Abstract
Moso bamboo, well known for its high growth rate, is being subjected to increasing amounts of nitrogen deposition. However, how anthropogenic management practices regulate the effects of N deposition on Moso bamboo stoichiometry remains poorly understood. We observed the effects of two years of simulated N deposition (30, 60 and 90 kg N ha−1yr−1) on the foliar stoichiometry of Moso bamboo plantations under conventional management (CM) and intensive management (IM). Young bamboo had significantly greater foliar N and P concentrations and N:P ratios than mature plants (P < 0.05). IM significantly increased the foliar N concentrations of young bamboo and P concentrations of mature bamboo but decreased mature bamboo foliar N:P ratios (P < 0.05). Nitrogen increased foliar N and P concentrations in IM bamboo plantations, but the positive effects were diminished when the addition rate exceeded 60 kg N ha−1yr−1. Nitrogen increased foliar N concentrations but aggravated P deficiency in CM bamboo plantations. The positive effects of N deposition on foliar stoichiometry were influenced by management practices and bamboo growth stage. The effects of N deposition on foliar stoichiometry combined with anthropogenic management practices can influence ecosystem production, decomposition, and subsequent N and P cycles in Moso bamboo plantations.
The quantitative relationships between foliar nitrogen (N) and phosphorus (P) concentrations, as well as the effects of N and P limitation on plant growth under global climate change, are increasingly concerning in ecological stoichiometry theory1,2. N and P are essential for plant growth, and the foliar N:P ratio is thought to be useful for assessing N versus P limitation in primary production in terrestrial ecosystems3,4. Stoichiometric homeostasis varies with plant species, some are able to maintain their nutrient balance other not5,6. Foliar N:P stoichiometry can be strongly affected by environmental nutrient availability7,8,9 and plant growth stage10,11. For example, Wu et al.10 reported that the foliar N and P concentrations of three wetland plants were highest in the early stages of growth and the smallest in the middle growth stage.
The increasing consumption of fossil fuels and application of agricultural fertilizers have largely enhanced the amount of anthropogenic available N being input into ecosystems worldwide in the last few decades12,13, and the ecological effects are attracting attention. Previous studies have reported that N addition increased the foliar N concentration and N:P ratio, and both increases and decreases have been found in the subsequent P uptake capacity of plants4,9,14, including in fast-growing arbor species such as Eucalyptus grandis15 and hybrid poplar16.
Moso bamboo (Phyllostachys pubescens Mazel ex H. de Lehaie) is a monopodial giant bamboo widely distributed in southern China and neighboring countries, such as Japan and Korea17. As a highly representative plantation forest in the subtropical region of China, the Moso bamboo forest is currently the most important source of non-wood forest products in China18. The forest covers an area of 3.87 million ha, representing 70% of the Chinese bamboo forest area and 80% of the global distribution of Moso bamboo18,19. Due to the enormous ecological and socioeconomic benefits, Moso bamboo forests have expanded rapidly in subtropical China, with an annual increase of 3% in recent decades18.
Being greatly different from the arbor species, the woody rhizomatous plant Moso bamboo is well known for its startlingly high growth rate. New bamboo usually grows to a height of 10–20 m and a diameter of 8–16 cm at breast height within 35–40 days after germination from the soil in the spring. Afterwards, the height, diameter and volume remain unchanged because of the scarce secondary cambium, and the bamboo begins to slowly accumulate dry matter20,21,22. New bamboo begins to grow leaves in May of the current year during which it emerges. These initial leaves fall the next spring, and new leaves quickly reemerge. These re-growing leaves will continue to fall biennially until a life span of two years is reached17. Moreover, due to long-term management practices, Moso bamboo plantations have been characterized by years of alternating high- and low-recruitment of new bamboo21,23,24. Thus, the Moso bamboo plantations are unevenly aged forests with a two-year interval. How this growth pattern of Moso bamboo influences foliar stoichiometry is still unclear.
To maintain high productivity in Moso bamboo plantations and maximize the economic benefits, increasing numbers of farmers are adopting intensive management (IM) practices, such as the regular removal of understory vegetation, plowing, and fertilization18,25. Typically, conventional management (CM) consists of regularly harvesting bamboo trunks and shoots, without any other management practices25. IM practices have increased bamboo production but reduced soil microbial functional diversity26,27. Moreover, the IM practice has also accelerated soil organic carbon mineralization28,29, influenced N and P release from decomposing litter30, altered soil nutrient availability31, and thus affected bamboo nutrient balance. Our previous investigation showed that IM practices increased Moso bamboo foliar N and P concentrations32.
The average bulk deposition of N has increased from 13.2 kg N ha−1yr−1 in the 1980s to 21.1 kg N ha−1yr−1 in the 2000s across China33. Subtropical China, with a maximum annual N deposition rate of 63.53 kg N ha−1yr−1 34, is predicted to become the region with the greatest N deposition in the world by 203035. However, knowledge of the effects of N deposition on plant stoichiometry in forest ecosystems in China remains limited36,37. Most Moso bamboo plantations are distributed in the center of the region, with the greatest N deposition in China, both at the present time and in future predictions30. Thus, the effects of increasing N deposition on the foliar stoichiometry of managed Moso bamboo plantations would be affected by anthropogenic management practices. However, thus far, a lack of information about these interactions has limited our understanding of the combined effects of N deposition and anthropogenic management practices on the Moso bamboo forest ecosystem.
