Abstract
Background
The impact of comorbidities on outcomes of patients with lower gastrointestinal bleeding (LGIB) remains unknown.
Objective
Investigate the prevalence of comorbidities and impact on outcomes of patients with LGIB.
Methods
The Nationwide Inpatient Sample 2010 was used to identify patients who had a primary discharge diagnosis of LGIB based on International Classification of Diseases, the 9th revision, clinical modification codes. The presence of comorbid illness was assessed using the Elixhauser index. Logistic regression models were used to assess the contributions of the individual Elixhauser comorbidities to predict in-hospital mortality.
Results
A total of 58,296 discharges with LGIB were identified. The overall mortality was 2.3 %. Among the patients who underwent colonoscopy, 17.3 % of patients had therapeutic intervention. As the number of comorbidities increased (i.e., 0, 1, 2, or ≥3), mortality increased (1.7, 2.0, 2.4, and 2.4 %, respectively). The mortality rate was highest in patients >65 years of age (2.7 %). Patients >65 years of age with two or more comorbidities had a mortality rate of 5 % as compared to 2.6 % in those with less than two comorbidities. Congestive heart failure (odds ratio, 1.67 [95 % confidence interval, 1.48–1.95]), liver disease (2.64 [1.83–3.80]), renal failure (1.99 [1.70–2.33]), and weight loss (2.66 [2.27–3.12]) were associated with a significant increase in mortality rate. Comorbidities increased hospital stay and costs.
Conclusions
Comorbidities were associated with increased the risk of mortality and health care utilization in patients with LGIB. Identification of comorbidities and development of risk-adjustment tools may improve the outcome of patients with LGIB.
Keywords: Lower gastrointestinal bleeding, Mortality, Comorbidities, Length of stay, Costs
Lower gastrointestinal bleeding (LGIB) is a common medical problem, and its incidence appears to be increasing. A population-based study suggested that the hospitalization rates for LGIB has increased by >50 % in a decade, from 20/100,000 in 1996 to 33/100,000 person in 2005 [1]. Additionally, LGIB was associated with higher mortality, longer hospitalization, and higher resource utilization than did upper GI complications [1].
The impact of comorbidities and outcomes in peptic ulcer bleeding (PUB) has been well studied [2–4]. In spite of the increasing incidence of LGIB, population-based studies from the USA, on the impact of comorbidities on outcomes of patients with LGIB, are not available. In the author's observations, patients with LGIB who die result most likely from deterioration of, or complications from, pre-existing comorbidity, rather than from continuous bleeding. However, given the lack of population-based studies from the USA, we sought to determine from a nationwide database, the impact of comorbidities on the outcomes of hospitalized patients with LGIB.
The aims of our study were to assess (1) the prevalence of various comorbidities in LGIB, (2) the impact of comorbidity on in-hospital mortality of patients with LGIB, and (3) the impact of comorbidity on length of hospital stay and hospitalization costs in patients with LGIB.
Methods
We obtained data from the Nationwide Inpatient Sample (NIS) which is the largest all-payer inpatient care database in the USA. The Agency for Healthcare Research and Quality maintains the Healthcare Cost and Utilization Project (HCUP), and it represents about 20 % of the stratified sample of US community hospitals. We obtained data including the basic demographic variables (including age, gender, race/ethnicity), primary and secondary diagnoses (up to 15), primary and secondary procedures (up to 15), total hospital charges, and length of stay (LOS). For our analysis, we used data from the HCUP NIS for the year 2010, which contains data from 45 states and 1,051 hospitals, accounting for over 8 million discharges.
We included all discharges in the 2010 Nationwide Inpatient Sample dataset, with patient age at admission between 18 and 90 years and a primary discharge diagnosis code, International Classification of Diseases 9th revision [ICD-9 CM] of LGIB. Discharge diagnosis of LGIB was ascertained using the International Classification of Diseases 9th revision [ICD-9 CM] codes which includes the various etiologies for LGIB including bleeding diverticulosis (Appendix 1). We based our codes on our previous study on timing of colonoscopy in LGI bleeding and another published study [5, 6].
