Abstract
In this study, we evaluated the clinical performance of anti-β2-glycoprotein 1 domain 1 antibodies (aβ2GP1-D1) in the diagnosis of antiphospholipid syndrome (APS). Sera from 229 subjects were tested, including 35 patients with primary APS, 51 patients with APS associated to other diseases, 30 patients with non-APS thrombosis, 32 patients with non-APS pregnancy-related morbidity, 42 patients with systemic lupus erythematosus, and 39 healthy controls (HC). Serum IgG aβ2GP1-D1, IgG/IgM anti-cardiolipin (aCL) and IgG/IgM aβ2GP1 were measured by a chemiluminescence assay. The levels of IgG aβ2GP1-D1 were significantly increased in patients with APS, compared with disease controls and HCs (p < 0.001). Significant correlation was identified between IgG aβ2GP1-D1 and IgG aβ2GP1 (p < 0.0001), indicating IgG aβ2GP1-D1 were the predominant domain-specific antibodies in IgG aβ2GP1 family. Importantly, aβ2GP1-D1, but not aβ2GP1 non-D1, was significantly correlated with thrombotic events. Interestingly, no significant correlation between IgG aβ2GP1-D1 and obstetric complications was observed. Additionally, significantly higher levels of IgG aβ2GP1-D1 were found in patients with triple aPL positivity, compared with patients with double and single aPL positivity. Our findings suggest a potential role of IgG aβ2GP1-D1 in identifying APS patients with high risk of thrombosis, shedding insight on the introduction of IgG aβ2GP1-D1 in China.
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by recurrent thrombosis in arteries and veins and/or pregnancy morbidity. A hallmark feature of APS is the presence of the antiphospholipid antibodies (aPLs). aPLs represent a heterogeneous population of autoantibodies that target phospholipids, phospholipid-binding plasma proteins, and/or plasma protein-phospholipid complexes. Among those aPLs, anti-β2-glycoprotein 1 (aβ2GP1) antibodies have been increasingly recognized as the most clinically relevant autoantibodies in APS1. The presence of aβ2GP1, along with the presence of lupus anticoagulant (LA) and anticardiolipin antibodies (aCL), has been included in the standard diagnostic criteria for patients with clinical suspicion of APS2.
aβ2GP1 antibodies are a heterogeneous population. β2GP1 is composed of 5 domains. Domain 5 contains a phospholipid-binding site, which allows this domain to interact with the anionic phospholipids on the plasma membrane. This interaction leads to exposure of domain 1 (D1) into the extracellular space, making it possible to generate domain-specific antibodies for each of the 5 domains3. Indeed, the D1 epitope becomes available for antibody binding only when β2GP1 transitions from a circular form to a fish-hook conformation4.
Iverson et al. characterized the autoantibodies to each of the 5 β2GP1 domains and found that most aβ2GP1 antibodies reacted with epitope(s) in D1, indicating D1 as the main immunogenic epitope targeted by aβ2GP1 antibodies from patients with APS5,6,7. De Laat et al. further demonstrated that the Gly40-Arg43 region in β2GP1-D1 was the critical epitope, and antibodies against this region were able to interfere with the coagulation process and were strongly correlated with thrombosis8. Additionally, Ioannou et al. confirmed D1 as the immunodominant epitope of β2GP1 in animal models of aPL-induced thrombosis, as treatment of recombinant D1 peptide protected C57BL/6 mice from human aPL-induced pathology9. Subsequent work from several groups demonstrated that antibodies to β2GP1-D1 were associated with increased risk of thrombotic and obstetric manifestations in patients with APS6,7,8,9,10,11, suggesting that aβ2GP1-D1 antibodies could represent a pathogenic subpopulation of aβ2GPI antibodies.
Although aβ2GP1-D1 antibodies have attracted particular interest for its prognostic potential for thrombosis and pregnancy complications, the number of studies is still limited, and its clinical value needs to be verified in patients with different ethnic/geographic background. To our knowledge, few, if any, studies have reported the role of aβ2GP1-D1 antibodies in Chinese patients with APS. It is of paramount importance to evaluate this, as this information will enhance our understanding of the clinical utility of the aβ2GP1-D1 antibodies.
Currently, the detection of aβ2GP1-D1 antibodies is mainly based on ELISA assays with different detection strategies6. A novel chemiluminescence immunoassay (CIA) based assay for detecting aβ2GP1-D1 antibodies has recently developed. This novel CIA assay showed good agreements with ELISA11,12,13. We and others have showed that CIA assays have good performance in detecting aCL and aβ2GP1 autoantibodies13,14. More importantly, CIA has been considered as a promising tool to improve the reproducibility and reduce inter-laboratory variations. In this study, we utilized the CIA assay to evaluate the role of aβ2GP1-D1 antibodies in the diagnosis of APS, with a particular interest in their prognostic value for thrombosis and pregnancy complications.
Results
Levels of IgG aβ2GP1-D1 Antibodies were Elevated in Patients with APS
The values expressed as chemiluminescent units (CU) of IgG aβ2GP1-D1 from all subjects is presented in Fig. 1. The levels of IgG aβ2GP1-D1 were significantly increased in patients with APS, compared with patients with non-APS thrombosis, non-APS PRM, and SLE (p < 0.001), as well as healthy controls (p < 0.001). No significant difference in the levels of IgG aβ2GP1-D1 antibodies was observed between patients with PAPS and APSAOD. When the manufacturer’s recommended cut off of 20 CU was applied, the presence of IgG aβ2GP1-D1 antibodies in patients with PAPS, APSAOD, non-APS thrombosis, non-APS PRM, and SLE were 48.6%, 45.1%, 0, 0, and 7.1%, respectively (Table 1).