In this study, we investigated the variation of foliar N and P concentrations in Moso bamboo at different ages under IM and CM practices after exposure to simulated N deposition for two years. The objectives are to examine (1) how foliar N and P stoichiometry responds to management practices, (2) how foliar N and P stoichiometry responds to increasing N deposition, and (3) whether the effects of N deposition on foliar stoichiometry depend on management practices and growth stage.
Results
Foliar N and P concentrations
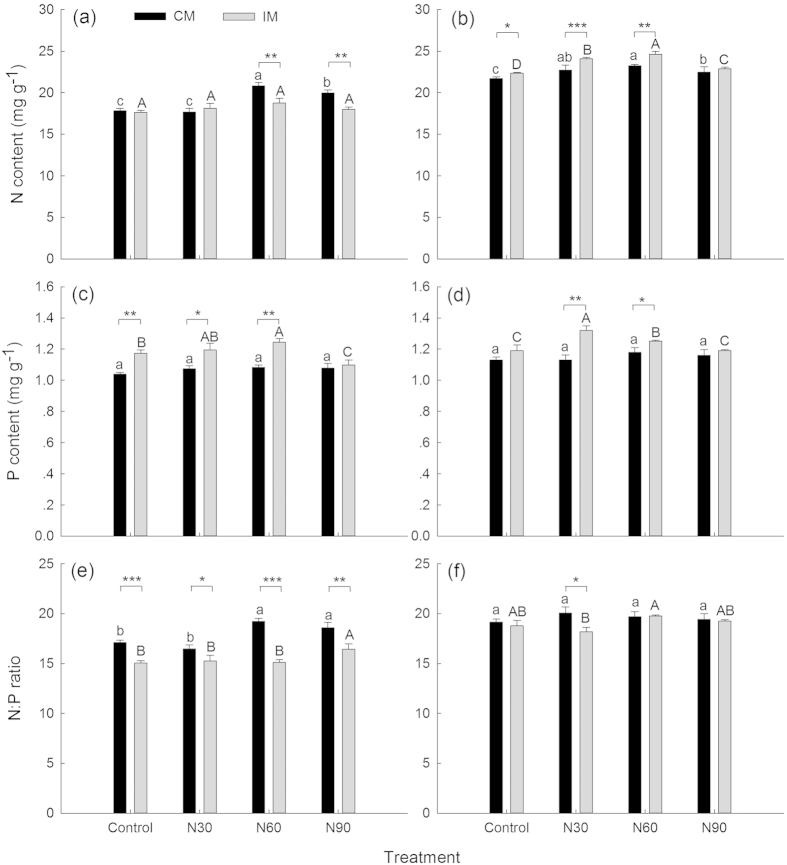
In CM plots, compared with the control, N addition significantly increased the foliar N concentration by 3.55–16.88% (Fig. 1a,b). However, these positive effects were diminished when the N addition rate was more than 60 kg N ha−1yr−1. Nitrogen addition did not significantly influence the foliar P concentration (Fig. 1c,d). In IM plots, compared with the control, N addition significantly increased the foliar N concentration of young bamboo by 2.33–10.25% (Fig. 1b) but did not affect that of mature bamboo (Fig. 1a). These positive effects on young bamboo foliar N were diminished when the N addition rate was more than 60 kg N ha−1yr−1. Nitrogen addition significantly increased the foliar P concentration by 5.04–10.92%, but excess N, such as 90 kg N ha−1yr−1, decreased the mature bamboo foliar P concentration by 6.48% (Fig. 1c,d).
Figure 1.
Mature Moso bamboo foliar N (a) and P (c) concentrations and the N:P ratio (e), and young Moso bamboo foliar N (b), and P (d) concentrations and the N:P ratio (f) under different management practices (CM: conventional management; IM: intensive management) and N treatments (control; N30: 30 kg N ha−1yr−1; N60: 60 kg N ha−1yr−1; N90: 90 kg N ha−1yr−1) (n = 3). Different lowercase letters indicate significant differences among N addition rates under CM treatments (P < 0.05). Different capital letters indicate significant differences among N addition rates under IM treatments (P < 0.05). Asterisks indicate significant differences between CM and IM at the same N addition rate (*P < 0.05, **P < 0.01, ***P < 0.001).
Young bamboo foliar N concentrations were significantly larger upon IM than upon CM when the N addition level was less than 60 kg N ha−1yr−1, but mature bamboo foliar N concentrations showed a contrary trend under the N60 and N90 treatments, in which the foliar N concentrations were 9.93–9.96% lower in CM compared with IM plots (Fig. 1a,b). Mature bamboo foliar P concentrations were significantly greater upon IM than upon CM by 11.81–14.77% when the N addition rate was less than 60 kg N ha−1yr−1. Young bamboo showed a similar pattern under the N30 and N60 treatments (Fig. 1c,d). Young bamboo foliar N concentrations were always significantly greater than those of mature bamboo under all treatments (P < 0.01), and there was a general trend toward P concentrations being greater in young bamboo than in mature plants (Fig. 1, Table 1). Pearson correlation analysis showed that there was a significant correlation between N and P concentrations only in young bamboo (Table 2).
