus other treatment modalities was associated with a decreased risk of death.
Discussion
HCC incidence continues to increase in the U.S. population. However, this analysis shows that the rate of rising incidence has slowed down significantly from 2006 to 2011. The deceleration in incidence of localized cases may indicate improved HCC primary prevention strategies (e.g., hepatitis B vaccination programs, screening of blood donors for hepatitis viruses, and decline in intravenous drug abuse) as well as advances in chronic hepatitis treatments, which limit progression to chronic liver diseases with high risk of HCC.1,2 If the current rate of deceleration continues, incidence of HCC is predicted to stabilize, and potentially decrease, within 3 years (by 2017). The current projections on HCC incidence represent an important resource for planning and evaluating cancer-control programs. However, because the estimates in this study are model based, there is a need to exercise caution when using them. Although our study showed an increase in the number of HCC cases diagnosed at an early stage of disease, the decrease in incidence of localized disease in more recent years suggests prevalent, rather than incident, disease. Interestingly, HCC incidence-based mortality trends also show a decreasing trend in recent years. The drop in mortality observed was for all genders and cancer stages. This finding is further supported by the estimation of long-term survival by stage, which revealed that, over the study period, the survival for all stages of HCC improved. Previous studies have suggested improving HCC survival in the U.S. population, but the decline in mortality rates reported in this study is a new finding. To clarify the importance of this new observation, it is imperative to differentiate between mortality and survival. Mortality rates define the number of people who die of a certain cause (e.g., HCC) in a year divided by the total number of people in the at-risk population, whereas survival rates estimate the percentage of people with a disease who are still alive at a given time point following diagnosis. We can only decrease the mortality rate by preventing death, or curing the disease, whereas survival rate can be increased by preventing death, curing the disease, making the diagnosis earlier, or slowing down progression of the disease. Though both mortality and survival rates are important, the mortality rates are a less-biased measure to quantify improvements in the management of disease. Commonly observed forms of bias noted in survival analysis, such as lead time (earlier diagnosis) and length time (less-severe cases diagnosed), do not affect mortality analysis.
Over the study period, there was a progressive increase in detection of HCC at early stages when it is potentially curable. These improvements may also be influenced by robust screening and clinical surveillance of individuals with known risk factors for HCC.16-19 Despite the encouraging findings, a majority of eligible cases received neither surgical nor local tumor destruction therapy. The low rate of intervention observed may reflect advanced disease not amenable for ablation, resection, or transplant. This suggests that major decreases in mortality are achievable with better application of early detection strategies and increase utilization of appropriate curative therapy. These will likely lead to improved overall survival in these patients.
Patients with early HCC are generally thought to have a good prognosis. The best median survival in this study was observed among cases that received LT for early stage disease (107 months; 95% CI: 100.3-113.7). Also, there was a significant increase in the utilization of LT. The increase in LT is likely related to modifications in LT guidelines with Model for End-Stage Liver Disease exception points resulting in prioritization of HCC patients over patients without HCC.
In this study, black patients with HCC had the worst overall survival and were independently associated with increased risk of death, compared to other races. Other reports also describe differences in overall HCC survival, as well as treatment-specific survival between racial groups.20-23 In one study of localized-stage disease treated with invasive therapy, blacks had a 12% higher mortality rate, whereas Asians or Pacific Islanders had a 16% lower mortality rate, when compared to whites.20 The survival advantage among Asians or Pacific Islanders undergoing curative treatment, especially surgery, could partially be explained by the lower prevalence of cirrhosis among HCC cases, given that hepatitis B virus (HBV) is the predominant etiology of HCC in this population. Recent studies have suggested that, in early-stage HCC, compared to cases with hepatitis C virus–associated HCC, HBV-associated HCC cases had better outcomes after surgery.17,24,25 A possible rationale for this is the better liver reserve and less hepatic inflammation among the HBV-associated HCC. Also, because some Asian or Pacific Islander groups are known to be at high risk of HCC as a result of endemic HBV infection, health care providers may be more stringent in implementing screening guidelines in individuals of this ethnic group, hence leading to the detection of HCC at stages where curative therapy is still possible. In addition, social factors, such as income, education, employment, and low access to health care among blacks, have been linked to the racial disparities in survival observed.21,26-28
Tumor size is a known risk factor for poor survival following resection of HCC. In this study, tumor size ≥5 cm was independently associated with increased risk of death. Similar to previous studies, vascular invasion was a strong predictor of mortality. The recognition that vascular invasion is independently associated with an adverse prognosis may be important both in risk stratification of patients and in clinical decision making.