Demographic information including age, race, and sex was obtained. Additionally, health insurance status, classified as private, Medicare, Medicaid, or other/unknown was also obtained. Medicare is a national social insurance program, administered by the US government providing health insurance for Americans aged 65 and older and younger people with disabilities as well as people with end-stage renal disease. Medicaid is a social government insurance health care program for families and individuals with low incomes.
Our primary outcome of interest was to study the impact of comorbidities on in-hospital mortality. We also studied the impact of comorbidities on length of hospital stay and total hospital costs.
We studied the burden of comorbid conditions using the Elixhauser co morbidity algorithm [7]. Elixhauser index has been suggested to be a superior risk-adjustment model than Charlson comorbidity score [8, 9].
Statistical analysis
All statistical analyses were performed using the survey commands in STATA MP 11.0 (STATA corp, College Station, TX) to adjust for the complex sampling design of the NIS. Hospital and discharge-level weights were applied to the NIS 2010 data as appropriate for all the analysis. A bivariate analysis was done using the chi square tests. Two-sided p values were reported with a significance level (p) of 0.05. Discharge-level weights published by HCUP were used to produce 95 % confidence intervals (CI) for point estimates and to reflect nationwide data during the study period.
We used the logistic regression models to assess the contributions of the individual Elixhauser comorbidities to predict in-hospital mortality. We adjusted for sociodemographic and clinical differences including age, sex, health insurance type, race, and endoscopic treatment at the time of colonoscopy.
A multivariable Cox proportional hazards models was used to predict hospital length of stay while linear regression models determined the effect of Elixhauser comorbidities on hospital cost. For all the described comorbidities, we defined the prevalence and the corresponding in-hospital mortality.
The national cost estimates were determined by multiplying total charges by a hospital wide, cost-to-charge ratio per hospital derived from center for Medicare- and Medicaidstandardized hospital accounting reports. The charge information represents the amount that hospitals billed for services but does not reflect how much hospital services actually cost or the specific amounts that hospitals received in payment [10].
Institutional review board approval exemption (IRB 13-243) was obtained from the Cleveland Clinic.
Results
Among a total of 39,008,298 discharges recorded in the NIS 2010 database, we found a total of 58,296 patients with the primary diagnosis of LGIB for the hospitalization. Among the various etiologies of LGIB, diverticulosis with bleeding was seen in 15,118 patients, angiodysplasia of the colon in 2,635 patients, hemorrhoids in 11,134 patients, colon polyps in 8,278 patients, colon cancer in 4,605 patients, colonic ischemia in 3,498 patients, inflammatory bowel disease in 2,215 patients, solitary rectal ulcer syndrome in 758 patients, dieulafoy lesion in 120 patients, and anal fissure in 525 patients.
Table 1 presents demographic and clinical characteristics of the patients with LGIB. The median age of patients with LGIB was 75 years, 28,040 (48.1 %) were men, 40, 690 (69.8 %) were white, and 40, 690 (69.8 %) had private health insurance coverage.
Table 1. Demographic characteristics of patients admitted with lower gastrointestinal bleeding.