Table 1. The significance level of effects of Moso bamboo growth stage on foliar stoichiometry under different treatments (n = 3, df = 1).
| Treatment | N |
P |
N:P |
|||
|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | F | Sig. | |
| CM | ||||||
| Control | 343.6 | *** | 48.6 | ** | 80 | ** |
| N30 | 410.6 | *** | 7.4 | ns | 81.1 | ** |
| N60 | 118.7 | *** | 24.7 | ** | 2.1 | ns |
| N90 | 32.9 | ** | 9.3 | * | 3.5 | ns |
| IM | ||||||
| Control | 6225.2 | *** | 0.481 | ns | 115.5 | *** |
| N30 | 392.1 | *** | 17.7 | * | 49.2 | ** |
| N60 | 240.8 | *** | 0.05 | ns | 716.8 | *** |
| N90 | 523.8 | *** | 22.8 | ** | 76.9 | ** |
CM: conventional management; IM: intensive management; N: nitrogen; P: phosphorus. Asterisks indicate that the young bamboo foliar N and P concentrations and N:P ratios are significantly larger than those of mature bamboo (*P < 0.05, **P < 0.01, ***P < 0.001). ns, no significance.
Table 2. Pearson correlation coefficients between the foliar nitrogen (N) and phosphorus (P) concentrations of Moso bamboo at different growth stages under conventional management (CM) and intensive management (IM) (n = 12).
*The correlation is significant at the α = 0.05 level.
Three-way analysis of variance (ANOVA) showed that foliar N concentrations were significantly influenced by three factors—management type, N addition and growth stage—both individually and interactively, except for management type (Table 3). Similarly, foliar P concentrations were also significantly influenced by these three factors, both individually and interactively, except for the interaction between growth stage, management type and N addition.
Table 3. The significance levels in three-way ANOVA for the effects of management type, N level, bamboo age and their interactions on foliar nitrogen (N), phosphorus (P) and N:P ratios of Moso bamboo.
| Source of variation | N |
P |
N:P |
||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | Sig. | df | F | Sig. | df | F | Sig. | |
| Management type | 1 | 0.0 | 0.9999 | 1 | 155.1 | 0.0001 | 1 | 151.1 | 0.0001 |
| Bamboo age | 1 | 1889.3 | 0.0001 | 1 | 83.5 | 0.0001 | 1 | 468.9 | 0.0001 |
| N addition level | 3 | 65.6 | 0.0001 | 3 | 15.5 | 0.0001 | 3 | 19.5 | 0.0001 |
| Management × Age | 1 | 87.9 | 0.0001 | 1 | 2.1 | 0.1587 | 1 | 54.3 | 0.0001 |
| Management × N level | 3 | 13.1 | 0.0001 | 3 | 12.4 | 0.0001 | 3 | 2.6 | 0.0656 |
| Age × N level | 3 | 14.6 | 0.0001 | 3 | 2.2 | 0.1122 | 3 | 6.3 | 0.0017 |
| Management × Age × N level | 3 | 9.7 | 0.0001 | 3 | 5.8 | 0.0027 | 3 | 16.8 | 0.0001 |
Foliar N:P ratios
In CM plots, compared with the control, N addition did not significantly influence young bamboo foliar N:P ratios (Fig. 1f), but it did significantly increase mature bamboo foliar N:P ratios by 8.64–12.38% in the N60 and N90 treatments (Fig. 1e). In IM plots, compared with the control, N addition did not significantly influence foliar N:P ratios except under N90 treatment for mature bamboo (Fig. 1e,f). Mature bamboo N:P ratios were always significantly greater in CM than in IM plots under all N addition levels (Fig. 1e), but this pattern was not observed for young bamboo except under N30 treatment (Fig. 1f). Young bamboo foliar N:P ratios were significantly larger than those for mature bamboo except in CM plots with N60 and N90 treatments (Table 1, Fig. 1e,f). Three-way ANOVA showed that the foliar N:P ratios were significantly influenced by the three factors both individually and interactively, except for the interaction between management type and N addition level (Table 3).
Discussion
Effects of management practices on foliar stoichiometry
Under N-free conditions, IM treatment significantly increased young bamboo foliar N (Fig. 1b) and mature bamboo foliar P (Fig. 1c), and it significantly decreased mature bamboo foliar N:P (Fig. 1e), being consistent with our previous investigation32. Widespread fertilization management is thought to result in an increase in the N:P ratios of the natural terrestrial ecosystem and a decline in soil N:P ratios in some cropland ecosystems because of greater solubility and loss of N9. In the present IM plots, owing to annual fertilization of 450 kg ha−1 (N:P2O5:K2O, 15:6:20, equating 67.5 kg N and 11.8 kg P ha−1yr−1), IM treatments significantly increased soil N and P contents and decreased the soil N:P ratio due to a greater increase in P content (+76.78%) than N (+9.35%) (Fig. S1), to some extent contributing to the changes in foliar stoichiometry.
In contrast to temperate ecosystems, tropical forests are generally considered N-rich but P-poor38. Han et al.39 also found that soil P was lower in subtropical areas than in temperate areas in China, which led to a similar pattern in foliar P, indicating a strong P limitation in subtropical China. In the present study, under N-free conditions, the significantly larger foliar P and smaller N:P ratios in both soil and mature bamboo, together with significantly larger foliar N in young bamboo upon IM treatments than upon CM, indicated that both N and P elements were deficient in the CM Moso bamboo forests. Our results corroborate the fact that current IM practices, especially fertilization, provide available N and P elements to Moso bamboo.