Figure 1. Levels of IgG aβ2GP1 D1 antibodies in Patients with APS and controls.
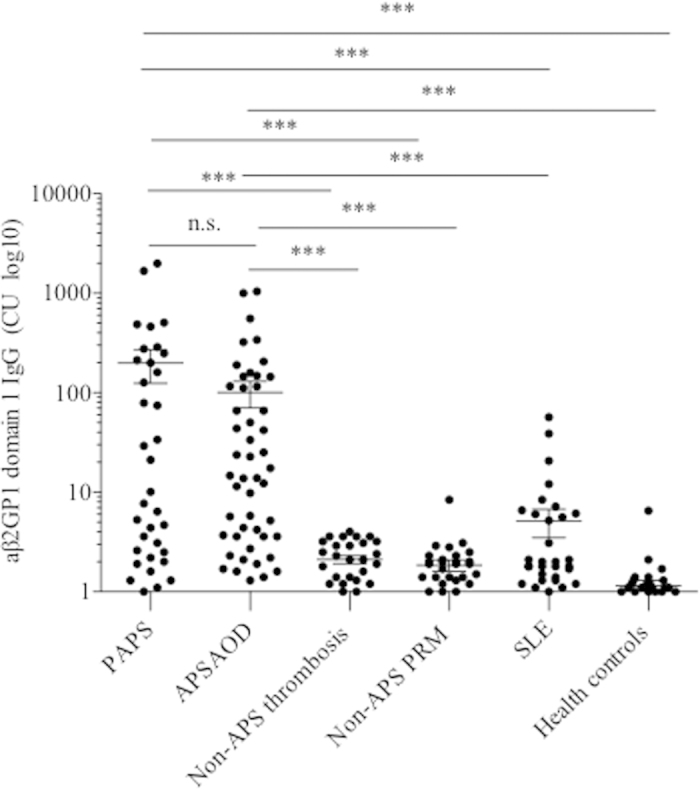
The values expressed as CU of IgG aβ2GP1 D1 from PAPS, APSOD, Non-APS thrombosis, Non-APS RPM, SLE, and healthy controls. CU, chemiluminescent units; PAPS, primary APS; APSAOD, APS associated to other diseases; non-APS RPM, non-APS pregnancy-related morbidity, SLE, systemic lupus erythematosus. ***p < 0.001.
Table 1. Demographic, clinical characteristics and aCLs profiles of patients with APS and controls.
| PrimaryAPS (n = 35) | APS associated toother diseases (n = 51) | Non-APSthrombosis (n = 30) | Non-APSPRM (n = 32) | SLE controls(n = 42) | Health controls(n = 39) | |
|---|---|---|---|---|---|---|
| Sex (female/male) | 25/10 | 42/9 | 10/20 | 32/0 | 39/3 | 14/25 |
| Median age at study (max, min) | 34 (9, 76) | 33 (5, 86) | 53.5 (14, 85) | 35 (24, 41) | 30 (12, 68) | 39 (25, 65) |
| Median duration/years (max, min) | 1.5 (1, 6) | 3 (1, 8) | 3 (1, 11) | 1 (1, 7) | 4 (1, 21) | N/A |
| SLEDAI | ||||||
| 0–4 (%) | N/A | 7 (15.9)**** | N/A | N/A | 6 (15.0) | N/A |
| 5–9 (%) | N/A | 7 (15.9)**** | N/A | N/A | 11 (26.0) | N/A |
| 10–14 (%) | N/A | 22 (50.0)**** | N/A | N/A | 12 (29.0) | N/A |
| ≥15 (%) | N/A | 8 (18.2)**** | N/A | N/A | 13 (31.0) | N/A |
| Arterial thrombosis, n (%) | 9 (25.7) | 19 (37.3) | 5 (16.7) | 0 (0.0) | 1 (2.3) | 0 (0.0) |
| Venous thrombosis, n (%) | 14 (40.0) | 26 (51.0) | 26 (86.7) | 1 (3.0) | 0 (0.0) | 0 (0.0) |
| Obstetric complications, n (%)* | 10/19 (52.6) | 18/35 (51.4) | 0/10 (0.0) | 32/32 (100.0) | 0/31 (0.0) | 0/14 (0.0) |
| aCL, n (%)** | 22 (62.9) | 28 (54.9) | 0 (0.0) | 1 (3.1) | 3 (7.1) | 0 (0.0) |
| aβ2GP1, n (%)** | 19 (54.3) | 38 (74.5) | 0 (0.0) | 2 (6.3) | 8 (19.0) | 1 (2.6) |
| LAC, n (%) | 25 (71.4) | 40 (78.4) | 2 (6.7) | 1 (3.1) | 5 (11.9) | 0 (0.0) |
| aβ2GP1 domain-1 IgG, n (%) | 17 (48.6) | 23 (45.1) | 0 (0.0) | 0 (0.0) | 3 (7.1) | 0 (0.0) |
| Non aβ2GP1 domain-1 IgG, n (%)*** | 2 (5.7) | 12 (23.5) | 0 (0.0) | 1 (3.1) | 5 (11.9) | 0 (0.0) |
*Percentage among married women of reproductive age. **IgG and/or IgM positive. ***aβ2GP1 IgG positive while aβ2GP1 domain-1 IgG negative. ****Percentage among patients with APSAOD with SLE. APS, antiphospholipid syndrome; RPM,pregnancy-related morbidity; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; aCL, anticardiolipin antibodies; aβ2GP1, anti-β2-glycoprotein I antibodies; LAC, lupus anticoagulant, N/A, not available.