| Characteristic | Value |
|---|---|
| Age; median (IQR), years | 75 (62–83) |
| Sex, male, % | 28,040 (48.1) |
| Race, % | |
| White | 40, 690 (69.8) |
| Black | 10, 260 (17.6) |
| Hispanic | 4,489 (7.7) |
| Asian or Pacific Islander | 1,341 (2.3) |
| Native American | 408 (0.7) |
| Other | 1,166 (2.0) |
| Insurance, % | |
| Private | 40, 690 (69.8) |
| Medicare | 10, 085 (17.3) |
| Medicaid | 4,081 (7.0) |
| Self-pay | 2,157 (3.7) |
| No charge | 175 (0.3) |
| Other | 1,108 (1.9) |
| Weekend admission, % | 14, 108 (24.2) |
| No. of comorbidities, % | |
| 0 | 873 (1.5) |
| 1 | 2,099 (3.6) |
| 2 | 3,731 (6.4) |
| ≥3 | 51, 593 (88.5) |
| In-hospital death (%) | 1,341 (2.3) |
| Endoscopic therapy (%) | 10,085 (17.3) |
| Length of stay, median (IQR), days | 3 (2–5) |
| Total hospital cost, median (IQR), $ | 19,176 (11,280–33,260) |
IQR interquartile range
Total charges converted to actual hospital cost
The proportion of patients with LGIB with three or more comorbidities was 51,592 (88.5 %). The overall in-hospital mortality of patients was 2.3 % (N=1,341). Among the patients presenting with LGIB, 10,085 (17.3 %) of patients underwent endoscopic control of bleeding at the time of their colonoscopy. The median length of stay in patients with LGIB was 3 days, and the median hospital cost for admission with LGIB was $19,176.
Outcomes
Mortality
Our primary outcome of interest was in-hospital mortality. Table 2 demonstrates the adjusted effect of each co morbidity on in-hospital mortality. We adjusted for age, sex, and race, and primary health insurer, type of admission, comorbidities, and use of endoscopic therapy.
Table 2. Elixhauser comorbidities: prevalence and relationship to in-hospital mortality rate for patients with lower gastrointestinal bleeding.
| Comorbidity | Prevalence (%) | Adjusted odds ratio (aOR) | 95 % CI | |
|---|---|---|---|---|
|
|
||||
| Lower | Upper | |||
| Congestive heart failure | 8.1 | 1.67 | 1.48 | 1.95 |
| Cardiac arrhythmias | 4.3 | 0.87 | 0.58 | 1.32 |
| Valvular disease | 0.4 | 0.43 | 0.06 | 3.11 |
| Hypertension | 6.0 | 0.12 | 0.05 | 0.32 |
| Pulmonary circulation disorders | 0.2 | 1.06 | 0.26 | 4.31 |
| Peripheral vascular disorders | 0.2 | 1.02 | 0.66 | 1.23 |
| Paralysis | 0.4 | 1.12 | 0.59 | 2.33 |
| Other neurological disorders | 2.0 | 1.99 | 1.24 | 3.18 |
| Chronic pulmonary disease | 3.4 | 1.333 | 0.82 | 1.61 |
| Diabetes mellitus, uncomplicated | 2.7 | 0.72 | 0.51 | 1.51 |
| Diabetes mellitus, complicated | 0.7 | 1.33 | 0.88 | 4.25 |
| Hypothyroidism | 0.9 | 1.83 | 0.92 | 1.94 |
| Renal failure | 18.1 | 1.99 | 1.70 | 2.33 |
| Liver disease | 2.9 | 2.64 | 1.83 | 3.80 |
| Peptic ulcer disease (with no bleeding) | 0.04 | 1.01 | 0.12 | 1.11 |
| AIDS | 0.02 | 0.76 | 0.11 | 5.45 |
| Lymphoma | 0.02 | 1.01 | 0.01 | 1.22 |
| Metastatic cancer | 1.6 | 1.63 | 0.36 | 1.12 |
| Solid tumor without metastasis | 0.2 | 1.04 | 0.20 | 1.44 |
| Rheumatoid arthritis/collagen vascular diseases | 0.9 | 0.49 | 0.12 | 1.98 |
| Coagulopathy | 0.4 | 1.06 | 0.33 | 1.55 |
| Obesity | 0.2 | 1.33 | 0.71 | 1.56 |
| Weight loss | 18.4 | 2.66 | 2.27 | 3.12 |
| Fluid and electrolyte disorders | 5.8 | 0.99 | 0.69 | 1.42 |
| Blood loss-related anemia | 6.3 | 1.79 | 0.91 | 1.98 |
| Iron deficiency-related anemia | 7.2 | 1.44 | 0.98 | 2.13 |
| Alcohol abuse | 2.9 | 1.20 | 0.57 | 2.54 |
| Drug abuse | 1.8 | 2.07 | 0.48 | 8.867 |
| Psychoses | 2.9 | 1.19 | 0.10 | 1.71 |
| Clinical depression | 0.7 | 1.01 | 0.08 | 1.22 |
Models were adjusted for age, sex, and race, and primary health insurer, type of admission, comorbidities, and use of endoscopic therapy
Congestive heart failure (odds ratio, 1.67 [95 % confidence interval, 1.48–1.95]), liver disease (2.64 [1.83–3.80]), renal failure (1.99 [1.70–2.33]), and weight loss (2.66 [2.27–3.12]) were associated with a significant increase in mortality rate. Also, the presence of neurological disorders other than paralysis (1.99 [1.24–3.18]) was associated with a significant increase in mortality rate. The presence of hypertension was negatively associated with mortality.