Plant N and P contents vary with the growth stage1,10,40. In the present study, the significantly greater foliar N and P in young bamboo than mature bamboo (Table 1, Fig. 1) indicated that young bamboo had a higher demand for N and P elements because after completing fast height and volume growth within two months, young bamboo begins a slow accumulation of dry matter18,22 and needs more N and P elements in its leaves to complete the primary production process. Moreover, when there is not sufficient N or P in CM plots, these elements are preferentially provided to young bamboo through underground rhizomes that connect young and mature bamboo and transfer nutrients among them17,21.
Mature bamboo had similar foliar N contents of 17.83 and 17.63 mg g−1 under CM and IM treatments without N addition, respectively (Fig. 1a), which are nearly identical to the levels reported by Elser et al.41 for 397 terrestrial plants (17.6 mg g−1), and slightly lower than those reported by Han et al.39 for 753 Chinese terrestrial plants (18.6 mg g−1) and by Reich and Oleksyn42 for 1251 world terrestrial plants (18.3 mg g−1). Foliar P concentrations of both young and mature bamboo under two management practices (1.04–1.19 mg g−1) were slightly lower than that of 753 Chinese terrestrial plants (1.21 mg g−1)39 but remarkably lower than those of 397 terrestrial plants (1.58 mg g−1)41 and 1251 world terrestrial plants (1.42 mg g−1)42. The growth rate hypothesis proposes that fast-growing organisms require relatively more P-rich RNA to support rapid rates of protein synthesis1. In the present study, as a fast-growing plant, Moso bamboo has a relatively low foliar P concentration compared to terrestrial plants across China39 and the world41,42, being inconsistent with the growth rate hypothesis, which may be due to the especially fast growth rate of Moso bamboo. Alternatively, the growth rate hypothesis alone may not dictate leaf stoichiometry43.
Effects of N deposition on foliar stoichiometry and interactions with management practices and growth stage
Our results showed that N addition significantly increased the foliar N concentration (except for mature bamboo under IM treatment), increased the foliar P concentration under IM treatment (except at the highest rate of N addition (90 kg N ha−1yr−1)), and generally increased foliar N:P ratios of mature bamboo. Moreover, these effects depended on the management treatment and the bamboo growth stage (Table 3).
In CM plots, foliar N concentrations of both young and mature bamboo significantly increased with the N addition rate but began to decline when the N addition rate exceeded 60 kg N ha−1yr−1 (Fig. 1a,b), indicating that the N element is deficient in conventionally managed bamboo forests. In IM plots, however, only young bamboo foliar N significantly increased with the N addition rate, and it declined when the N addition rate exceeded 60 kg N ha−1yr−1 (Fig. 1a,b), indicating that the N element was still lacking for young Moso bamboo, even though fertilization (equating 67.5 kg N ha−1yr−1) significantly increased the soil N content under IM treatment (Fig. S1a). The positive effects of N addition on foliar N were also observed in previous studies4,14,15 and were consistent with our current findings. However, excessive N deposition (>60 kg N ha−1yr−1), in turn, could restrain N uptake. Aber et al.44 thought that the foliar N concentration would increase in the initial response to N input and then would decline upon excessive N deposition because of the limited availability of other resources essential for growth. In addition, excessive N input (high N addition combined with annual fertilization (67.5 kg N ha−1yr−1)) and atmospheric N deposition ((30.9 kg N ha−1yr−1)45 in IM plots) could decrease the activity of extracellular enzymes and thus decrease the net mineralization rate of soil N46.
Nitrogen addition can increase the P uptake capacity of plants by stimulating the activity of phosphatase in the rhizosphere47,48. In IM plots, foliar P concentrations significantly increased with the N addition rate (≤60 kg N ha−1yr−1) and were significantly greater than upon CM. However, in CM plots, this increase in foliar P concentrations with N addition did not happen (Fig. 1c,d) because P is deficient in the soil of CM plots, whereas annual fertilization (equating 11.8 kg P ha−1yr−1) provides abundant P to IM plots. The significantly lower soil P content in CM plots compared with IM plots supports this assumption (Fig. S1). The current result indicates that the extent to which N deposition affects Moso bamboo foliar P concentrations depends on the soil P condition, which is mainly controlled by fertilization in Moso bamboo plantations. In addition, our previous study on the effects of N deposition on Moso bamboo litter decomposition found that IM practices weakened the positive effects of N deposition on N loss but enhanced the positive effects on P loss in the early decomposition stage30. Moreover, IM practices decreased the diversity and biomass of understory plants21,30. These effects of IM practices together regulate the effects of N deposition on foliar stoichiometry.