Correlation between the Levels of IgG aβ2GP1-D1 Antibodies and the Levels of IgG aβ2GP1, IgG aCL Antibodies and LAC
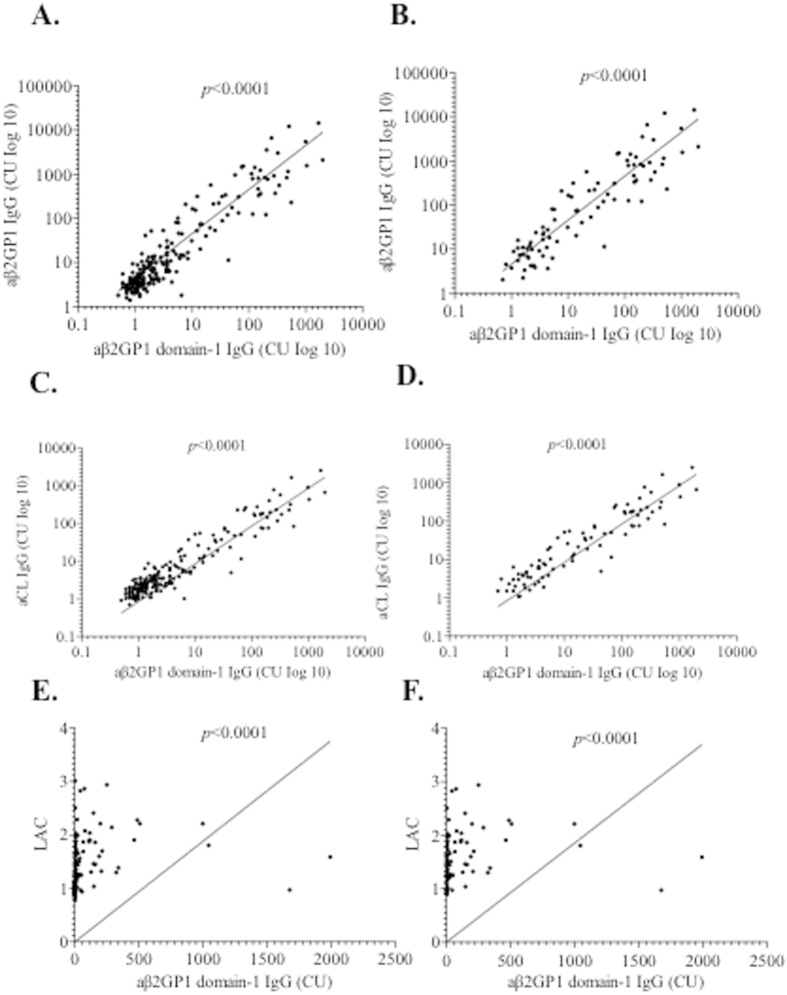
As shown in Fig. 2, the levels of IgG aβ2GP1-D1 antibodies were significantly correlated with the levels of IgG aβ2GP1 antibodies in all subjects (p < 0.0001) (Fig. 2A) and in patients with APS (p < 0.0001) (Fig. 2B). Interestingly, 16 out of 86 APS patients (16/86, 18.6%) were positive for IgG aβ2GP1 and negative for IgG aβ2GP1-D1 (Fig. 2B). One out of 86 APS patients was positive for IgG aβ2GP1-D1 and negative for IgG aβ2GP1 (Fig. 2B). Notably, among the 16 APS patients that were positive for IgG aβ2GP1 and negative for IgG aβ2GP1-D1, the majority of the patients (13/16, 81.3%) only showed low levels of IgG aβ2GP1 (<100 CU) (Fig. 2B). In addition, significant correlations were also observed between the levels of IgG aβ2GP1-D1 antibodies and the levels of IgG aCL antibodies (Fig. 2C,D), and between levels of IgG aβ2GP1-D1 antibodies and LAC (Fig. 2E,F).
Figure 2.
Correlation between levels of IgG aβ2GP1 D1 antibodies and the levels of IgG aβ2GP1 antibodies (A,B), between levels of IgG aβ2GP1 D1 antibodies and the levels of IgG aCL antibodies (C,D), and between levels of IgG aβ2GP1 D1 antibodies and the levels of LAC (E,F) in all subjects (A,C,E) and in patients with APS (B,D,F). The values expressed as CU of IgG aβ2GP1 and IgG aβ2GP1 D1. CU, chemiluminescent units; aCL, anticardiolipin antibodies; aβ2GP1, anti-β2-glycoprotein I antibodies; LAC, lupus anticoagulant.