Length of stay and hospital costs
Table 3 demonstrates the adjusted effect of each comorbidity on the length of hospital stay (LOS). We adjusted for age, sex, and race, and primary health insurer, type of admission, comorbidities, and use of endoscopic therapy.
Table 3. Multivariate associations of comorbidities with length of hospital stay in patients with lower gastrointestinal bleeding.
| Comorbidity | Hazard ratioa | 95 % CI | P value | |
|---|---|---|---|---|
|
|
||||
| Lower | Upper | |||
| Congestive heart failure | 1.68 | 1.48 | 1.96 | 0.03 |
| Cardiac arrhythmias | 1.04 | 0.69 | 1.56 | 0.86 |
| Valvular disease | 0.68 | 0.10 | 4.82 | 0.70 |
| Hypertension | 0.18 | 0.07 | 0.48 | 0.001 |
| Pulmonary circulation disorders | 1.31 | 0.33 | 5.26 | 0.70 |
| Peripheral vascular disorders | 1.02 | 0.11 | 1.60 | 0.95 |
| Paralysis | 1.01 | 0.12 | 1.51 | 0.92 |
| Other neurological disorders | 1.24 | 0.78 | 1.96 | 0.34 |
| Chronic pulmonary disease | 0.58 | 0.32 | 1.06 | 0.08 |
| Diabetes mellitus, uncomplicated | 0.11 | 0.02 | 1.77 | 0.26 |
| Diabetes mellitus, complicated | 1.08 | 0.03 | 2.06 | 0.90 |
| Hypothyroidism | 0.18 | 0.03 | 1.29 | 0.09 |
| Renal failure | 1.55 | 1.33 | 1.81 | <0.0001 |
| Liver disease | 2.16 | 1.51 | 3.08 | <0.0001 |
| Peptic ulcer disease (with no bleeding) | 1.00 | 0.09 | 1.08 | 0.99 |
| AIDS | 0.63 | 0.09 | 4.50 | 0.65 |
| Lymphoma | 1.14 | 0.05 | 1.82 | 0.99 |
| Metastatic cancer | 0.95 | 0.54 | 1.66 | 0.85 |
| Solid tumor without metastasis | 1.02 | 0.01 | 1.06 | .96 |
| Rheumatoid arthritis/collagen vascular diseases | 0.67 | 0.17 | 2.69 | 0.57 |
| Coagulopathy | 1.03 | 0.01 | 6.42 | 0.923 |
| Obesity | 1.05 | 0.03 | 7.91 | 0.95 |
| Weight loss | 1.86 | 1.59 | 2.17 | <0.0001 |
| Fluid and electrolyte disorders | 1.13 | 0.79 | 1.61 | 0.52 |
| Blood loss-related anemia | 1.11 | 0.01 | 2.22 | 0.90 |
| Iron deficiency-related anemia | 1.03 | 0.22 | 8.79 | 0.89 |
| Alcohol abuse | 1.56 | 0.75 | 3.27 | 0.24 |
| Drug abuse | 1.80 | 0.44 | 7.38 | 0.41 |
| Psychoses | 1.23 | 1.07 | 1.81 | 0.02 |
| Clinical depression | 1.00 | 0.06 | 1.21 | 0.91 |
Cox proportional hazards regression: forward selection with inclusion and selection criteria of p<0.2. Length of hospital stay analyzed as a time-varying covariate
Models were adjusted for age, sex, and race, and primary health insurer, type of admission, comorbidities, and use of endoscopic therapy
Congestive heart failure, liver disease, renal failure, presence of psychosis, and weight loss were associated with a significant increase in LOS. The presence of hypertension was negatively associated with LOS.