In IM plots, the foliar P concentrations started to decline when the N addition rate exceeded 60 kg N ha−1yr−1 (Fig. 1c,d), indicating that moderate N deposition (≤60 kg N ha−1yr−1) can favor P uptake but excessive N deposition (> 60 kg N ha−1yr−1) will restrain P uptake. The soil acidification induced by high N may partly explain this result. The soil of our bamboo stands is acidic even in the CM plots (Fig. S1d) because of the severe N deposition in this region45. In the IM plots, high N addition (≥60 kg N ha−1yr−1), combined with annual fertilization (67.5 kg N ha−1yr−1) and atmospheric N deposition (30.9 kg N ha−1yr−1)45, can largely increase the solution loss of NO3- and cations from soil and increase H+ content in soil through the reaction of NH4+ nitrification49,50, resulting in a significant decline of soil pH compared with low N addition (≤30 kg N ha−1yr−1) and CM plots (Fig. S1d). The decline in soil pH can decrease the mineralization rates of soil P and available P49,51, and it can subsequently lead to a decline in foliar P. In addition, excessive N deposition can increase the dissolution of aluminum ions, which can also inhibit P uptake49,52. Previous studies also demonstrated that high N deposition decreased foliar P concentrations of Abies balsamea and Betula papyrifera var. cordifolia in subalpine forests of the northeastern United States53. Wessman et al.54 suggested that foliar chemistry (such as N and P concentrations) responses to N deposition could be regarded as predictors of the earliest stages of N saturation. Our results showing that N and P concentrations began to decline when the N addition rate was more than 60 kg N ha−1yr−1 imply that the N deposition level of 60 kg N ha−1yr−1 might be presumed as a threshold for positive/negative ecological effects on Moso bamboo growth or as a marker for whether Moso bamboo forests have reached N saturation.
Nitrogen deposition can increase the N:P ratio of plants and soils in terrestrial ecosystems9,55. In the present study, N addition significantly influenced soil N and P contents and N:P ratios (Fig. S1). However, our Pearson correlation analysis showed that soil N and P contents and N:P ratios did not have significant correlations with foliar N and P concentrations or N:P ratios (P > 0.05, data not shown), except for soil N and foliar N under IM treatment (P = 0.025, data not shown). This result likely occurred because the foliar stoichiometry of Moso bamboo is controlled by multiple environmental factors aside from soil nutrients. Mature bamboo foliar N:P ratios remained stable at a value of 15 in IM plots under moderate N addition (≤60 kg N ha−1yr−1) and were always significantly lower than that in CM plots regardless of the N addition rate (Fig. 1e), supporting the assumption that there is a positive relationship between high N input and high N:P ratios. This relationship is strengthened by P limitation7 and also suggests that increasing N deposition would aggravate P limitation in CM Moso bamboo forests. These results indicate that the effects of N addition on the mature bamboo foliar N:P ratio are regulated by the effects of management practices.
Young bamboo foliar N:P ratios in the range of 18 to 20 did not show significant variation among N addition rates or management treatments (Fig. 1f), indicating that young bamboo had an intrinsically higher foliar N:P ratio than mature bamboo. Young bamboo contains higher foliar N and P concentrations than mature bamboo (Fig. 1) for more dry matter accumulation21,22. Moreover, the demand for N is greater than for P, which may result in a larger stable N:P ratio in young bamboo than in mature bamboo. Our findings indicate that the stable foliar N:P ratio may change with the Moso bamboo growth stage, being low for mature bamboo and high for young bamboo, and the effects of N addition on the foliar N:P ratio also vary with the Moso bamboo growth stage. Three-way ANOVA confirmed this conclusion.
Increasing N deposition can act together with increasing atmospheric CO2 to improve the growth and productivity of forests and thus enhance carbon sequestration capacity56, which is favorable to IM Moso bamboo forests. In CM Moso bamboo forests, however, P limitations would significantly constrain the N deposition fertilization effects that improve C storage in biomass by promoting growth9. Therefore, it is recommended to apply fertilizer, especially phosphate fertilizer, to CM Moso bamboo forests.
Conclusion
Nitrogen deposition generally increased the foliar N and P concentrations and N:P ratios of intensively managed Moso bamboo but aggravated the P deficiency of conventionally managed Moso bamboo. When N deposition exceeded 60 kg N ha−1yr−1, these positive effects were diminished but negative effects could occur. Moreover, these positive effects were regulated by management practices and depended on the Moso bamboo growth stage. Findings from this study suggested that the combination of anthropogenic management practices should be taken into account when estimating the ecological consequences of increasing N deposition.
Materials and Methods
Study site
The study site was located in Qingshan Town, Lin’an City (30°14′N, 119°42′E), Zhejiang Province, China. The area has a monsoonal subtropical climate with four distinct seasons. The mean annual precipitation is 1,420 mm, and the mean annual temperature is 15.6 °C, ranging from 24 °C in July to 3 °C in January. The area receives an average of approximately 1,847 hours of sunshine per year and features an average of 230 frost-free days per year.
The conventionally managed Moso bamboo plantations were originally established in the late 1970s from native evergreen broadleaf forest in sites with similar topography (southwest slope of approximately 6 degrees) and soil type (Ferrisols derived from granite)30. The IM practices, including annual fertilization, plowing, and weeding with herbicide, have been conducted since 2001. In September of each year, compound fertilizer (N:P2O5:K2O, 15:6:20, 450 kg ha−1) is manually and evenly scattered on the soil surface and followed with a deep plowing to 0.3 m, which is equivalent to an annual addition of 67.5 kg N, 11.8 kg P, and 74.7 kg K per hectare. CM practices only selectively and regularly harvest bamboo trunks and shoots, which is also done in IM. Except for harvesting, conventionally managed bamboo forests did not receive any other management practices30.