Association between aβ2GP1-D1 Antibodies and Clinical Symptoms
It has been shown that aβ2GP1-D1 antibodies are associated with thromboembolic events, and to a lesser extent, with pregnancy morbidity6,7,8,9,10,11. Thus, the odds ratios (OR) were calculated to evaluate the association of aβ2GP1-D1 antibodies with those clinical manifestations in Chinese patients with APS. A significant association between aβ2GP1-D1 antibodies and thrombotic events was identified (OR, 3.27; 95% CI, 1.59–6.71) (Table 2). In addition, higher levels of aβ2GP1-D1 antibodies (>40 CU and >100 CU) were associated with higher OR values (4.48 and 3.67, respectively) (Table 2). In contrast, no significant associations were found between IgG aβ2GP1 non-D1 antibodies and thrombotic events (Table 2). Significant correlations were also observed between IgG aβ2GP1 and thrombotic events (OR, 2.75; 95% CI, 1.48–5.10), IgG aCL and thrombotic events (OR, 3.62; 95% CI, 1.77–7.41), and LAC and thrombotic events (OR, 3.28; 95% CI, 1.78–6.03) (Table 2). Interestingly, no significant correlation between IgG aβ2GP1-D1 antibodies and obstetric complications was observed (Table 2).
Table 2. Correlations between aPLs and thrombosis or obstetrical complications in all patients.
| aPLs | Odds ratio (95% confidence interval) |
|
|---|---|---|
| Thrombosis | Obstetrical complications | |
| Anti-Domain Ι aβ2GP1 IgG (>20 CU) | 3.27 (1.59–6.71) | 1.55 (0.64–3.74) |
| Anti-Domain Ι aβ2GP1 IgG (>40 CU) | 4.48 (1.80–11.14) | 1.79 (0.60–5.38) |
| Anti-Domain Ι aβ2GP1 IgG (>100 CU) | 3.67 (1.12–11.97) | 1.52 (0.33–7.10) |
| Non-Domain Ι aβ2GP1 IgG | 1.37 (0.57–3.28) | 0.75 (0.27–2.12) |
| aβ2GP1 IgG | 2.75 (1.48–5.10) | 1.27 (0.60–2.67) |
| aβ2GP1 IgM | 1.88 (0.51–6.87) | 1.12 (0.22–5.79) |
| aCL IgG | 3.62 (1.77–7.41) | 1.96 (0.78–4.93) |
| aCL IgM | 1.62 (0.58–4.55) | 0.45 (0.11–1.83) |
| LAC | 3.28 (1.78–6.03) | 1.18 (0.57–2.46) |
Levels of IgG aβ2GP1-D1 antibodies in APS patients with different aPL profiles
The levels of IgG aβ2GP1-D1 antibodies were also evaluated in APS patients with the triple aPL positivity, double aPL positivity, and single aPL positivity, as triple aPL positivity has been considered as a risk factor for aPL-mediated clinical manifestations6. Importantly, significantly higher levels of IgG aβ2GP1-D1 antibodies were found in patients with triple aPL positivity, compared with patients with double and single aPL positivity (Fig. 3). Additionally, patients with double aPL positivity exhibited significantly higher levels of IgG aβ2GP1-D1 antibodies, compared with patients with single aPL positivity (Fig. 3).
Figure 3. Levels of IgG aβ2GP1 D1 antibodies in triple (LAC+, IgG aCL+, IgG aβ2GP1+), double (LAC+/aCL+, LAC+/aβ2GP1+ or aCL+/aβ2GP1+), and single aPLs positive (positive for any of the aPLs) groups in patients with APS.
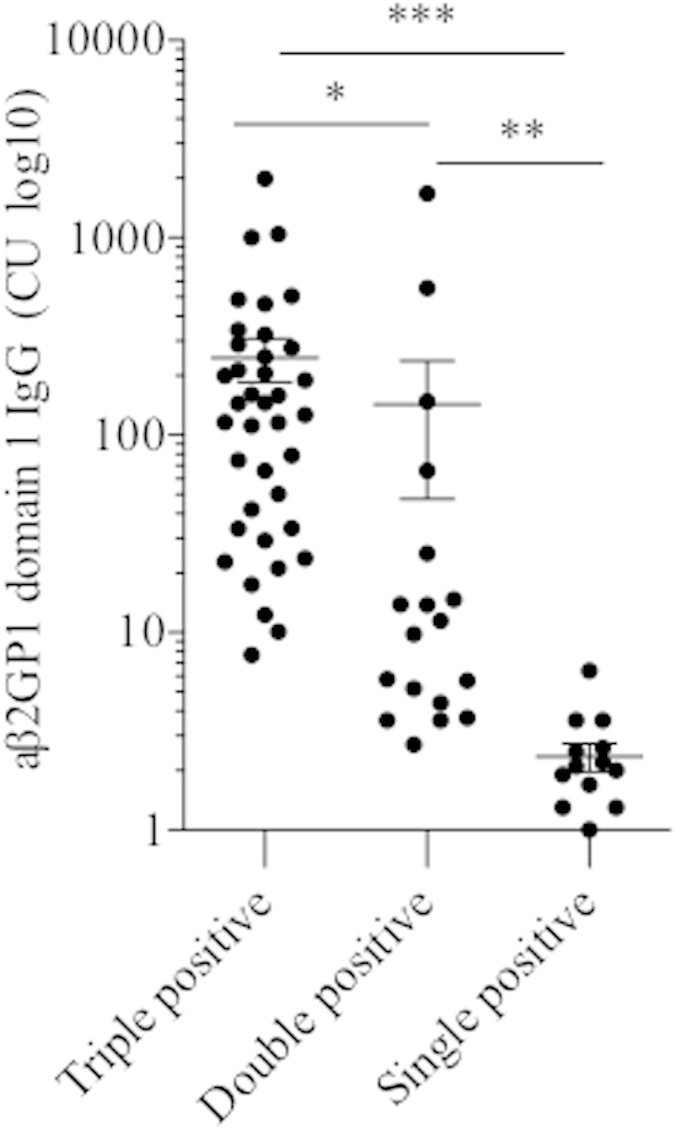
The values expressed as CU of IgG aβ2GP1 D1 antibodies. CU, chemiluminescent units; aCL, anticardiolipin antibodies; aβ2GP1, anti-β2-glycoprotein I antibodies; LAC, lupus anticoagulant. *p < 0.05, **p < 0.01,***p < 0.001.