Table 4 demonstrates the adjusted effect of each comorbidity on the hospitalization costs. The same factors that determined the LOS were also associated with increase in hospitalization costs.
Table 4. Univariate and multivariate associations of comorbidities with hospitalization cost in patients with lower gastrointestinal bleeding.
| Comorbidity | Univariate P value | Difference in cost ($) | 95 % CI | |
|---|---|---|---|---|
|
|
||||
| Lower | Upper | |||
| Congestive heart failure | 0.04 | 1,787 | 1,567 | 7,925 |
| Cardiac arrhythmias | 0.19 | −1,567 | −3,921 | 786 |
| Valvular disease | 0.08 | −7,510 | −15,862 | 841 |
| Hypertension | <0.0001 | −7,561 | −9,564 | −5,558 |
| Pulmonary circulation disorders | 0.69 | −1,751 | −10,413 | 6,910 |
| Peripheral vascular disorders | 0.578 | 3,810 | −9,581 | 17,202 |
| Paralysis | 0.14 | −6,761 | −15,746 | 2,223 |
| Other neurological disorders | <0.0001 | 7,610 | 3,859 | 11,361 |
| Chronic pulmonary disease | <0.0001 | 8,846 | 6,125 | 11,568 |
| Diabetes mellitus, uncomplicated | 0.15 | −9,977 | −12,962 | 6,992 |
| Diabetes mellitus, complicated | 0.39 | −2,945 | −9,705 | 3,813 |
| Hypothyroidism | 0.22 | −17,345 | −22,961 | 11,730 |
| Renal failure | <0.0001 | 6,718 | 5,522 | 7,915 |
| Liver disease | <0.0001 | 5,039 | 2,220 | 7,859 |
| Peptic ulcer disease (with no bleeding) | 0.43 | −21,872 | −76,242 | 32,497 |
| AIDS | 0.001 | 12,216 | 4,828 | 19,604 |
| Lymphoma | 0.42 | −14,224 | −48,613 | 20,163 |
| Metastatic cancer | <0.0001 | 8,074 | 4,070 | 12,079 |
| Solid tumor without metastasis | 0.23 | −8,054 | −21,248 | 5,139 |
| Rheumatoid arthritis/collagen vascular disease | 0.55 | −7,970 | 2,420 | 13,519 |
| Coagulopathy | 0.03 | 7,873 | 983 | 14,762 |
| Obesity | 0.14 | −11,666 | −20,936 | 2,397 |
| Weight loss | <0.0001 | 6,419 | 5,132 | 7,706 |
| Fluid and electrolyte disorders | 0.01 | 2,567 | 532 | 4,602 |
| Blood loss-related anemia | 0.99 | 32 | −5,080 | 5,145 |
| Iron deficiency-related anemia | 0.003 | 7,222 | 2,470 | 11,974 |
| Alcohol abuse | 0.003 | 6,464 | 2,223 | 10,705 |
| Drug abuse | 0.11 | 4,781 | −1,114 | 10,678 |
| Psychoses | 0.002 | 5,383 | 2,052 | 8,713 |
| Clinical depression | 0.02 | 8,273 | 1,519 | 15,026 |
Models were adjusted for age, sex, and race, and primary health insurer, type of admission, comorbidities, and use of endoscopic therapy
Of the 58,296 discharges 10,085 (17.3 %) received endoscopic intervention, while the others 48,211 (82.7 %) did not receive endoscopic intervention. For patients who were not intervened endoscopically, the overall in-hospital mortality rate was 1.9 % (N=1,108), length of stay [median (IQR)] was 2 (1–3) days, and hospital cost of $10,166 (9,233–17,268). The lower mortality rate, LOS, and cost in patients who did not receive endoscopic therapy are more likely due to the fact that these patients were less sick and less likely to receive endoscopic interventions.