Experimental design and sampling
In November 2012, 24 plots of 20 × 20 m were established. The initial stand and soil characteristics of both the 12 CM plots and 12 IM plots are summarized in Song et al.30. Each plot was surrounded by a 20 m-wide buffer zone to avoid disturbing nearby plots. According to the local N deposition rate of 30.9 kg N ha−1yr−1 45 and the widely used method (double and triple the local N deposition rate) in previous studies simulating N deposition57,58, three N addition treatments, low-N (N30, 30 kg N ha−1yr−1), medium-N (N60, 60 kg N ha−1yr−1), high-N (N90, 90 kg N ha−1yr−1), and a control (no added N) with three replicates were conducted randomly in each Moso bamboo plantation management type. Starting in January 2013, quantified NH4NO3 was weighed according to the N addition rate, mixed with 10 L of water and sprayed evenly onto the forest floor of each plot using an electric sprayer at the beginning of every month for a total of 12 equal applications over the entire year throughout the experiment period. These water-based additions amounted to an increase of 0.3 mm of rainfall per year. Each control plot received 10 L of N-free water.
Sample collection
In the local region, bamboo trunks are usually harvested after growing for 4 years. The present Moso bamboo stands only include two growth stages: young bamboo growing out in the spring of 2014 (one-year-old) and mature bamboo growing out in the spring of 2012 (three-year-old). Therefore, the two-year-old leaves (growing out during the spring of 2013 from mature bamboo emerging in 2012) and one-year-old leaves (growing out during the spring of 2014 from young bamboo emerging in 2014) all fell in the spring of 2015.
After two years of N addition, sampling was performed in January 2015 to examine the short-term responses of foliar stoichiometry to N deposition. The diameter at breast height (DBH) of each bamboo plant in each plot was measured, and the mean DBHs of young and mature bamboo were calculated in each plot. Five young and five mature bamboo plants with the DBH closest to the mean were selected from each plot, and the leaves in the middle and upper parts of the canopy were sampled and mixed according to the corresponding bamboo age. The leaf samples were collected and transported to the laboratory in insulated cases at 4 °C and then were oven-dried at 105 °C for 30 minutes and 65 °C to a constant weight. Oven-dried leaves were milled for analysis of N and P content.
At the same time, to better explain the change in foliar stoichiometry under different treatments, the top 20 cm portions of the soils were also collected randomly from five spots in each plot with a soil auger (3.5 cm diameter) and then mixed together. The soil samples were air-dried for a few months in the laboratory for chemical analysis (Fig. S2). Soil pH was measured using a pH meter (FE20, Mettler Toledo, Switzerland) after shaking the soil water (1:2.5 w/v) suspension for 30 min. The total N concentrations of both plant and soil samples were determined using a CN automatic analyzer (Sumigraph NC-80, Shimadzu, Japan). The P concentrations of both plant and soil samples were determined using a modified Kjeldahl method followed by colorimetric analysis59.
Data and statistical analysis
One-way ANOVA and least significant difference (LSD) multiple comparisons were used to determine the significance of differences between foliar N and P concentrations and mass-based N:P ratios among the four simulated N treatments in each plantation management type and growth stage, or between two plantation management types in each N treatment and growth stage, or among growth stages in each management type and N treatment. Similar statistical analyses were also used on soil N and P contents and N:P ratios among the four simulated N addition treatments in each plantation management type as well as between two plantation management types in each N treatment. The results showed that the assumptions of homogeneity of variance were met. A three-way ANOVA method was used to test the effect of the interaction of three factors (i.e., N addition, management practices and growth stage) on foliar stoichiometry. Pearson correlation analysis was performed to test the correlation of foliar N and P concentrations between young and mature bamboo, and of soil and leaf stoichiometry. All analyses were conducted using SPSS (Statistical Package for the Social Sciences) 16.0 for Windows (SPSS Inc., Chicago, Illinois).
Additional Information
How to cite this article: Song, X. et al. Management practices regulate the response of Moso bamboo foliar stoichiometry to nitrogen deposition. Sci. Rep. 6, 24107; doi: 10.1038/srep24107 (2016).
Supplementary Material
Acknowledgments
This study was funded by the “948” Project of the State Forestry Bureau of China (Grant No. 2013-4-55), the National Natural Science Foundation of China (Grant No. 31270517, 31470529), the Pandeng Project for Young & Middle-aged discipline leaders of Zhejiang Province (Grant No. pd2013234) and the Start-up Funds (Z109021502) of Northwest A&F University.
Footnotes
Author Contributions X.S. designed the study. X.S., H.G. and Q.L. performed the data collection and analysis. X.S., M.W. and G.Z. interpreted the results and wrote the paper.
