Abstract
The KCL035 human embryonic stem cell line was derived from an embryo donated for research that carried a mutation in the HBB gene, which is linked to the β-thalassemia syndrome. The ICM was isolated using laser microsurgery and plated on γ-irradiated human foreskin fibroblasts. Both the derivation and cell line propagation were performed in an animal product-free environment. Pluripotent state and differentiation potential were confirmed by in vitro assays.
Resource table
| Name of stem cell line | KCL035 |
| Institution | King's College London, London UK |
| Derivation team | Neli Kadeva, Victoria Wood, Glenda Cornwell, Stefano Codognotto, Emma Stephenson |
| Contact person and email | Dusko Ilic, email: dusko.ilic@kcl.ac.uk |
| Date archived/stock date | Oct 28, 2011 |
| Type of resource | Biological reagent: cell line |
| Sub-type | Human pluripotent stem cell line |
| Origin | Human embryo |
| Key marker expression | Pluripotent stem cell markers: NANOG, OCT4, TRA-1-60, TRA-1-81, alkaline phosphatase (AP) activity |
| Authentication | Identity and purity of line confirmed |
| Link to related literature (direct URL links and full references) |
http://www.ncbi.nlm.nih.gov/pubmed/22029654
http://www.ncbi.nlm.nih.gov/pubmed/22722371 |
| Information in public databases | KCL035 is a National Institutes of Health (NIH) registered hESC line NIH Registration Number: 0227 NIH Approval Number: NIHhESC-13-0227 http://grants.nih.gov/stem_cells/registry/current.htm?id=667 |
| Ethics | The hESC line KCL035 is derived under license from the UK Human Fertilisation and Embryology Authority (research licence numbers: R0075 and R0133) and also has local ethical approval (UK National Health Service Research Ethics Committee Reference: 06/Q0702/90). Informed consent was obtained from all subjects and the experiments conformed to the principles set out in the WMA Declaration of Helsinki and the NIH Belmont Report. No financial inducements are offered for donation. |
1. Resource details
| Consent signed | Feb 17, 2011 |
| Embryo thawed | Oct 12, 2011 |
| UK Stem Cell Bank Deposit Approval | Sep 13, 2012 Reference: SCSC12-38 |
| Sex | Female 46, XX |
| Grade | Research |
| Disease status (Fig. 1) |
The mutation in the HBB gene. |
| Karyotype (aCGH) | Imbalance relative to the control DNA was found at 8p23.1 (7,256,229–7,729,370). Imbalance in this region has been found in normal individuals and is established as benign copy number variation. |
| DNA fingerprint (Table 1) |
Allele sizes (in bp) of 17 microsatellite markers specific for chromosomes 13, 18 and 21 |
| Viability testing | Pass |
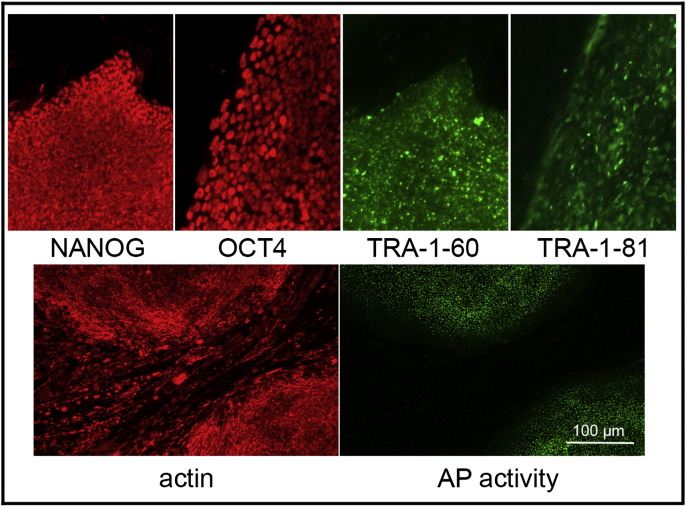
| Pluripotent markers (immunostaining) (Fig. 2) |
NANOG, OCT4, TRA-1-60, TRA-1-81, AP activity |
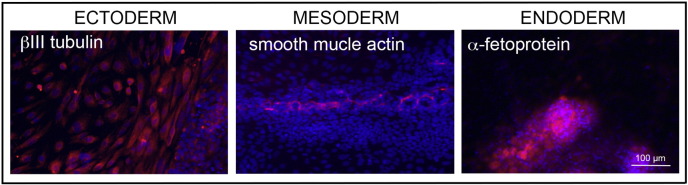
| Three germ layers differentiation in vitro (immunostaining) (Fig. 3) |
Endoderm: AFP (α-fetoprotein) Ectoderm: TUBB3 (tubulin, β3 class III) Mesoderm: ACTA2 (actin, α2, smooth muscle) |
| Sibling lines available | KCL030 |
We generated KCL035 clinical grade hESC line following protocols, established previously (Ilic et al., 2012, Stephenson et al., 2012). The expression of the pluripotency markers was tested after a freeze/thaw cycle (Fig. 2). Differentiation potential into three germ layers was verified in vitro (Fig. 3).