We then calculated the mortality rate, LOS, and total hospital costs for patients based on the number of comorbidities. As the number of comorbidities in patients with LGIB increased from 0 to 1, to 2, and to 3 or more, the median age of LGIB increased. Interestingly, most patients with LGIB had three or more comorbidities. Also, the risk of mortality and the hospitalization costs also increased (Table 5).
Table 5. Relationship between the number of Elixhauser comorbidities and outcomes (n=55,957).
| Characteristic | No. of Elixhauser comorbidities | |||
|---|---|---|---|---|
|
|
||||
| 0 | 1 | 2 | ≥3 | |
| No. of patients | 880 | 2,112 | 3,633 | 49,332 |
| Median age, y (IQR) | 44 (24–62) | 57 (42–75) | 65 (51–80) | 76 (64–84) |
| In-hospital death, no. (%) | 15 (1.7) | 42 (2.0) | 89 (2.4) | 1,208 (2.4) |
| Median length of stay, d (IQR) | 2 (1–3) | 2 (1–3) | 2 (2–4) | 3 (2–5) |
| Median hospitalization cost, $ (IQR) | 11,386 (6,199–19,381) | 13,429 (7,792–22,712) | 15,365 (9,121–26,203) | 19,902 (11,808–34,382) |
Figure 1 represents the mortality based on stratification by age. When stratified by age, the mortality rate was highest in patients >65 years of age (2.7 %) [N=1,574]. Patients >65 years of age with 2 or more comorbidities had a mortality rate of 5 % [N=2,915] as compared to 2.6 % [N=1,516] in those with less than two comorbidities.
Fig. 1.
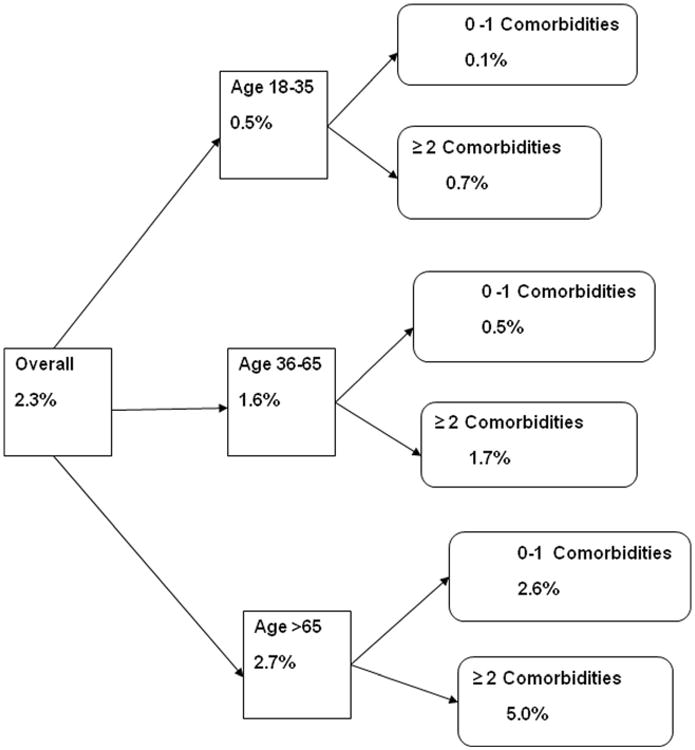
Probability of death for Patients with Lower Gastrointestinal Bleeding stratified by age, and number of comorbidities. Patients > 65 years of age with 2 or more comorbidities had a mortality rate of 5 % as compared to 2.6 % in those with less than 2 comorbidities
Discussion
This nationwide database study showed that presence of comorbidities were associated with increased mortality, longer hospital stay and increased hospitalization costs in patients with LGIB. In particular, congestive heart failure, liver disease, renal failure, and weight loss were associated with a significant increase in mortality rate, LOS, and hospitalization costs. Our study has demonstrated that risk adjustment of comorbidities is important in patients with LGIB, as they are prevalent and associated with increased mortality and utilization of health care resources.