References
- Sterner R. W. & Elser J. J. Ecological Stoichiometry: the biology of elements from molecules to the biosphere (Princeton University Press, 2002). [Google Scholar]
- Elser J. J., Fagan W. F., Kerkhoff A. J., Swenson N. G. & Enquist B. J. Biological stoichiometry of plant production metabolism, scaling and ecological response to global change. New Phytol. 186, 593–608 (2010). [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Cleveland C. C., Asner G. P. & Bustamante M. M. C. Controls over foliar N: P ratios in tropical rain forests. Ecology 88, 107–118 (2007). [DOI] [PubMed] [Google Scholar]
- Huang W. et al. Effects of elevated carbon dioxide and nitrogen addition on foliar stoichiometry of nitrogen and phosphorus of five tree species in subtropical model forest ecosystems. Environ. Pollut. 168, 113–120 (2012). [DOI] [PubMed] [Google Scholar]
- Yu Q. et al. Stoichiometric homeostasis predicts plant species dominance, temporal stability, and responses to global change. Ecology 96, 2328–2335 (2015). [DOI] [PubMed] [Google Scholar]
- Meunier C. L., Malzahn A. M. & Boersma M. A new approach to homeostatic regulation: towards a unified view of physiological and ecological concepts. Plos ONE 9, e107737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güsewell S. N. P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–66 (2004). [DOI] [PubMed] [Google Scholar]
- Hall S. R., Smith V. H., Lytle D. A. & Leibold M. A. Constraints on primary producer N:P stoichiometry along N:P supply ratio gradients. Ecology 86, 1894–1904 (2005). [Google Scholar]
- Sardans J., Rivas-Ubach A. & Peñuelas J. The C:N:P stoichiometry of organisms and ecosystems in a changing world, A review and perspectives. Perspect. Plant Ecol. 14, 33–47 (2012). [Google Scholar]
- Wu T., Wu M., Liu L. & Xiao J. Seasonal variations of leaf nitrogen and phosphorus stoichiometry of three herbaceous species in Hangzhou Bay coastal wetlands, China. Chin. J. Plant Ecol. 34, 23–28 (2010). [Google Scholar]
- Zeng D., Jiang L., Zeng C., Wang W. & Wang C. Reviews on the ecological stoichiometry characteristics and its applications. Acta Ecol. Sinica. 33, 5484–5492 (2013). [Google Scholar]
- Falkowski P. et al. The global carbon cycle: a test of our knowledge of Earth as a system. Science 290, 291–296 (2000). [DOI] [PubMed] [Google Scholar]
- Galloway J. N. et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008). [DOI] [PubMed] [Google Scholar]
- Knops J. M. H., Naeemw S. & Reich P. B. The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Glob. Chang. Biol. 13, 1960–1971 (2007). [Google Scholar]
- Liu Y., Zhang J., Chen Y., Chen L. & Liu Q. Effect of nitrogen and phosphorus fertilization on biomass allocation and C:N:P stoichiometric characteristics of Eucalyptus grandis seedlings. Chin. J. Plant Ecol. 37, 933–941 (2013). [Google Scholar]
- Hansen E. A., McLaughlin R. A. & Pope P. E. Biomass and nitrogen dynamics of hybrid poplar on two different soils: implications for fertilization strategy. Can. J. For. Res. 18, 223–230 (1988). [Google Scholar]
- Li R., Werger M. J. A., de Kroon H., During H. J. & Zhong Z. Interactions between shoot age structure, nutrient availability and physiological integration in the giant bamboo Phyllostachys pubescens. Plant boil. 2, 437–446 (2000). [Google Scholar]
- Song X. et al. Carbon Sequestration by Chinese Bamboo Forests, and Their Ecological Benefits: Assessment of Potential, Problems, and Future Challenges. Environ. Rev. 19, 418–428 (2011). [Google Scholar]
- Li H. & Lei Y. Estimation and evaluation of forest biomass carbon storage in China (China Forestry Press, 2010). [Google Scholar]
- Li R., Werger M. J. A., During H. J. & Zhong Z. Carbon and nutrient dynamics in relation to growth rhythm in the giant bamboo Phyllostachys pubescens. Plant Soil 201, 113–123 (1998). [Google Scholar]
- Zhou G., Jiang P. & Xu Q. Carbon fixing and transition in the ecosystem of bamboo stands (Science Press, 2010). [Google Scholar]
- Peng B. & Song J. High efficient cultivation of bamboo (Fujian Science & Technology Press, 2004). [Google Scholar]
- Li R., Werger M. J. A., During H. J. & Zhong Z. Biennial variation in production of new shoots in groves of the giant bamboo Phyllostachys pubescens in Sichuan, China. Plant Ecol. 135, 103–112 (1998). [Google Scholar]
- Chen X. Cultivation and utilization of Mosobamboo (China Forestry Press, 1996).