Discussion
Previous studies have shown that abs specific to β2GP1-D1 are associated with thrombosis and pregnancy morbidity6,7,8,9,10,11. Two recent studies provide a proof of concept on the pathogenic role of aβ2GP1-D1 antibodies15,16. Agostinis et al. showed that a single-chain fragment variable (scFv) directed against β2GP1-D1 induced thrombosis and fetal loss in naïve rats/mice15. The other study demonstrated that aβ2GP1-D1-rich polyclonal IgG fractions from serum of patient with APS induced significantly larger thrombi in vivo compared with aDI-poor counterpart16. The pathogenic potential of aβ2GP1-D1 antibodies may come from their ability to trigger TLR4-NF-κB pathway, as β2GP1-D1 shares a high degree of homology with an extracellular epitope of human TLR417. Given the significance of aβ2GP1-D1 antibodies, it is of paramount importance to characterize the clinical relevance of aβ2GP1-D1 antibodies in Chinese patients with APS.
In this study, we found that the levels of IgG aβ2GP1-D1 antibodies were significantly elevated in patients with APS. In addition, IgG aβ2GP1-D1 antibodies were the predominant domain-specific antibodies in IgG aβ2GP1 family. More importantly, aβ2GP1-D1 antibodies, but not aβ2GP1 non-D1 antibodies, were significantly correlated with thrombotic events. In contrast, no significant correlation between IgG aβ2GP1-D1 antibodies and obstetric complications was observed. Our findings suggest that aβ2GP1-D1 antibodies could serve as a promising biomarker to identify patients at risk of thrombosis in China.
We used the CIA assay in the entire study, rendering the results more reliable. Previously, we showed that the CIA assay had good performance characteristics and good agreements with a commercial ELISA from the same manufacturer14. As a variety of different assays have been used in detecting aβ2GP1-D1 antibodies (e.g., competitive inhibition ELISA with different D1 antigen, direct ELISA with different D1 antigen), the comparability of results across different studies might result in substantial variations18.
In the present study, IgG aβ2GP1-D1 antibodies were detected in 48.6% of patients with PAPS and 45.1% of patients with APSAOD. Mondejar et al. from Spain reported that IgG aβ2GP1-D1 antibodies were present in 31% of patients with PAPS and 46% of patients with APSAOD using the CIA assay13. The prevalence of IgG aβ2GP1-D1 antibodies in APSAOD patients was similar between the two studies, but the prevalence of IgG aβ2GP1-D1 antibodies in PAPS patients was higher in our study. Interestingly, a recent meta-analysis on 548 patients with APS from 11 different centers showed that the prevalence of IgG aβ2GP1-D1 antibodies was 44.0% (241/548)19, similar to what we found in this study.
It is worth mentioning that 13 patients with APS (7 patients with PAPS and 6 patients with APSAOD) were LAC positive but aβ2GP1 negative. This discrepancy may be due to the existence of other antibodies20. Indeed, when we tested those patients for anti-prothrombin/phosphatidylserine (aPS/PT) antibodies (QUANTA Lite® aPS/PT, INOVA Diagnostic), 2 patients out of 7 (28.6%) with PAPS and 3 patients out of 6 (50.0%) with APSAOD exhibited positive for IgM aPS/PT antibodies (Zhang et al. unpublished data).
It has been suggested that the majority of the IgG aβ2GP1 antibodies bind to epitopes located in β2GP1-D21,22. In our study, we found IgG aβ2GP1-D1 antibodies were present in 81.4% of APS patients with positive aβ2GP1 antibodies, supporting D1 as the major epitope in β2GP1. Interestingly, in a multicenter study on patients with APS from Europe and the United States, IgG aβ2GP1-D1 antibodies were detected in 55% of patients with positive aβ2GP1 antibodies10, which is lower than that in our study. It is likely that the discrepancies are caused by different assays on IgG aβ2GP1 and IgG aβ2GP1-D1 antibodies detection, as we utilized the CIA assay, while they used the ELISA assay10. Interestingly, another study from Italy using CIA for IgG aβ2GP1 and IgG aβ2GP1-D1 determination showed that IgG aβ2GP1-D1 antibodies accounted for 69% of IgG aβ2GP1 antibodies11. Interestingly, one patient was negative for IgG aβ2GP1, but was positive for IgG aβ2GP1-D1. A possible explanation for this discrepancy could be due to the different epitopes recognized by IgG aβ2GP1 and IgG aβ2GP1-D1, as D1 epitope becomes available when β2GP1 transitions from a circular form to a fish-hook conformation4.