A previous study had reported correlation between number of comorbidities and impact on LGIB [5]. Increasing age and comorbid illness were significant predictors of in-hospital mortality [5]. We observed a similar relationship between the number of CCI and outcomes in our previous study [6]. However, no previous study has systematically studied the impact of individual comorbidities in LGIB outcomes. The contribution of a particular comorbidity to outcomes may be different based on the underlying type of comorbidity and generalizing the outcomes just based on number of comorbidities rather than the type of comorbidities is inadequate. This prompted us to study the impact of individual comorbidities on LGIB outcomes in our current study.
In author's observations, patients with LGIB are often elderly and have multiple comorbidities. Although patients with LGIB have appropriate resuscitation and management of bleeding, the mortality of LGIB is still significant. Our study has demonstrated that the presence of underlying co morbidity plays a significant role in determining mortality. Also, mortality in patients with LGIB appears to increase as the number of comorbidities increases.
We observed that cardiovascular disease, liver disease, and renal disease were common and predicted mortality and overall health care utilizations in patients with LGIB. These conditions have been clearly demonstrated to impact outcomes in patients with PUB [11, 12]. It appears that the same factors that determine worse outcomes in PUB also play a significant impact on LGIB even when adjusting for close to 30 comorbidities. Thus, systemic factors that impair platelet function and coagulation status appear to worsen overall outcomes irrespective of appropriate treatment of bleeding endoscopically.
Patients with liver disease have several risk factors which increase bleeding risk including thrombocytopenia, and abnormal coagulation factors, and the presence of concomitant renal insufficiency can also be seen which increases bleeding risk [13, 14]. Unless, we manage the underlying liver disease with appropriate resuscitation measures, outcomes of patients with LGIB will not improve. The presence of weight loss as a factor that impacts outcomes would be probably because of the fact that underlying causes of LGIB would be more likely to be colon cancer or a malignant process in these patients.
We also observed that hypertension was negatively associated with mortality rate and health care resource use. This may have resulted from the inverse relationship between disease severity and coding of hypertension [15, 16]. Moreover, we demonstrated a steady rise in mortality and utilization of health care resources as the number of comorbidities increased in out cohort of patients. Approximately, 90 % of patients with LGIB had three or more comorbidities. Among patients with multiple comorbidities, the probability of death was dependent on the age of the patient. The mortality rate was highest in patients >65 years of age (2.7 %). Patients >65 years of age with two or more comorbidity had a mortality rate of 5 % as compared to 2.6 % in those with less than two comorbidities in contrast to younger patients in whom the mortality rate was low, irrespective of comorbidity status. The question, whether optimization of comorbidities can improve the outcomes of LGIB, still remains unanswered.
Our study had several limitations. This was a retrospective database study and often in these types of studies, the diagnosis of LGIB and identification of comorbidity are dependent on the accuracy of coding. Even though we did not validate the diagnosis, routine validation is routinely performed by the Agency for Healthcare Research and Quality [17]. A previous study has also attempted to validate the ICD-9 codes for LGIB and found to be accurate [18].