- Liu J. et al. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manage. 262, 1131–1137 (2011). [Google Scholar]
- Xu Q., Jiang P. & Xu Z. Soil microbial functional diversity under intensively managed bamboo plantations in southern China. J. Soils Sediments 8, 177–183 (2008). [Google Scholar]
- Zhou G., Jiang P. & Mo L. Bamboo: a possible approach to the control of global warming. Int J Nonlinear Sci. Numer. Simul. 10, 547–550 (2009). [Google Scholar]
- Zhou G., Xu J. & Jiang P. Effect of management practices on seasonal dynamics of organic carbon in soils under bamboo plantations. Pedosphere 16, 525–531 (2006). [Google Scholar]
- Jiang P., Wang H., Wu J., Xu Q. & Zhou G. Winter mulch increases soil CO2 efflux under Phyllostachys praecox stands. J. Soils Sediments 9, 511–514 (2009). [Google Scholar]
- Song X., Zhou G., Gu H. & Qi L. Management practices amplify the effects of N deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 395, 391–400 (2015). [Google Scholar]
- Wu J., Zhou G., Qian X., Yang F. & Wu X. Distribution of nutrient elements in different organs of Phyllostachys pubescens under different managements. J. Zhejiang For. Coll. 22, 486–489 (2005). [Google Scholar]
- Gu H. et al. Ecological stoichiometry of Phyllostachys pubescens leaves with extensive and intensive management. J. Zhejiang A&F Univ. 32, 661–667 (2015). [Google Scholar]
- Liu X. et al. Enhanced nitrogen deposition over China. Nature 494, 459–462 (2013). [DOI] [PubMed] [Google Scholar]
- Lü C. & Tian H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res. 112, 10–15 (2007). [Google Scholar]
- Reay D. S., Dentener F., Smith P., Grace J. & Feely R. A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 1, 430–437 (2008). [Google Scholar]
- Yang L., Wang G., Yang Y. & Yang Y. Responses of leaf functional traits and nitrogen and phosphorus stoichiometry in Abies fabiri seedlings in Gongga Mountain to simulated nitrogen deposition. Acta Ecol. Sinica. 31, 44–50 (2012). [Google Scholar]
- Li M. et al. Photosynthetic characteristics, biomass allocation, C, N and P distribution of Schima superba seedlings in response to simulated nitrogen deposition. Acta Ecol. Sinica. 33, 1569–1572 (2013). [Google Scholar]
- Matson P. A., McDowell W. H., Townsend A. R. & Vitousek P. M. The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46, 67–83 (1999). [Google Scholar]
- Han W., Fang J., Guo D. & Zhang Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 168, 377–385 (2005). [DOI] [PubMed] [Google Scholar]
- Elser J. J. et al. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550 (2000). [Google Scholar]
- Elser J. J. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 (2000). [DOI] [PubMed] [Google Scholar]
- Reich P. B. & Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 101, 11001–11006 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzek V. & Vitousek P. M. N:P stoichiometry and protein: RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis. Ecol. Lett. 12, 765–771 (2009). [DOI] [PubMed] [Google Scholar]
- Aber J. D., Nadelhoffer K. J., Steudler P. & Melillo J. M. Nitrogen Saturation in Northern Forest Ecosystems. BioScience 39, 378–386 (1989). [Google Scholar]
- Xie Y., Zhang S., Zhao X., Xiong Z. & Xing G. Seasonal variation patterns of NH4+-N/NO3−-N ratio and δ15 NH4+ value in rainwater in Yangtze river delta. Chin. J. Appl. Ecol. 19, 2035–2041 (2008). [PubMed] [Google Scholar]
- Aber J. et al. Nitrogen saturation in temperate forest ecosystems. BioScience 48, 921–934 (1998). [Google Scholar]
- Phoenix G. K. et al. Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol. 161, 279–289 (2004). [Google Scholar]
- Fujita Y., Robroek B. J. M., de Ruiter P. C., Heil G. W. & Wassen M. J. Increased N affects P uptake of eight grassland species: the role of root surface phosphatase activity. Oikos 119, 1665–1673 (2010). [Google Scholar]
- Matson P. A., McDowell W. H., Townsend A. R. & Vitousek P. M. The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46, 67–83 (1999). [Google Scholar]
- Xu R. et al. Amelioration principles and technologies for acidified red soils (Science Press, 2013). [Google Scholar]
- Paré D. & Bernier B. Phosphorus-fixing potential of Ah and H horizons subjected to acidification. Can. J. For. Res. 19, 132–134 (1989). [Google Scholar]
- Macklon A. E. S. & Sin A. Modifying effects of non-toxic levels of aluminum on the uptake and transports of phosphate in ryegrass. J. Exp. Bot. 43, 915–923 (1992). [Google Scholar]
- Lang G. E., Reiners W. A. & Shellito. G. A. Tissue chemistry of Abies balsamea and Betula papyrifera var. cordifolia from subalpine forests of northeastern United States. Can. J. For. Res. 12, 311–318 (1982). [Google Scholar]
- Wessman C. A., Aber J. D., Peterson D. L. & Melillo. J. M. Remote sensing of canopy chemistry and nitrogen cycling in temperate forest ecosystems. Nature 335, 154–156 (1988). [Google Scholar]
- Stevens C. J. et al. The impact of nitrogen deposition on acid grasslands in the Atlantic region of Europe. Environ. Pollut. 159, 2243–2250 (2011). [DOI] [PubMed] [Google Scholar]
- Maurer S., Egli P., Spinnler D. & Körner C. Carbon and water fluxes in Beech Spruce model ecosystems in response to long-term exposure to atmospheric CO2 enrichment and increased nitrogen deposition. Funct. Ecol. 13, 748–755 (1999). [Google Scholar]
- Mo J., Brown S., Xue J., Fang Y. & Li Z. Response of litter decomposition to simulated N deposition in disturbed rehabilitated and mature forests in subtropical China. Plant Soil 282, 135–151 (2006). [Google Scholar]
- Fang H., Mo J., Peng S., Li Z. & Wang H. Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern china. Plant Soil 297, 233–242 (2007). [Google Scholar]
- Song X. et al. Interactive effects of elevated UV-B radiation and N deposition on Moso bamboo litter decomposition. Soil Biol. Biochem. 69, 11–16 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.