Multiple studies have highlighted a strong association between IgG aβ2GP1-D1 antibodies and thrombosis6,7,8,10,11,13. In this study, we found a significant correlation between IgG aβ2GP1-D1 antibodies with thrombotic events in Chinese patients with APS (OR, 3.27; 95% CI, 1.59–6.71). Strikingly, there was a further increase in the OR value when IgG aβ2GP1-D1 antibodies cutoff value was raised to >40 (OR, 4.48; 95% CI, 1.80–11.14). It is also noteworthy to mention that IgG aCL, IgG aβ2GP1, and LAC were also found significantly associated with thrombotic events in our study. De Laat et al. reported an OR of 3.5 (95% CI, 2.3–5.4) between IgG aβ2GP1-D1 antibodies and thrombotic events in an international multicenter study10, which is similar to what we observed. However, they only observed a week but significant association between LAC and thrombotic events (OR, 1.8; 95% CI, 1.1–3.1), and no association between aCL and thrombotic events (OR, 1.1; 95% CI, 0.6–2.1). As the patients from their study were selected based on positivity in the IgG/IgM aβ2GP1 ELISA, the bias might lead the results to favor the aβ2GP1 antibodies. Notably, antibodies to aβ2GP1-Domain 4/5 (D4/5) have also been characterized23,24,25,26. However, no associations were identified between aβ2GP1-Dm4/5 and thromboembolic events27. Interestingly, a recent study suggested that asymptomatic aPL carriers had higher levels of IgG aβ2GP1-D4/5, and an aβ2GP1-D1 to aβ2GP1- D4/5 ratio of ≥1.5 was predictive of systemic autoimmunity28. Thus, further studies are needed to determine the levels of IgG aβ2GP1-D4/5 in Chinese patients with APS using CIA, especially the ratio of aβ2GP1-D1 to aβ2GP1- D4/5.
Increasing evidence suggest that multiple positivity of aPLs are important parameters for risk assessment29,30. Interestingly, we observed that, 4 patients with PAPS had multiple thrombosis, and three of them exhibited triple-positive aPL profile and one showed positive LAC. Additionally, 3 patients with PAPS had both thrombosis and obstetric complications, and 2 of them exhibited triple-positive aPL profile and one showed positive LAC. In patients with APSAOD, 12 patients showed multiple thrombosis, and 5 of them showed triple-positive aPL profile, 5 of them displayed double-positive aPL profile, and the rest 2 patients exhibited positive LAC. In addition, 11 patients with APSAOD had both thrombosis and obstetric complications, and 5 of them exhibited triple-positive aPL profile, and the rest 6 patients showed double-positive aPL profile (data not shown). Notably, in patients with PAPS, 2 patients out of 35 (5.7%, one patient with LAC+/aCL+ and the other patient with aCL+/aβ2GP1+) exhibited double-positive aPL profile, while in patients with APSAOD, 15 patients out of 51 (29.4%, 11 patients with LAC+/aβ2GP1+ and 4 patients with aCL+/aβ2GP1+) showed double-positive aPL profile (data not shown). More importantly, in this study, we found significantly higher levels of IgG aβ2GP1-D1 antibodies in patients with triple-positive aPL profile, further supporting the importance of IgG aβ2GP1-D1 antibodies in evaluation of the APS clinical risks.
In contrast to the association between aβ2GP1-D1 antibodies with thrombosis, we did not observe any significant correlation between IgG aβ2GP1-D1 antibodies and obstetric complications, which differs from previous studies6,7,8,10,11. Moreover, no significant associations were observed between IgG aCL, IgG aβ2GP1, or LAC and obstetric complications. Different ethnic/geographic backgrounds might contribute to this discrepancy. Further studies with more APS patients with obstetric complications are needed.
It should be noted, however, that several limitations exist in this study. First, the diagnosis of patients with APS in this study requires the presence of at least one of the aPLs (LA, aCL, and aβ2GP1 autoantibodies)2, which might exclude the seronegative APS patients31. Second, we used sera from homogenous Chinese Han population. A multicenter study with different ethnic backgrounds is needed for generalizing our data to wider populations. Third, thrombosis is unusual in young non-APS subjects. Thus, patients with non-APS thrombosis were younger than patients with APS, as we wanted to reflect the real epidemiology in patients with non-APS thrombosis. Last, as mentioned before, more APS patients with obstetric complications are needed to assess the association of IgG aβ2GP1-D1 antibodies and obstetric complications.
In summary, our data suggest a potential role of IgG aβ2GP1-D1 antibodies in identifying APS patients with high risk of thrombosis, and thus could serve as a promising biomarker in clinical and therapeutic decision-making process. Our findings might shed insight on the introduction of IgG aβ2GP1-D1 antibodies in the laboratory diagnosis of APS in Chinese hospitals.