Fig. 2.
Expression of pluripotency markers. Pluripotency is confirmed by immunostaining (Oct4, Nanog, TRA-1-60, TRA-1-81) and alkaline phosphatase (AP) activity assay. Actin stress fibers, visualized with rhodamine-phalloidin (red), are present in both feeders and hES cell colonies, whereas AP activity (green) is detected only in hES cells. Scale bar, 100 μm.
Fig. 3.
Differentiation of three germ layers in vitro is confirmed by detection of markers: smooth muscle actin (red) for mesoderm, β-III tubulin (red) for ectoderm and α-fetoprotein (red) for endoderm. Nuclei are visualized with Hoechst 33342 (blue). Scale bar, 100 μm.
2. Materials and methods
2.1. Consenting process
We distribute Patient Information Sheet (PIS) and consent form to the in vitro fertilization (IVF) patients if they opted to donate to research embryos that were stored for 5 or 10 years. They mail signed consent back to us and that might be months after the PIS and consent were mailed to them. If in the meantime new versions of PIS/consent are implemented, we do not send these to the patients or ask them to re-sign; the whole process is done with the version that was given to them initially. The PIS/consent documents (PGD-V.8) were created on Jul. 01, 2010. HFEA Code of Practice that was in effect at the time of document creation: Edition 8 — R.2 (http://www.hfea.gov.uk/2999.html). The donor couple signed the consent on Feb. 17, 2011. HFEA Code of Practice that was in effect at the time of donor signature: Edition 8 — R.2. HFEA Code of Practice Edition 8 — R.2 was in effect Apr. 07, 2010–Apr. 06, 2011.
2.2. Embryo culture and micromanipulation
Embryo culture and laser-assisted dissection of inner cell mass (ICM) were carried out as previously described in details (Ilic et al., 2012, Stephenson et al., 2012). The cellular area containing the ICM was then washed and transferred to plates containing mitotically inactivated human neonatal foreskin fibroblasts (HFF).
2.3. Cell culture
ICM plated on mitotically inactivated HFF was cultured as described (Ilic et al., 2012, Stephenson et al., 2012). TE cells were removed mechanically from outgrowth (Ilic et al., 2007, Ilic et al., 2010). hESC colonies were expanded and cryopreserved at the third passage.
2.4. Viability test
Straws with the earliest frozen passage (p.2–3) are thawed and new colonies are counted three days later. These colonies are then expanded up to passage 8, at which point cells were part frozen and part subjected to standard battery of tests (pluripotency markers, in vitro and in vivo differentiation capability, genetics, sterility, mycoplasma).
2.5. Pluripotency markers
Pluripotency was assessed using two different techniques: enzymatic activity assay [alkaline phosphatase (AP) assay] and immunostaining as described (Ilic et al., 2012, Stephenson et al., 2012).
2.6. Differentiation
Spontaneous differentiation into three germ layers was assessed in vitro as described (Ilic et al., 2012, Stephenson et al., 2012).
2.7. Genotyping
DNA was extracted from hESC cultures using a Chemagen DNA extraction robot according to the manufacturer's instructions. Amplification of polymorphic microsatellite markers was carried out as described (Ilic et al., 2012). Allele sizes were recorded to give a unique fingerprint of each cell line.