Our analysis has numerous strengths, including the comparison of incidence and IB mortality trends as well as ability to examine trends by sex and stage. However, these results need to be interpreted with caution because of limited information on the method of diagnosis, as well as patient comorbidities, HCC etiology, and treatment. SEER does not report on the presence or extent of liver cirrhosis, which may affect prognosis. However, our survival analyses focused on all-cause mortality and were therefore not influenced by the type of underlying liver disease. We carried out a survival analyses on a large population data set. Given the nature of data used, it is important to consider the possibility of patient selection bias, miscoding, missing, or inaccurate data entry. However, some studies have shown that data entry errors and misclassification bias is very minimal in the SEER database.5,8,27,29 The increase in the number of patients reported in the 2000s with HCC, compared to the previous decades, may be a result of detection bias given the increase in early-stage cancer and improved staging over the study period. The resulting detection bias may lead to a spurious increase in incidence. On the contrary, our study showed a deceleration in the incidence of HCC in the last decade. Although the data derived from the SEER data set offer a perspective that is generalizable to many practice patterns, the data do not include detailed information on the characteristics of adjuvant treatment regimens. As such, the data set do not contain data on the type of chemotherapy used. Although we should not entirely exclude lead-time bias as a contributory factor, the magnitude of the overall survival gains (4-fold increase in overall median survival from the 1970s to the 2000s) cannot be explained solely by lead-time bias resulting from earlier diagnosis. Finally, data in the current study include patient data only through 2011. More data in the sorafenib era are required to further appreciate the gains in overall mortality or survival for metastatic disease.
In conclusion, HCC diagnosis and management are changing in the U.S. population, with favorable results on stage, treatment, mortality, and survival. Our results indicate a deceleration in the incidence of HCC around 2006. Since 2009 and for the first time in four decades, there was no increase in IB mortality and incidence rates for HCC in the U.S. population. The nonsignificant increase in incidence and incidence-based mortality in recent years suggest that the peak of the HCC epidemic may be near. These novel findings warrant further investigation and characterization, because such research could enlighten future cancer prevention and intervention programs aimed at decreasing HCC mortality. A significant survival improvement in HCC was also noted from 1973 to 2010, which seems to be driven by earlier detection of HCC at a curative stage, and greater utilization of curative modalities (especially LT). The small percentage of eligible cases receiving curative treatment suggests that there is room for further improvement in survival, with implementation of the HCC control guidelines. Early diagnosis through better screening measures, together with patient and physician education on up-to-date guidelines and treatment protocols, should continue to be a priority to improve patient outcomes.
Supplementary Material
Acknowledgments
Potential conflict of interest: Dr. Lim consults and received grants from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, and Janssen; he consults for Merck and received grants from Abbott, Achillion, Genentech, and Vertex.
Abbreviations
- APCs
annual percentage changes
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IB
incidence based
- ICD
International Classification of Disease
- LT
liver transplantation
- NCI
National Cancer Institute
- RFA
radiofrequency ablation
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Supporting Information: Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.27388/suppinfo.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States From 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207. x–xi. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, M A, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 8.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and Survival in Surveillance, Epidemiology, and End Results registries, 1992-2008. Hepatology. 2012;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan SL, editors. International Classification of Diseases for Oncology. 3rd. Geneva: World Health Organization; 2000. [Google Scholar]
- 10.Percy C, Van Holten V, Muir CS, editors. International Classification of Diseases for Oncology. 2nd. Geneva: World Health Organization; 1990. [Google Scholar]
- 11.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology. 2004;39:798–803. doi: 10.1002/hep.20096. [DOI] [PubMed] [Google Scholar]
- 12.Koudah S, El Mouhadi S, Arrivé L. Fibrolamellar hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2012;36:5–6. doi: 10.1016/j.clinre.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820–830. doi: 10.1016/j.jamcollsurg.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–1158. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population. Am J Surg. 2008;195:829–836. doi: 10.1016/j.amjsurg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Sherman M. Screening for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:101–118. doi: 10.1016/j.bpg.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–623.e1. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Wong R, Corley D. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig Dis Sci. 2009;54:2031–2039. doi: 10.1007/s10620-008-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 22.Crosse K, Umeadi OG, Anania FA, Laurin J, Papadimitriou J, Drachenberg C, Howell CD. Racial differences in liver inflammation and fibrosis related to chronic hepatitis C. Clin Gastroenterol Hepatol. 2004;2:463–468. doi: 10.1016/s1542-3565(04)00162-4. [DOI] [PubMed] [Google Scholar]
- 23.Mathur Ak, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 24.Yuen MF. Revisiting the natural history of chronic hepatitis B: impact of new concepts on clinical management. J Gastroenterol Hepatol. 2007;22:973–976. doi: 10.1111/j.1440-1746.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- 25.Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858–867. doi: 10.1007/s00268-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 26.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, et al. Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754.e1. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Sloane DA, Guo C, Howell CD. Risk Factors for primary hepatocellular carcinoma in black and white Americans in 2000. Clin Gastroenterol Hepatol. 2006;4:355–360. doi: 10.1016/j.cgh.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Moyer LA, Boyle CA, Pollock DA. Validity of death certificates for injury-related causes of death. Am J Epidemiol. 1989;130:1024–1032. doi: 10.1093/oxfordjournals.aje.a115403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