In spite of the above limitations, our study has several strengths and clinical implications. Identification of comorbidities that impact outcomes is important. The contribution of a particular comorbidity to outcomes may be different based on the underlying type of comorbidity and generalizing the outcomes just based on number of comorbidities rather than the type of comorbidities is inadequate. Our study identified comorbidities and provides an opportunity to optimize the comorbidities in future studies with LGIB to see if we can improve the overall outcomes. We were able to identify the high-risk comorbidities among the existing comorbidities, and future studies to develop and validate management algorithms using these needs are to be performed. To our knowledge, ours is the first population-based study from the USA to demonstrate the significance of the impact of individual comorbidities on LGIB outcomes.
To conclude, comorbidities were associated with increased in the risk of mortality and health care utilization in patients with LGIB. Comorbidities significantly impact outcomes particularly in patients >65 years of age with LGIB. Future studies need to address development of risk scoring systems based on comorbidities and appropriate triage of patients to improve outcomes.
Supplementary Material
Acknowledgments
Grant support The study is supported by a research grant from the American College of Gastroenterology (to U.N).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00384-014-1915-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declared no financial conflict of interest.
Contributor Information
Preethi G. K. Venkatesh, Digestive Disease Institute, The Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, USA
Basile Njei, Department of Medicine, University of Connecticut Health Center, Farmington, CT, USA.
Madhusudhan R. Sanaka, Digestive Disease Institute, The Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, USA
Udayakumar Navaneethan, Email: udhaykumar81@gmail.com, Digestive Disease Institute, The Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, USA.
References
- 1.Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633–1641. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 2.Leontiadis GI, Molloy-Bland M, Moayyedi P, Howden CW. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(3):331–345. doi: 10.1038/ajg.2012.451. [DOI] [PubMed] [Google Scholar]
- 3.Parasa S, Navaneethan U, Sridhar AR, Venkatesh PG, Olden K. End-stage renal disease is associated with worse outcomes in hospitalized patients with peptic ulcer bleeding. Gastrointest Endosc. 2013;77(4):609–616. doi: 10.1016/j.gie.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh PG, Parasa S, Njei B, Sanaka M, Navaneethan U. Increased mortality with peptic ulcer bleeding in patients with both compensated and decompensated cirrhosis. Gastrointest Endosc. 2013 doi: 10.1016/j.gie.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004–1010. doi: 10.1016/j.cgh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navaneethan U, Njei B, Venkatesh PG, Sanaka MR. Timing of colonoscopy and outcomes in patients with lower GI bleeding: a nationwide population-based study. Gastrointest Endosc. 2014;79(2):297–306. doi: 10.1016/j.gie.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 10.Cost-to-Charge Ratio Files. Agency for Healthcare Research and Quality; Rockville, MD: Sep, 2012. Healthcare Cost and Use Project (HCUP) www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. [Google Scholar]
- 11.Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal hemorrhage. Gut. 1996;38:316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang JY, Elders A, Majeed A, et al. Recent trends in hospital admissions and mortality rates for peptic ulcer in Scotland 1982–2002. Aliment Pharmacol Ther. 2006;24:65–79. doi: 10.1111/j.1365-2036.2006.02960.x. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–1046. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 14.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JS, Iezzoni LI, Daley J, Greenberg L. How severity measures rate hospitalized patients. J Gen Intern Med. 1996;11(5):303–311. doi: 10.1007/BF02598273. [DOI] [PubMed] [Google Scholar]
- 16.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data: the role of length of stay and comorbidities. JAMA. 1988;260(15):2240–2246. [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality. Agency for Healthcare Research and Quality; May, 2011. [Accessed 31 May 2011]. Healthcare Cost and Utilization Project (HCUP) database. Web site. http://www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 18.Lanas A, García-Rodríguez LA, Polo-Tomás M, et al. The changing face of hospitalization due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33(5):585–591. doi: 10.1111/j.1365-2036.2010.04563.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