2.8. Array comparative genomic hybridization (aCGH)
aCGH was performed as described in details (Ilic et al., 2012).
Author disclosure statement
There are no competing financial interests in this study.
Fig. 1.
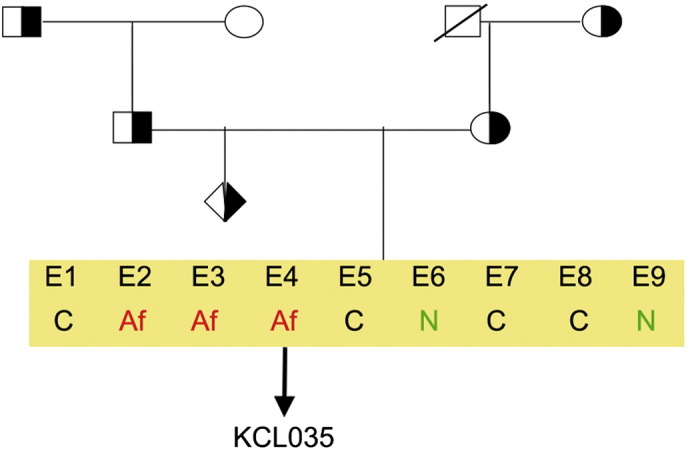
Genetic pedigree tree. Both parents were carrying mutation in HBB gene. The affected embryos were donated for research. Af, affected; C, carrier; N, normal.
Table 1.
Genotyping. Microsatellite markers specific for chromosomes 13, 18, 21, X and Y were amplified. The allele sizes in bp for markers on chromosomes 13, 18, and 21 are listed in the table.
| Chr | Marker | Allele 1 | Allele 2 |
|---|---|---|---|
| 13 | D13S252 | 303 | 303 |
| D13S325 | 285 | 293 | |
| D13S628 | 441 | 446 | |
| D13S634 | 397 | 401 | |
| 18 | D18S386 | 356 | 356 |
| D18S390 | 372 | 372 | |
| D18S391 | 209 | 221 | |
| D18S535 | 482 | 482 | |
| D18S819 | 412 | 416 | |
| D18S976 | 472 | 483 | |
| D18S978 | 215 | 219 | |
| 21 | D21S11 | 240 | 249 |
| D21S1409 | 212 | 224 | |
| D21S1411 | 304 | 312 | |
| D21S1435 | 184 | 188 | |
| D21S1437 | 319 | 321 |
Acknowledgments
This work was supported by the UK Medical Research Council grants G0701172 and G0801061. We thank Dr. Yacoub Khalaf, Director of the Assisted Conception Unit of Guy's and St Thomas' NHS Foundation Trust and his staff for supporting the research program. We are especially indebted to Prof Peter Braude and to the patients who donated embryos.
References
- Ilic D., Genbacev O., Krtolica A. Derivation of hESC from intact blastocysts. Curr. Protoc. Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01a02s1. (Chapter 1: Unit 1 A.2) [DOI] [PubMed] [Google Scholar]
- Ilic D., Caceres E., Lu S., Julian P., Foulk R., Krtolica A. Effect of karyotype on successful human embryonic stem cell derivation. Stem Cells Dev. 2010;19(1):39–46. doi: 10.1089/scd.2009.0136. [DOI] [PubMed] [Google Scholar]
- Ilic D., Stephenson E., Wood V., Jacquet L., Stevenson D., Petrova A., Kadeva N., Codognotto S., Patel H., Semple M., Cornwell G., Ogilvie C., Braude P. Derivation and feeder-free propagation of human embryonic stem cells under xeno-free conditions. Cytotherapy. 2012;14(1):122–128. doi: 10.3109/14653249.2011.623692. [DOI] [PubMed] [Google Scholar]
- Stephenson E., Jacquet L., Miere C., Wood V., Kadeva N., Cornwell G., Codognotto S., Dajani Y., Braude P., Ilic D. Derivation and propagation of human embryonic stem cell lines from frozen embryos in an animal product-free environment. Nat. Protoc. 2012;7(7):1366–1381. doi: 10.1038/nprot.2012.080. [DOI] [PubMed] [Google Scholar]