Methods
Subjects and Specimen Collections
Sera from 229 subjects were collected and analyzed in this study (Table 1). All the subjects were Chinese Han population. These subjects included 35 patients with primary APS (PAPS), 51 patients with APS associated to other diseases (APSAOD) (43 patients with SLE, 1 patient with both SLE and Sjögren’s syndrome (SS), 4 patients with connective tissue diseases, 1 patient with primary SS, 1 patient with Waldenstrom macroglobulinemia and 1 patient with tuberculous pleurisy), 30 patients with non-APS thrombosis, 32 patients with non-APS pregnancy-related morbidity (PRM), 42 patients with systemic lupus erythematosus (SLE), and 39 healthy controls (HC). HC were defined as no signs of infection or inflammation or other significant illnesses. APS was diagnosed according to the Sydney revised Sapporo guidelines2. Specifically, a combination of one positive clinical criterion and one positive laboratory criterion (LAC, aCL or aβ2G1 antibodies determined by ELISA) on two different occasions separated by 12 weeks were used for the diagnosis2. For patients with PAPS, treatments of patients at the time of serum collection include Aspirin (10/35, 28.6%), Warfarin (15/35, 42.9%), Heparin (7/35, 20.0%), Glucocorticoids (2/35, 5.7%), and Hydroxychloroquine (1/35, 2.9%). For patients with APSAOD, treatments of patients at the time of serum collection include Aspirin (19/51, 37.3%), Warfarin (21/51, 41.2%), Heparin (14/51, 27.5%), Glucocorticoids (24/35, 47.1%), and Hydroxychloroquine (15/51, 29.4%). The median time intervals between clinical events and the time of serum collection were 3 years (0.2–8 years) for patients with thrombosis and 1.5 years (0.3–6 years) for patients with obstetric complications. Clinical and laboratory features were collected from all the subjects. The presence of arterial and venous thrombosis in patients with PAPS, APSAOD, non-APS thrombosis, non-APS PRM, and SLE were 25.7% and 40.0%, 37.3% and 51.0%, 16.7% and 86.7%, 0 and 3.0%, and 2.3% and 0, respectively. The incidence of obstetric complications in patients with PAPS, APSAOD, non-APS thrombosis, non-APS PRM, and SLE were 52.6%, 51.4%, 0, 100%, and 0, respectively. LAC was determined by updated guidelines, as previously described21. The presence of LAC in patients with PAPS, APSAOD, non-APS thrombosis, non-APS PRM, and SLE were 71.4%, 78.4%, 6.7%, 3.1%, and 11.9%, respectively. Study protocols were reviewed and approved by the Ethical Committee of Peking Union Medical College Hospital (PUMCH) and informed consents were obtained from all participants. The study was conducted in accordance with the approved guidelines. All sera were stored at −20 °C until analysis.
Serum aPL Antibodies Determination
Serum IgG and IgM aCL and IgG and IgM aβ2GP1 antibodies were determined by CIA (QUANTA Flash® assays, INOVA Diagnostic, Inc, San Diego, CA) according to the manufacturer’s instructions, as previously described14,32. Serum IgG aβ2GP1-D1 antibodies were measured by CIA from QUANTA Flash® β2GPI Domain 1 (INOVA Diagnostic, Inc, San Diego, CA). The principle and procedures of the QUANTA Flash® β2GPI Domain 1 was previously described by Pengo et al.14. The cutoff values were set based on the recommendations by the manufacturer.
Statistical Analysis
Prism 5.02 (GraphPad Software, San Diego, California, USA) was utilized for all statistical tests. Data of IgG aβ2GP1 (CU) and IgG aβ2GPI-D1 antibodies were transformed into log10 to create the Gaussian distribution. One-way ANOVA was used to calculate the difference between groups. Spearman’s correlation test was performed to analyze the correlation between IgG aβ2GP1 and IgG aβ2GPI-D1 antibodies. p values of less than 0.05 were considered statistical significant.
Additional Information
How to cite this article: Zhang, S. et al. Evaluation of the diagnostic potential of antibodies to beta2-glycoprotein 1 domain 1 in Chinese patients with antiphospholipid syndrome. Sci. Rep. 6, 23839; doi: 10.1038/srep23839 (2016).
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China Grants No. 81373188, 81172857 (to YL), 81302592 (to SZ), the Chinese National High Technology Research and Development Program, Ministry of Science and Technology Grants No. 2011AA02A113, the National Science Technology Pillar Program in the 12nd Five-year Plan No. 2014BAI07B00, the capital health research and development of special grants No. 2014-1-4011 (to YL).
Footnotes
Author Contributions Z.S., F.Z. and Y.L. designed the study. Z.S. and Z.W. performed the experiments, analyzed the data, and drafted the manuscript. Y.L. interpreted the data and wrote the manuscript. S.C., J.L., X.W., L.L., W.Z. and J.Z. participated in sample and data collection. All authors have read and approved the final manuscript.
References
- Meroni P. L., Borghi M. O., Raschi E. & Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 7, 330–339 (2011). [DOI] [PubMed] [Google Scholar]
- Miyakis S. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4, 295–306 (2006). [DOI] [PubMed] [Google Scholar]
- de Groot P. G. & Meijers J. C. β(2)-Glycoprotein I: evolution, structure and function. J Thromb Haemost 9, 1275–1284 (2011). [DOI] [PubMed] [Google Scholar]
- de Laat B., Derksen R. H., van Lummel M., Pennings M. T. & de Groot P. G. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood 107, 1916–1924 (2006). [DOI] [PubMed] [Google Scholar]
- Iverson G. M., Victoria E. J. & Marquis D. M. Anti-beta2 glycoprotein I (beta2GPI) autoantibodies recognize an epitope on the first domain of beta2GPI. Proc Natl Acad Sci USA 95, 15542–15546 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M., Norman G. L., Meroni P. L. & Khamashta M. Autoantibodies to domain 1 of beta 2 glycoprotein 1: a promising candidate biomarker for risk management in antiphospholipid syndrome. Autoimmun Rev 12, 313–317 (2012). [DOI] [PubMed] [Google Scholar]
- Chighizola C. B., Gerosa M. & Meroni P. L. New tests to detect antiphospholipid antibodies: anti-domain I beta-2-glycoprotein-I antibodies. Curr Rheumatol Rep 16, 402 (2014). [DOI] [PubMed] [Google Scholar]
- de Laat B., Derksen R. H., Urbanus R. T. & de Groot P. G. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood 105, 1540–1545 (2005). [DOI] [PubMed] [Google Scholar]
- Ioannou Y. et al. In vivo inhibition of antiphospholipid antibody-induced pathogenicity utilizing the antigenic target peptide domain I of beta2-glycoprotein I: proof of concept. J Thromb Haemost 7, 833–842 (2009). [DOI] [PubMed] [Google Scholar]
- de Laat B. et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J Thromb Haemost 7, 1767–1773 (2009). [DOI] [PubMed] [Google Scholar]
- Pengo V. et al. Antiphospholipid syndrome: antibodies to Domain 1 of β2-glycoprotein 1 correctly classify patients at risk. J Thromb Haemost 13, 782–787 (2015). [DOI] [PubMed] [Google Scholar]
- Bertolaccini M. L. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev 13, 917–930 (2014). [DOI] [PubMed] [Google Scholar]
- Mondejar R. Role of antiphospholipid score and anti-β2-glycoprotein I Domain I autoantibodies in the diagnosis of antiphospholipid syndrome. Clin Chim Acta 431, 174–178 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Evaluation of the Clinical Performance of a Novel Chemiluminescent Immunoassay for Detection of Anticardiolipin and Anti-Beta2-Glycoprotein 1 Antibodies in the Diagnosis of Antiphospholipid Syndrome. Medicine (Baltimore) 94, e2059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis C. et al. A non-complement-fixing antibody to β2 glycoprotein I as a novel therapy for antiphospholipid syndrome. Blood 123, 3478–3487 (2014). [DOI] [PubMed] [Google Scholar]
- Pericleous C. Proof-of-concept study demonstrating the pathogenicity of affinity-purified IgG antibodies directed to domain I of β2-glycoprotein I in a mouse model of anti-phospholipid antibody-induced thrombosis. Rheumatology (Oxford) 54, 722–727 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti T. et al. Autoantibodies specific to a peptide of β2-glycoprotein I cross-react with TLR4, inducing a proinflammatory phenotype in endothelial cells and monocytes. Blood 120, 3360–3370 (2012). [DOI] [PubMed] [Google Scholar]
- Devreese K. M. Standardization of antiphospholipid antibody assays. Where do we stand? Lupus 21, 718–721 (2012). [DOI] [PubMed] [Google Scholar]
- Rodríguez-García V., Ioannou Y., Fernández-Nebro A., Isenberg D. A. & Giles I. P. Examining the prevalence of non-criteria anti-phospholipid antibodies in patients with anti-phospholipid syndrome: a systematic review. Rheumatology (Oxford) 54, 2042–2050 (2015). [DOI] [PubMed] [Google Scholar]
- Roubey R. A. Autoantibodies to phospholipid-binding plasma proteins: a new view of lupus anticoagulants and other “antiphospholipid” autoantibodies. Blood 84, 2854–2867 (1994). [PubMed] [Google Scholar]
- Pengo V. et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 7, 1737–1740 (2009). [DOI] [PubMed] [Google Scholar]
- Iverson G. M. et al. Use of single point mutations in domain I of beta 2-glycoprotein I to determine fine antigenic specificity of antiphospholipid autoantibodies. J Immunol 169, 7097–7103 (2002). [DOI] [PubMed] [Google Scholar]
- Iverson G. M. et al. Patients with atherosclerotic syndrome, negative in anti-cardiolipin assays, make IgA autoantibodies that preferentially target domain 4 of beta2-GPI. J Autoimmun 27, 266–271 (2006). [DOI] [PubMed] [Google Scholar]
- Arvieux J. et al. Distinguishing features of anti-beta2 glycoprotein I antibodies between patients with leprosy and the antiphospholipid syndrome. Thromb Haemost 87, 599–605 (2002). [PubMed] [Google Scholar]
- Ambrozic A. et al. Anti-beta(2)-glycoprotein I antibodies in children with atopic dermatitis. Int Immunol 14, 823–830 (2002). [DOI] [PubMed] [Google Scholar]
- Andreoli L. et al. Anti-β2-glycoprotein I IgG antibodies from 1-year-old healthy children born to mothers with systemic autoimmune diseases preferentially target domain 4/5: might it be the reason for their ‘innocent’ profile? Ann Rheum Dis 70, 380–383 (2011). [DOI] [PubMed] [Google Scholar]
- Pengo V. et al. Antibodies to Domain 4/5 (Dm4/5) of β2-Glycoprotein 1 (β2GP1) in different antiphospholipid (aPL) antibody profiles. Thromb Res 136, 161–163 (2015). [DOI] [PubMed] [Google Scholar]
- Andreoli L. et al. Clinical characterization of antiphospholipid syndrome by detection of IgG antibodies against β2 -glycoprotein i domain 1 and domain 4/5: ratio of anti-domain 1 to anti-domain 4/5 as a useful new biomarker for antiphospholipid syndrome. Arthritis Rheumatol 67, 2196–2204 (2015). [DOI] [PubMed] [Google Scholar]
- Pengo V. et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 8, 237–242 (2010). [DOI] [PubMed] [Google Scholar]
- Otomo K. et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum 64, 504–512 (2012). [DOI] [PubMed] [Google Scholar]
- Cousins L. et al. Antibodies to domain I of β-2-glycoprotein I and IgA antiphospholipid antibodies in patients with ‘seronegative’ antiphospholipid syndrome. Ann Rheum Dis 74, 317–319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M. et al. Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies. Clin Chim Acta 413, 719–726 (2012). [DOI] [PubMed] [Google Scholar]